Abstract
Photobiomodulation-based (LLLT) therapies show tantalizing promise for treatment of skin diseases. Confidence in this approach is blighted however by lamentable inconsistency in published experimental designs, and so complicates interpretation. Here we interrogate the appropriateness of a range of previously-reported treatment parameters, including light wavelength, irradiance and radiant exposure, as well as cell culture conditions (e.g., serum concentration, cell confluency, medium refreshment, direct/indirect treatment, oxygen concentration, etc.), in primary cultures of normal human dermal fibroblasts exposed to visible and near infra-red (NIR) light. Apart from irradiance, all study parameters impacted significantly on fibroblast metabolic activity. Moreover, when cells were grown at atmospheric O2 levels (i.e. 20%) short wavelength light inhibited cell metabolism, while negligible effects were seen with long visible and NIR wavelength. By contrast, NIR stimulated cells when exposed to dermal tissue oxygen levels (approx. 2%). The impact of culture conditions was further seen when inhibitory effects of short wavelength light were reduced with increasing serum concentration and cell confluency. We conclude that a significant source of problematic interpretations in photobiomodulation reports derives from poor optimization of study design. Further development of this field using in vitro/ex vivo models should embrace significant standardization of study design, ideally within a design-of-experiment setting.
Introduction
Photobiomodulation (PBM) or low-level light therapy (LLLT)1 is a rapidly expanding field, showing encouraging results for treatment of cutaneous disorders2, 3 and a wider range of health conditions4–6.
In dermatology, application of PBM spans the cosmetic and medical fields including androgenetic alopecia7, 8, alopecia areata9, skin rejuvenation10, wound healing11 and alleviation of psoriasis vulgaris and atopic dermatitis symptoms12, 13, where many commercialized professional and home-use devices exist3, 14.
Therapeutic action of PBM is attributed to non-thermal photochemical or photobiological action of light via interaction with a range of endogenous photoreceptors and chromophores contained in human skin3, 15, 16. Photoreceptors and mediators of the photobiological action of visible and NIR light include cytochrome c oxidase, nitrosated and flavo- proteins, opsins and ion-gated channels3, 17–19. Meanwhile, investigations on potential action mechanisms of PBM using in vitro and ex vivo models continue, but often reporting very contradictory results2, 7, 20, 21.
A remarkably wide range of optical parameters has been applied to both in vitro and ex vivo model systems (variable across 2 orders of magnitude), where negative, positive and neutral outcomes are reported for visible and near-infrared (NIR) light despite very similar optical settings3, 8, 9, 11. For example, exposure of human epidermal keratinocytes to similar doses of short wavelength visible light (i.e., 420 and 450 nm) were reported to alternately induce and reduce expression of differentiation markers20, 22.
Clarity on how one particular optical setting truly impacts on cell behavior is further compromised in published reports that don’t even disclose parameters applied or if they do sadly report them incorrectly3.
It now appears clear that gene expression patterns can be influenced in a dynamic time-varying manner by a single light treatment of defined parameters further suggesting that experimental outcomes are tightly coupled to treatment regime23.
Previously we have demonstrated that variations in treatment protocol and cell culture conditions result in different outcomes despite using the same light parameter24. In particular, interaction between components of cell culture medium and blue light (450 nm) can lead to cytotoxicity in human dermal fibroblasts when irradiated in the DMEM culture medium, but that this effect can be prevented by medium refreshment directly after light treatment24.
Therefore, we hypothesized that in vitro cell response to light is not exclusively defined by optical parameters and regimes, but rather also depends on treatment protocols. Variations in the latter may account for the myriad inconsistencies in the PBM literature3.
A number of factors can potentially affect PBM experimental outcomes such as cell confluency, passage, donor, donor age, body site, as well as differences in gene expression programs between primary cells and cell lines and many others even more difficult to apprehend such as the lack of standardization of the composition of bioactive compounds in FBS batch25, 26. Additional factors will also involve the biological diversity of any one histologically-distinct cell type e.g., different subtypes of skin dermal fibroblast (DF)27, 28 including the highly proliferative and low metabolically active papillary, and the less proliferative, more metabolically active reticular dermal fibroblasts29 and other cutaneous fibroblast subpopulations such as dermal sheath and dermal papilla fibroblasts of the hair follicle29 with strikingly different morphology, metabolism and gene expression. It is expected that these different cutaneous fibroblast subtypes will respond differently to light30, 31.
Therefore robust assessment of the therapeutic value of PBM requires multidimensional investigations, where optical (wavelength, radiant exposure, irradiance) and biological factors (cell type, subtype), as well as treatment protocols (environment of the cells during culture and treatment, supplements/components in growth medium, treatment iteration etc.) all need to be evaluated.
The aim of this study was to investigate the impact of several key factors such as wavelength, irradiance, radiant exposure, serum concentration, cell culture confluency, environmental oxygen concentration, light-based treatment regime and cell culture protocols on the response to light of human dermal fibroblasts in vitro.
Results
Impact of optical parameters on the response of human dermal fibroblasts to light treatment
Accordingly to a body of research on PBM, the wavelength of light and the radiant exposure exert the most profound effect on cell response to optical radiation, moreover reports indicating importance of irradiance in defining cell behavior upon irradiation can also be found in literature2, 32. Indeed, our results demonstrate that wavelength (p < 0.001) and radiant exposure (p < 0.001) of light had the strongest impact on the metabolic activity of human DF. At the same time, irradiance showed no or little effect (p = 0.6) (Fig. 1A–C). We observed that, on average, shorter wavelengths (450, 500 and 530 nm) have a large impact on fibroblast metabolic activity, while long wavelengths (>550 nm) show negligible effects within the tested radiant exposures when cells were cultured at 20% environmental oxygen (atmospheric level).
Figure 1.
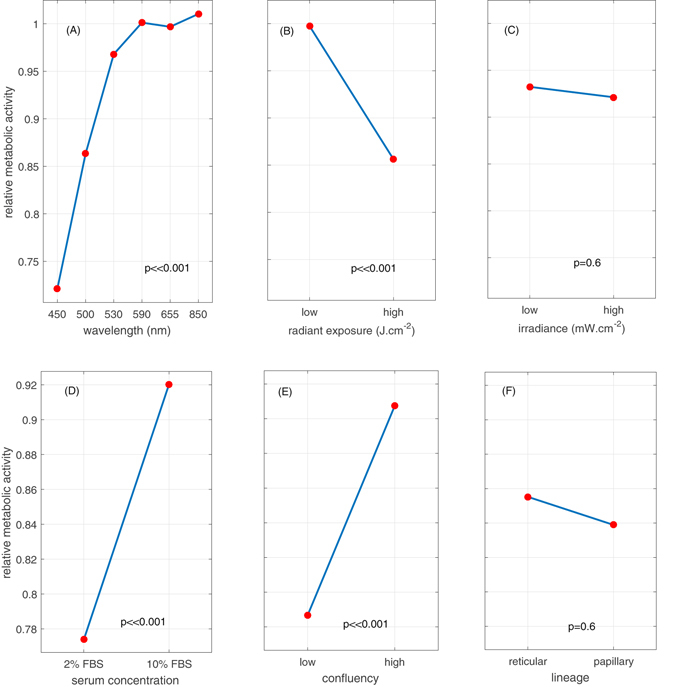
Main effects plot of the wavelength, radiant exposure, irradiance, serum concentration, initial cell confluency and lineage on the relative metabolic activity of fibroblasts. Main effects plot of the wavelength (A), radiant exposure (B) and irradiance (C) on the relative metabolic activity of human dermal fibroblasts (reticular and papillary). Data points correspond to grand means, i.e. the average value of the metabolic activity for each level of one selected factor (wavelength, dose or irradiance) while all the levels of the two other factors are averaged. Statistical significance was evaluated using ANOVA (N = 3, 2 lineages and 3 replicates). The levels of radiant exposure correspond to 2 J.cm−2 (low) and 30 J.cm−2 (high), the levels of irradiance depended on wavelength and are reported in Table 1 in the Materials and Methods Section. Main effects plot of serum concentration (D), initial confluency (E) and lineage (F) on the relative response of the metabolic activity of human dermal fibroblasts after light treatment (450 nm, 2 to 60 J.cm−2). Data points correspond to grand means, i.e. the average value of the metabolic activity for each level of one selected factor (serum concentration, confluency, lineage) while all the levels of the two other factors are averaged. Statistical significance was evaluated using ANOVA (N = 2, 2 lineages and 3 replicates). The initial ratio at the moment of seeding between ‘high’ and ‘low’ confluency groups is 5. The control group is has an average relative metabolic activity of 1.
Wavelength and radiant exposure of light were strongly linked (Fig. 2A p < 0.001), whereby a radiant exposure-dependent cell behavior was seen with the short visible wavelengths (<550 nm). Low light exposure (2 J.cm−2) exhibited essentially neutral effects on fibroblast metabolic activity at all wavelengths, while cell inhibitory effects were seen at high exposures of light at 450, 500 and 530 nm (Fig. 2).
Figure 2.
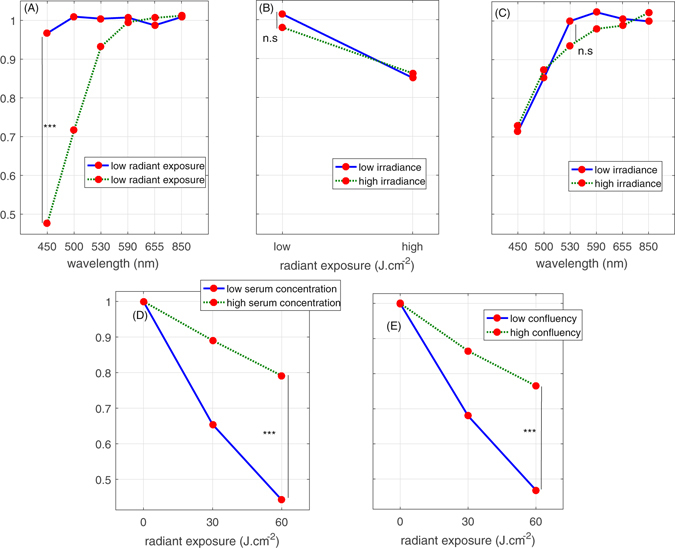
Interactions plot between the wavelength, radiant exposure, irradiance, cell confluency and serum concentration and their relative impact on human dermal fibroblasts metabolic activity. Interactions between wavelength and radiant exposure (A), irradiance and radiant exposure (B) and irradiance and wavelength (C) and their relative impact on human dermal fibroblasts metabolic activity (reticular and papillary). Data points correspond to grand means, i.e. the average value of the metabolic activity for each level of two selected factors (wavelength and radiant exposure, radiant exposure and irradiance or wavelength and irradiance) while all the levels of the remaining factor are averaged. Statistical significance was evaluated using ANOVA (N = 3, 2 lineages and 3 replicates). The levels of radiant exposure correspond to 2 J.cm−2 (low) and 30 J.cm−2 (high), the levels of irradiance depended on wavelength and are reported in Table 1 in the Materials and Methods Section. Interactions between levels of radiant exposure of 450 nm light and serum concentration (D), and initial confluency (E) and their impact on the relative response of the metabolic activity of human dermal fibroblasts. Data points correspond to grand means, i.e. the average value of the metabolic activity for each level of two selected factors (radiant exposure and serum concentration or radiant exposure and confluency) while all the levels of the remaining factor are averaged. Statistical significance was evaluated using ANOVA (N = 2, 2 lineages and 3 replicates). The initial ratio at the moment of seeing between ‘high’ and ‘low’ confluency groups is 5. The control group has an average relative metabolic activity of 1.
By contrast, no interaction was observed between irradiance levels and any of the optical factors (i.e. wavelength and radiant exposure, Fig. 2B,C) in terms of cell metabolic activity, specifically meaning that a choice of irradiance at a fixed dose and a wavelength did not impact cell response.
Impact of biological factors on the response of human dermal fibroblasts to light treatment
To assess the impact of several biological factors on the response of DFs to light we selected a single wavelength (450 nm) as it exerted the strongest effect on the relative metabolic activity of the target cells, and varied radiant exposure from 2 J.cm−2 to high 60 J.cm−2 (Fig. 1D–F).
Fibroblast confluency and serum concentration strongly influence how specific light parameters affect cell behavior (Fig. 1D,E). In particular, lower cell confluency or lower serum concentration resulted in stronger light-associated inhibition of cell metabolic activity (Fig. 2D,E). Both lineages of fibroblasts (i.e., reticular and papillary) responded similarly to test light parameters (Fig. 1F).
As expected, serum concentration and confluency were related. High serum concentrations drove higher fibroblast proliferation and so confluency, given equal initial seeding density (Fig. 3, A B control bars).
Figure 3.
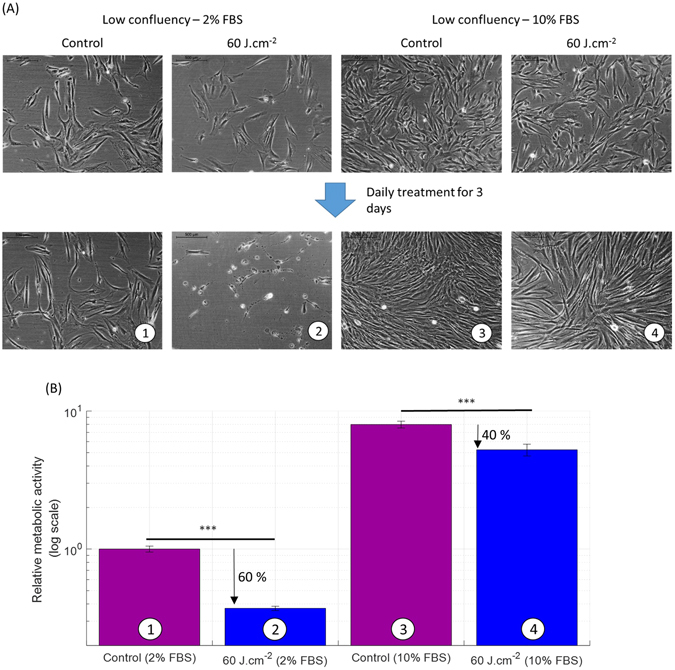
Serum concentration in the culture medium reduces the inhibitory effect of blue light (450 nm) on the metabolic activity of human dermal fibroblasts. (A) Phase-contrast images of human reticular fibroblasts on day 0 (8 days after seeding) at low initial confluency and cultured in DMEM with two serum concentrations 2% and 10% (upper line), and after three daily light treatments on day 4 at a low initial confluency (lower line). Papillary fibroblasts were found to behave similarly (not shown). A second donor responded similarly (not shown). (B) Relative metabolic activity of human reticular fibroblasts after light treatment at 450 nm, 50 mW.cm−2 with 60 J.cm−2 (log scale). We observe a 60% reduction in 2% FBS against 40% reduction in 10% FBS case compared to control. The high confluency level showed similar variation as a function of the change in FBS concentration (not shown), except for the cytotoxic effects observed in low serum/low confluency only. Statistical significance was evaluated using ANOVA. A second donor responded similarly (not shown as the scale is absolute). The control group has an average relative metabolic activity of 1. (N = 2, 2 lineages and 3 replicates).
In order to isolate the effect of serum concentration alone on the impact of light treatment, cells both at low and high confluency were treated using the same light parameter and protocol at the two culture conditions, 2% FBS and 10% FBS. At 60 J.cm−2 (Fig. 3B) the reduction of the metabolic activity of the fibroblasts grown in low serum was much stronger than at high serum, (60% versus 40%, respectively). This effect was also reflected in changes in cell morphology. Light treatment (450 nm, 60 J.cm−2) in low serum and low confluency fibroblasts triggered cytotoxic effects (Fig. 3A), while cells in high serum and low confluency (Fig. 3A) and in combinations of high confluency/low serum and high confluency/high serum (data not shown) exhibited no cytotoxic effects.
To further investigate the effect of defined light parameters on DF metabolic activity, experiments were carried out at two different cell confluency levels: low (20%, 5,000 cells per 1.77 cm2 well area at seeding) and a very high (90%, 30,000 cells per 1.77 cm2 well area at seeding) (Fig. 4A). Remarkably, the same light parameter applied to what is typically assumed in the PBM literature as ‘identical’ cells, albeit at different confluencies, resulted in opposite effects (Fig. 4B). This was clearly different at two dose levels used, i.e., 2 and 30 J.cm−2 (only the higher dose is shown). It appears that DF at high confluencies (>90%) may be more ‘protected’ in response to 450 nm light, at least in context of metabolic activity.
Figure 4.
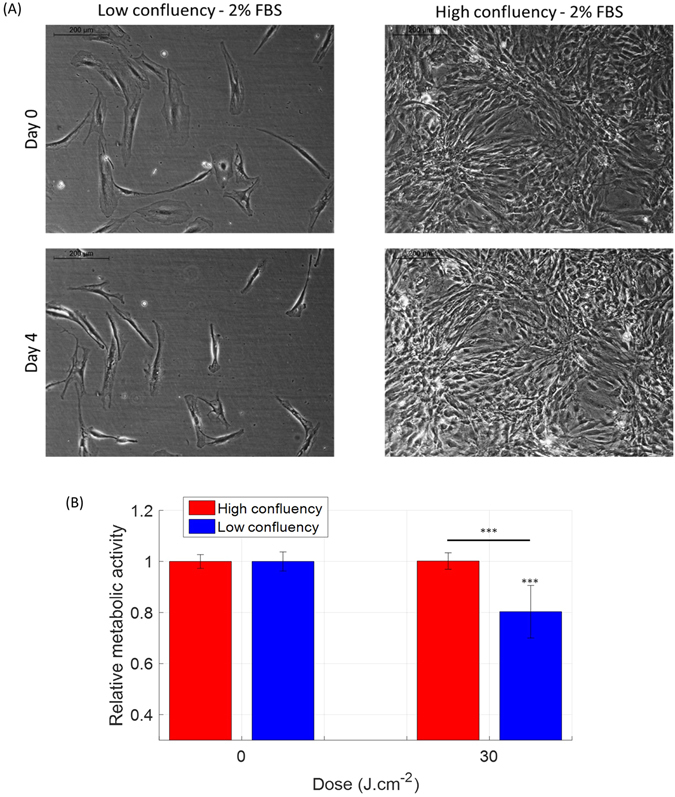
An increase in the cell confluency of human dermal fibroblasts reduces the inhibitory effect of blue light (450 nm) on their metabolic activity. (A) Phase-contrast images of human papillary fibroblasts on day 0 (8 days after seeding) at a low initial confluency (upper left) and high initial confluency (upper right), and after three light treatments on day 4 at a low initial confluency (lower left) and high initial confluency (lower right). Reticular fibroblasts were found to behave similarly (not shown). (B) Relative metabolic activity of human papillary fibroblasts after light treatment at 450 nm, 50 mW.cm−2 with 30 J.cm−2 in function of the confluency level. Statistical analysis was evaluated using student t-tests between the control group and the treated group (N = 3, 2 lineages and 3 replicates), with following thresholds *p < 0.05, **p < 0.01, ***p < 0.001. The control group has an average relative metabolic activity of 1. The significance of the interaction between the radiant exposure and the confluency level was much lower than 0.001.
Impact of the treatment regimes and protocols on dermal fibroblast metabolic activity
The choice of treatment regime, in particular, the number of consecutive treatments, impacts on the cell response to light, reflected in both cell metabolic activity and cell morphology. More specifically, a single exposure to 2 J.cm−2 and 30 J.cm−2 of 450 nm light resulted in an increase in metabolic activity, while 2, 3 or 4 consecutive treatments (Fig. 5B) reversed the effect, leading to decrease in cell metabolism.
Figure 5.
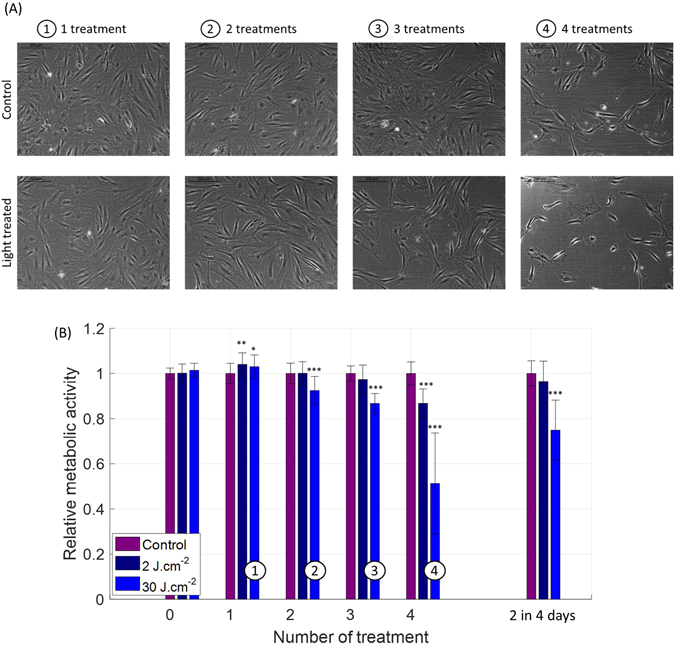
Response of human dermal fibroblasts to variable number of treatments with blue light (450 nm) occurring on consecutive days. (A) Phase-contrast images of human reticular fibroblasts 24 h after the last treatment, when 1, 2, 3 or 4 daily treatments (30 J.cm−2) were performed (lower line), together with the images of the control group (upper line). (B) Relative metabolic activity of human papillary and reticular fibroblasts (merged) after light treatment for 2 radiant exposures of 450 nm light (at a fixed 50 mW.cm−2 irradiance) and with variable number of treatments occurring on consecutive days. An irradiance of 50 mW.cm−2 was used. Statistical analysis was evaluated using student t-tests between the control group and treated group (N = 2, 2 lineages and 3 replicates), with following thresholds *p < 0.05, **p < 0.01, ***p < 0.001. The control group has an average relative metabolic activity of 1. The significance of the interaction between the radiant exposure and the number of iterative treatment was much lower than 0.001.
Observed changes in metabolic activity were accompanied by morphological alterations (Fig. 5A), such as shrinkage of cells, which became more pronounced with an increase in the number of exposures. After 4 treatments, cells were significantly smaller than the corresponding control cells. This observed steady shrinking of cell volume could reflect the observed decrease in cell metabolic activity. A similar effect was also present in cells grown at low confluency (Fig. 4A).
Reducing light treatment frequency, from daily, every 24 hours, to every other day, every 48 hours, did not change the observed drop in metabolic activity or effect of cell morphology (Fig. 5B), suggesting that the initial exposure triggers a chain of molecular reactions lasting longer than 24 hours.
Secondly, the treatment protocol used, in particular the culture medium components exerted a significant impact on the outcome of the light exposure to short visible wavelengths (Fig. 6A).
Figure 6.
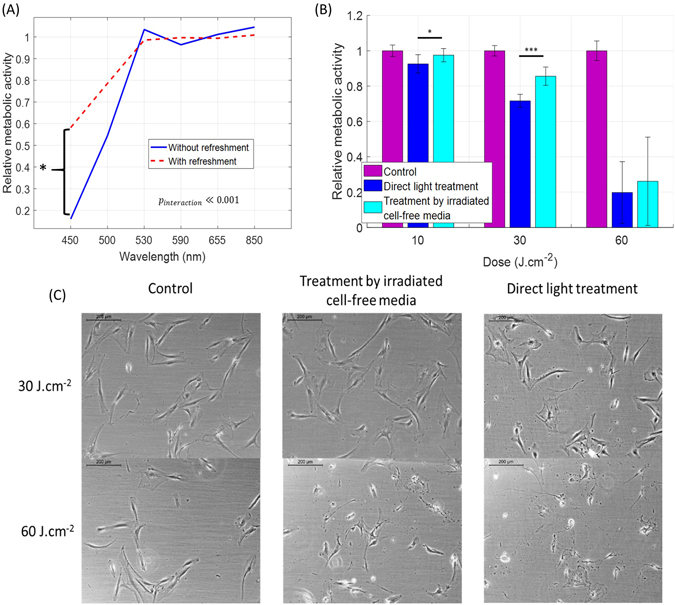
The inhibitory effect of blue light (450 nm) on the metabolic activity of human dermal fibroblasts is mediated by components present in the culture medium. (A) Relative metabolic activity of fibroblasts after light treatment as a function of the wavelength and the treatment regime (one or more consecutive irradiations) and protocol (direct light exposure or contact with pre-exposed culture medium). Metabolic activity after treatment was normalized to that of the control sample. Number of donors N = 3, number of technical replicates n = 3, data on papillary and reticular pools were merged together. The significance of the interaction between wavelength and treatment method was evaluated with ANOVA (p ≪ 0.001). (B) Relative metabolic activity of the fibroblasts after light treatment for two treatment protocols: direct light exposure (blue) and pre-exposure of DMEM alone followed by pouring on top of the cells (cyan). (C) Phase-contrast pictures of human reticular fibroblasts 24 h after the last treatment, in control group, treated group (30 J.cm−2 and 60 J.cm−2) and treated by irradiated cell-free media. An irradiance of 50 mW.cm−2 was used. Statistical analysis was evaluated using student t-tests between the control group and treated group (N = 3, reticular and 3 replicates), with following thresholds *p < 0.05, **p < 0.01, ***p < 0.001. The control group is has an average relative metabolic activity of 1.
Previously, we have shown that exposure of DF to blue light of 450 nm (30 J.cm−2) in culture media (DMEM), without its subsequent refreshment, resulted in cytotoxic effects 24 hours after treatment. Here we observed a similar effect at the wavelength up to 530 nm, suggesting that a wide range of short visible wavelengths are absorbed by components of the cell culture media, leading to generation of molecules further impacting cell physiology.
We next assessed whether this effect was dependent on cell-free elements of the culture conditions, and found that DF exposure to cell-free media, irradiated using 30 J.cm−2 and 60 J.cm−2, resulted in reduced metabolic activity of the cells. The magnitude of this affect however, was lower than when cells in culture were directed irradiated (Fig. 6B), though fibroblast morphology was impaired only at 60 J.cm−2 (Fig. 6C).
Impact of the oxygen level in cell culture medium on cellular response to light
Recent evidence suggests the existence of gradients of physiological oxygen levels throughout the different layers of human skin layers, where oxygen level in dermis (outside capillary loops) can be as low as 1–5%, compared to 20% atmospheric level33, 34. We therefore studied the effects of lowering oxygen concentration from 20% to 2% in our fibroblast cultures; i.e., to a physiologically-relevant concentration of oxygen in terms of DF interaction with visible light in vivo.
The reduction from 20% to 2% oxygen concentration did not result in noticeable changes in the metabolic activity or morphology in control fibroblast (papillary and reticular) cultures, (Fig. 7A).
Figure 7.
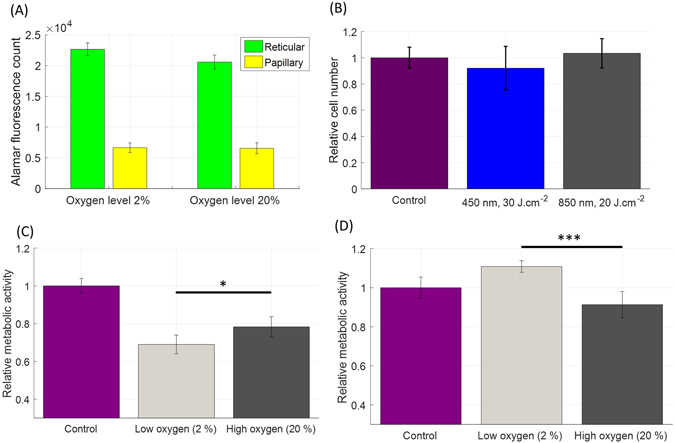
Impact of blue (450 nm) and NIR (850 nm) light treatment on the metabolic activity of human dermal fibroblasts when exposed to dermal tissue oxygen levels (approx. 2%). (A) Fluorescence counts (Alamar Blue) measured in reticular and papillary fibroblasts (facelift, female, 64 yo) in low (2%) and high (20%) oxygen levels. No strong change in Alamar fluorescence count is apparent. (B) Relative cell counts of human dermal fibroblasts (reticular and papillary) after light treatment in hypoxic conditions (2% oxygen). Cells were grown in 35-mm individual dishes and treated with light once a day for three consecutive days. Cell counting was performed 24 h after last treatment. Material originating from two different donors was included in the experiment (N = 2, reticular and papillary, 3 repeats, 12 counts per bar). (C) Relative metabolic activity of human papillary fibroblasts in response to 30 J.cm−2 at 450 nm in low and high environmental oxygen levels (N = 2, papillary and 3 replicates). (D) Relative metabolic activity of human reticular fibroblasts in response to 20 J.cm−2 at 850 nm in low and high environmental oxygen levels (N = 2, reticular and 3 replicates). Irradiance levels were 50 mW.cm−2 (450 nm) and 80 mW.cm−2 (850 nm).
By contrast, lowering oxygen concentration resulted in dramatic differences in fibroblast response to light. In particular, blue light (450 nm) at 30 J.cm−2 resulted in even stronger inhibition of metabolic activity of papillary fibroblasts than when grown in 20% oxygen. Remarkably, NIR light (850 nm) at 20 J.cm−2 stimulated metabolic activity of human reticular fibroblasts at physiological levels of oxygen (i.e. 2%), the effect that was not observed at 20% oxygen level. These effects were not associated with any significant change on the relative cell number (Fig. 7B).
Discussion and Conclusions
The goal of this study was to investigate the impact of optical parameters, cell culture protocols and treatment regimes on the metabolic activity of primary human dermal fibroblasts in vitro culture in response to visible and near-infrared radiation.
To achieve this we systematically studied the impact of wavelength, irradiance, dose, number of consecutive irradiations, serum- and oxygen concentration, cell confluency, medium refreshment and indirect treatment with irradiated medium alone in a design-of-experiment approach. The latter was specifically chosen in order to disentangle the effects caused by a direct interaction of photons of light with cells from the potential contribution of multiple ‘environmental’ factors.
We also aimed to explore whether the response of DF to identical optical parameters would change when cultured at conditions that more closely resemble those in vivo, i.e. low proliferation, cell confluency, serum and oxygen levels, as we felt that this is crucial when considering application of new technologies to in vivo clinical settings.
Several interesting and unexpected findings resulted from the approach, new to the world of photobiomodulation, which cause us to step back and re-assess how we interpret PBM data derived from in vitro studies.
First, all investigated parameters in this study, with the exception of irradiance, significantly impacted skin fibroblast metabolic responses to light.
Secondly, cell culture and treatment conditions (including confluency, serum- and environmental oxygen concentrations and treatment protocols) all significantly influence cellular responses to optical radiation, even when the identical wavelength, irradiance and dose were applied.
While medium-to-high confluency scenario is convention cell culture practice worldwide, in vivo DF are sparsely distributed throughout papillary and reticular dermal compartments. In an attempt to observe cells in a more natural situation, cultured at low confluency, we noticed that three consecutive daily treatments using short-wavelength visible light (450 nm at 30 J.cm−2) reduced their metabolic activity to a greater extent than when maintained at medium-to-high confluency.
Similarly, we observed that DF grown in lower FBS concentration 2%, though still higher than a fibroblast in a non-wounded skin environment would experience, coped less efficiently with induced stress, and were more inhibited by several consecutive exposures to 450 nm light at 30 J.cm−2. This response was clearly different from that of fibroblasts cultured at 10% FBS who were less inhibited and even protected from light-associated reactive oxygen species (ROS), as evidenced by normal metabolic activity and morphology. Similarly to low confluency, lower serum exposure may be more physiologically relevant.
The latter two findings may suggest that ‘non-physiological’ highly confluent cell cultures of DF in 10% FBS may be ‘more protected’ from potential stressors induced by light, such as ROS35, 36, than the cells in vivo. Two factors could potentially play a role here. First factor is a lower proliferative activity and thus lower number of mitotic cells (known to be more vulnerable to noxious factors37, 38 of highly confluent cell cultures of DF. Secondly, we also hypothesize that the higher the number of cells exposed to the light treatment event the stronger the antioxidant defense may be against any ROS created by irradiation of the cell medium constituents, as the latter was kept constant in all experiments regarding of cell number per well.
In addition to cell confluency and serum concentration, we assessed whether continuing to expose fibroblasts to their irradiated medium post-treatment negatively impacted on their viability. Specifically, we have shown that high radiant exposures of short visible wavelengths (450 and 500 nm, at 30 and 60 J.cm−2) suppressed fibroblast metabolic activity, and even resulted in some cytotoxicity when the medium was not refreshed after irradiation. Importantly, irradiation of cell-free culture medium that was then exposed to the cells resulted in an inhibitory effect, suggesting that ROS released after light absorption by riboflavin known to be present in the culture medium may have been at least in part responsible for this observed inhibitory effect. Previously it was shown that riboflavin mediated cytotoxic effects in human skin melanoma cells after irradiation by blue light (470 nm)39. However, it may not be completely artefactual, as it is also known that flavins are present in normal human skin dermis40 where they are likely to respond to clinical PBM modalities. However, we observed greater effects with the irradiated cell plus medium combination (than with the irradiated medium treatment alone), suggesting that there is a distinct cellular–derived component to the light response, indicating that photons of visible light can indeed directly interact with DF cells. Previously, interesting effects were observed in cells irradiated by ionizing radiation where oxidative stress was transferred from the irradiated cell to non-irradiated cells, via the release of components in the medium and even via the irradiated supernatant alone, which received a name ‘bystander effect’41, 42. Several signaling molecules mediated the transfer of oxidative stress. Small molecules, such as Ca2+ or peptides, were transmitted via gap junction intercellular communication to neighboring cells. Larger molecules, which could also be released by the irradiated cells, could propagate the damage and include lipid peroxide products and cytokines and reach neighboring cells by diffusion43, 44.
Next to cell culture conditions, what is less clear in PBM literature is if and how the experimental outcome depends on a number of consecutive treatments. Our observation is that the effect of shorter wavelengths (450 nm–590 nm) is accumulated over increasing number of consecutive daily and every other day treatments, suggests that the light can have prolonged effects on dermal fibroblasts, beyond cessation of irradiation, via the initiation of downstream cascade of cell signaling.
A key finding of our study was the observation that oxygen concentration markedly impacted on the response of dermal fibroblasts to both short visible- and to near-infrared light. Lowering oxygen to physiological level (i.e. 2%) resulted in fibroblast stimulation by near-infrared wavelengths: an observation not seen when cells were exposed to conventional cell culture oxygen levels (i.e. 20%), and so our protocol may allow researchers to appropriately investigate the photobiomodulation effects on skin and hair health. Indeed, researchers have shown the importance of environmental oxygen concentration at the genomic and proteomic levels in cells from various body locations. Physiological levels of oxygen in the body are organ-dependent and generally different from the standard oxygen concentration not only in human skin compartments but also in brain, lungs, liver and other body organs45. This therefore raises the need to distinguish between ‘normoxia’ and ‘physioxia’, and how this could impact PBM studies conducted using in vitro cell culture.
Skin temperature in itself could represent another condition to optimize in the pursuit of in vivo approximation. In the case of skin, the temperature is commonly assumed to be around 34 °C45. However, finding the right skin temperature might be complex for at least two reasons. First, there is evidence showing that skin temperature is highly variable within the body and dependent on the ambient temperature46. Second, related to light treatment in vivo, there will be thermal effects involved due to the strong optical absorption of visible and NIR radiations by skin chromophores (melanin, blood).
Similarly, our data suggests that in vitro protocols may be more physiological if irradiation is done with cells in culture medium rather than in a buffer such as PBS, given that in vivo (both steady state and during wounding/wound-healing) interstitial fluids and extracellular matrix will contain a complex mixture of essential minerals, ions, growth factors, and other nutrients and ligands’, which likely contribute to the total cellular response to light. However, the lack of in vivo ‘flow’ conditions in our static in vitro setup suggests that refreshment of medium after light treatment may be advisable. The downside being the loss of important autocrine cytokines and growth factors. In particular, we know that it may be important in the context of photobiomodulation, where latent growth factors have been shown to be activatable via infrared radiation4.
Interestingly, some of our study findings differ from those previously reported in the literature. First, our results did not show any strong effect of long visible and NIR radiation under environmental partial oxygen pressure. This observation is not surprising by itself, as the literature contains large discrepancies between the optical parameters and experimental outcomes used hitherto, impeding progress of PBM modality as previously reported3. More specifically, the majority of previous studies showed a measurable effect of red and NIR wavelengths in vitro and on ex vivo systems4–6, 19, 47. Other studies, however, found no effect of long visible and NIR wavelengths on skin cells20, 48. While not excluding the impact of cell culture conditions and oxygen levels on these, often opposite, experimental outcomes, we still hypothesize that human dermal fibroblasts, positioned deeper than epidermal melanocytes and keratinocytes, are more shielded from the blue part of the electromagnetic spectrum but are much more exposed to red and NIR components, and therefore could have adapted to them. Consequently one could suspect a stronger resistance of dermal fibroblasts to visible and NIR radiation, particularly in the long wavelength range, due to evolutionary reasons. The same reasoning may suggest that fibroblast originating from more deeply located tissues are more sensitive to light photons.
Second, we did not spot any irradiance effect in vitro despite some existing evidence in the literature both for in vitro and in vivo cases49, 50. However, irradiance effects have not been yet very clearly established in vitro in photobiomodulation. Although we might expect them in vivo, as light will be absorbed by skin chromophores (melanin, blood) and create bulk heat, it is less straightforward to expect irradiance effect in vitro. It might also have been that that our irradiance levels are not separated ‘well enough’ (only a factor 2 to 8 depending on the wavelength).
Third, we did not see any strong effect on the relative cell number after light treatment. However, the literature does contain evidence that short visible wavelengths exert anti-proliferative effects20, 49. The underlying reason of observed differences is that our biological model (low confluency, low serum and low oxygen) was tailored to resemble in vivo conditions of the dermal fibroblasts, where their proliferative activity is very low under non-wounded conditions. Combined with the replenishment of the media after light treatment, we barely can notice any proliferation for the duration of the experiments with light. Therefore, we were not expecting to be able to detect an effect on the relative cell number after treatment with light.
We conclude that in vitro experimental design factors including cell confluency, serum concentration, culture conditions like oxygen level, impact cell behavior33, 51 and so are critical to how we best define cell reactions to light i.e., either inhibitory, neutral or stimulatory, even when responding to light of the identical characteristics. Therefore, we recommend that for all in vitro and ex vivo investigations on photobiomodulation researchers should not only carefully select their experimental conditions as close to the physiological in vivo setting as possible but in any event report these together with their experimental findings. The implications of our study findings are that we recommend that confluency, serum- and environmental oxygen concentration should ideally be much lower than those currently used and reported worldwide in conventional dermal fibroblast culture protocols.
Experimental Procedure and Methods
Light-based devices
A proprietary LED-based device was designed to accommodate a single 24-well culture plate (Fig. 8) and had two sub-modules. Each module illuminated 4 rows of 6 wells. Each row of 6 wells was irradiated with the same wavelength. Rows of sub-module 1 were illuminated using 450, 500, 530 nm, while rows of sub-module 2 used 590, 655 and 850 nm light (Fig. 8A,B). Each sub-module had one row of wells without light treatment, which served as a control. Light leakage from well-to-well and thus light contamination was prevented by using blocking apertures between each individual LEDs (Fig. 8C) and by selecting black 24-well plate (Porvair Science, 303012).
Figure 8.
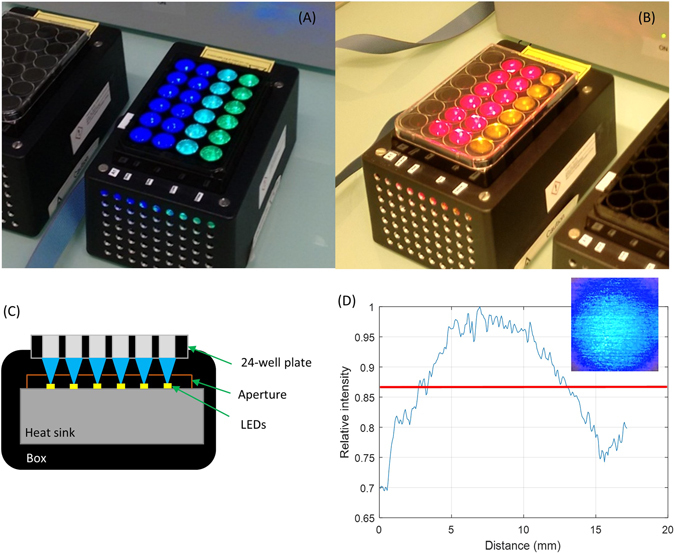
LED-based devices used in the experiments. (A) Photograph of sub-module 1 with the LEDs emitting 450, 500 and 530 nm wavelengths and (B) sub-module 2 with the LEDs emitting 590, 655 and 850 nm wavelengths. (C) Cross-section of the design a module, in the direction of a row of 6 wells of the 24-well plate (D) Typical profile of one single light beam in the axial x-y direction (at 450 nm) under one of the wells of the 24-well plate (the size of 1 well is 15 mm diameter) measured using a standard camera.
The light beam under each well was created by a single LED (Lumileds LUXEON Rebel Color: Royal Blue 450 nm LXML-PR01–0500, Cyan 500 nm LXML-PE01–0070, Green 530 nm LXML-PM01–0090, Amber 590 nm LXML-PL01–0040 and Deep Red 655 nm LXM3-PD01–0300; OSRAM OSLON Black Series SFH4715S 850 nm) (Fig. 8C). The homogeneity of the illumination in x-y plane under each individual well, measured using a camera was within ±15% of the average and was found to be acceptable (Fig. 8D). The exposure time and irradiance settings were controlled via a computer interface. Optical power at the level of the target, i.e., at the bottom of the well-plate, was measured using Ophir Nova II power meter (Ophir Photonics) equipped with detector (PD300–3W). Bottom-illumination of the well-plate avoided unnecessary absorption of light by the support of the module and any potential increase in temperature, both of which can be a problem in case of top-based illumination.
Primary human dermal fibroblast isolation and culture
Human dermal fibroblasts were isolated from fresh surplus residual human facial skin within 8 hours of surgery. Human skin was obtained after facelift surgery from healthy donors adhering to the Declaration of Helsinki principles under Human Tissue Transfer Agreement, between Philips Electronics Nederland B.V. acting through Philips Research and corresponding clinics, where clinics were responsible for having a full written informed consent from all patients. All experimental protocols were approved by the Philips Internal Committee of Biomedical Experiments (ICBE). Philips Electronics Nederland B.V., acting through Philips Research Eindhoven, confirms that it was its responsibility to ensure that approvals by appropriate local and/or national Ethics Committees and relevant authorizations were obtained whenever such approvals were required for the experiment(s).
Papillary and reticular fibroblasts lineages were extracted from superficial and deep dermis, respectively. Cells were initially cultured in DMEM supplemented with penicillin/streptomycin (1%), Glutamax (1%) and fetal bovine serum (FBS) (at 10%). The concentration of FBS was later very gradually reduced to 2% to quiesce fibroblast proliferation but not metabolic activity.
Pairs of reticular and papillary were donor sample-matched, unless otherwise indicated. The total number of donor used in the experiments was 4, the age of the donors was between 47 to 64 years old, the gender of the samples included both male and female and the passage was kept between passage 4 and 8 for all experiments.
Light treatment protocols
The cells were treated daily over 3 consecutive days, during which the culture medium was refreshed after each light treatment, unless stated. Light treatment was conducted outside the culture incubator but never extended beyond a maximum of 20 minutes at room temperature. Any increase in temperature of the cell substratum due to the light treatment was assessed using a FLIR infrared camera (SC600). Thermal increase never exceeded 37 °C (i.e., body and culture incubator temperature) for all tested wavelengths and at all treatment time intervals. We observed very slight heating, originating from the light treatment (maximum of 2oC at 530 nm and 850 nm). This increase did not correlate with the effects that we observed on the fibroblast metabolic activity. Additionally, as treatment is done outside the incubator, the temperature of cells never exceeded the physiological range of skin temperature46. In a typical experiment the same 24-well plate contained both control and treated groups, as one row of the 24-well plate was always kept as control, i.e. not irradiated. This helped to control for any effect due to the treatment being performed outside the incubator. Indeed, both control and treated groups were always under the same ambient conditions. Light treatment was performed using transparent DMEM medium without phenol red (Sigma, D5921).
To assess any potential indirect impact of light on dermal fibroblasts via possible interactions with irradiated DMEM culture medium components, a cell-free DMEM medium was similarly irradiated using the same wavelengths and doses of light and immediately brought in contact with the test cells. The time of cell ‘exposure’ to light-irradiated cell-free medium was equal to that of treatment when light was applied directly on the cells.
Measurement of dermal fibroblast metabolic activity
The metabolic activity of the dermal fibroblasts was assessed using the Alamar Blue® assay52 (Thermofisher, DAL1100). It was chosen for reported smallest variability across the different cell lineages, compared to MTT assay, often considered as the gold standard for determination of cell viability and proliferation53. MTT assay measures cell viability in terms of reductive activity as enzymatic conversion of the tetrazolium compound to water insoluble formazan crystals by dehydrogenases occurring in the mitochondria of living cells although reducing agents and enzymes located in other organelles such as the endoplasmic reticulum are also involved.
In contrast to MTT assay, in Alamar Blue® kit, the conversion of active component, resazurin, to fluorescent resorufin occurs mostly in the mitochondria and the quantity of resorufin generated can therefore be used as indicator of metabolic activity54.
On the day of assessment Alamar Blue® was added to fresh DMEM (10% in volume). The solution was then incubated in contact with the cells for 3 to 5 hours depending on the confluency of cells. The fluorescence generated was read using a plate reader FLUOstar Omega II (ex: 544 nm, em: 590 nm). The experimental outcome of the light-based treatments was expressed in terms of relative metabolic activity, defined as a ratio between the means of the treated groups and the control group.
Assessment of viability and cell number
Automatic cell counting, Vi-CELL XR (Beckman Coulter Life Sciences), was used to assess the cell number. Cells were grown in 35-mm individual dishes and treated with light once a day for three consecutive days. The cells were counted 24 h after the last treatment. For each condition, the cells of 3 individual dishes were pooled together before counting. Cells counting was repeated three times. Material originating from two donors was included in the experiment (N = 2, reticular and papillary, 3 repeats, 12 counts per bar). Trypan blue is automatically added to assess viability as well.
Assessment of cell morphology
Conventional phase-contrast light microscopy (Leica DMIL LED, obj. x10) was used daily to assess the morphological change of the dermal fibroblasts along treatment duration of three days.
Design of experiment and statistical analysis
Previously, we described the statistical approach for a systematic assessment of the impact of optical and biological factors on the response of human dermal fibroblasts to light24. It is based on a factorial design of experiment, allowing identification of the factors having a significant impact on the variation of a variable under investigation, i.e., Alamar Blue® reading in case of this study. Such factors span a multidimensional space and are related to light treatment (i.e., optical variables) and the target and its environment (biological variables). Optical variables included: wavelength (nm), irradiance (mW.cm−2), radiant exposure or dose (J.cm−2), and biological variables included: serum concentration (% FBS), confluency (cells per units of surface), oxygen level (%) and treatment protocols (with or without replenishment of media after treatment, treatment with irradiated cell-free media).
Each factor was then varied among pre-defined levels, in our case – this was equal to 2, except for wavelength (where 6 discrete wavelengths were used). The level intervals were chosen to be large enough to show its impact, if present, on the variable of interest. The selected levels of the optical parameters are given in Table 1, while the levels of the biological factors are given in the Results section.
Table 1.
Light parameters used for treatment of human dermal fibroblasts following a factorial design of experiment.
| Parameter | Wavelength (nm) | Irradiance (mW.cm−2) | Radiant exposure (J.cm−2) | Parameter | Wavelength (nm) | Irradiance (mW.cm−2) | Radiant exposure (J.cm−2) |
|---|---|---|---|---|---|---|---|
| Blue-Low-Low | 450 | 10 | 2 | Yellow-Low-Low | 590 | 3 | 2 |
| Blue-Low-High | 450 | 10 | 30 | Yellow-Low-High | 590 | 3 | 10 |
| Blue-High-Low | 450 | 50 | 2 | Yellow-High-Low | 590 | 7 | 2 |
| Blue-High-High | 450 | 50 | 30 | Yellow-High-High | 590 | 7 | 10 |
| Cyan-Low-Low | 500 | 10 | 2 | Red-Low-Low | 655 | 10 | 2 |
| Cyan-Low-High | 500 | 10 | 30 | Red-Low-High | 655 | 10 | 30 |
| Cyan-High-Low | 500 | 40 | 2 | Red-High-Low | 655 | 65 | 2 |
| Cyan-High-High | 500 | 40 | 30 | Red-High-High | 655 | 65 | 30 |
| Green-Low-Low | 530 | 10 | 2 | IR-Low-Low | 850 | 10 | 2 |
| Green-Low-High | 530 | 10 | 30 | IR-Low-High | 850 | 10 | 30 |
| Green-High-Low | 530 | 30 | 2 | IR-High-Low | 850 | 80 | 2 |
| Green -High-High | 530 | 30 | 30 | IR-High-High | 850 | 80 | 30 |
The pre-defined factors were then varied within the same experiment in a full factorial design mode. This allowed for a fair estimation of the effect of each factor independently, as well as any interaction between them. As a consequence, the number of experiments to be performed is equal to the number of levels to the power of the number of factors. This provided statistical power when assessing the effect of one single factor on the variation of our readout, as the variation of the response due to each of its level is weighted by the same combinations of all the other factors and levels. The resulting variation is therefore most probably due to the variation of this single factor itself.
Visually two representations for data were used, namely the main effects plot and the interaction plot. While the first shows the effects of each factor separately, the second one shows the interaction between the factors and how it impacts the variable of interest, i.e., metabolic activity. The numerical analysis is performed using ANOVA. When indicated, a Student’s t-test was used to compare two different conditions.
Our measurement method and setup were analyzed through a gage R&R approach. Operator, measuring instrument and method (plate reader, etc.), microplate type (black/white), illumination system and technical repeatability were tested. The results of the ANOVA analysis directly determined our selection of parameter and method to reduce the variability and reach satisfying level of reproducibility and repeatability55.
Acknowledgements
The costs made to conduct this study were paid by European Marie-Curie Actions Programme, Grant agreement no. 607886, where Charles Mignon is an Early Stage Researcher and Natallia E. Uzunbajakava, Natalia V. Botchkareva and Desmond J. Tobin are the members of a scientific supervisory team.
Author Contributions
Mr. Charles Mignon performed experiments, data analysis and wrote the manuscript. Mrs. Bianca Raafs gave technical support by performing isolation of reticular and papillary dermal fibroblasts and assisting Charles Mignon with cell culture. Dr. Natalia V. Botchkareva, MD, Dr. ir. Natallia E. Uzunbajakava, and Prof. Dr. Desmond J. Tobin jointly conceived the study, supervised its analysis and edited the manuscript.
Competing Interests
Natallia E. Uzunbajakava is an employee of Philips Electronics Nederland B.V. and received salary for this study.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carroll, J. Pubmed to adopt ‘Photobiomodulation Therapy’ as a MeSH term. http://blog.thorlaser.com/pubmed-adopts-photobiomodulation-therapy-as-a-mesh-term/ (2015).
- 2.Chung H, et al. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mignon C, Botchkareva NV, Uzunbajakava NE, Tobin DJ. Photobiomodulation devices for hair regrowth and wound healing: a therapy full of promise but a literature full of confusion. Exp Dermatol. 2016;25:745–9. doi: 10.1111/exd.13035. [DOI] [PubMed] [Google Scholar]
- 4.Arany PR, et al. Photoactivation of Endogenous Latent Transforming Growth Factor-β1 Directs Dental Stem Cell Differentiation for Regeneration. Sci. Transl. Med. 2014;6:238ra69–238ra69. doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anders JJ, Geuna S, Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol Res. 2004;26:233–239. doi: 10.1179/016164104225013914. [DOI] [PubMed] [Google Scholar]
- 6.Hashmi JT, et al. Role of Low-Level Laser Therapy in Neurorehabilitation. Am. Acad. Phys. Med. Rehabil. 2011;2:1–25. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanzafame RJ, et al. The growth of human scalp hair mediated by visible red light laser and LED sources in males. Lasers Surg. Med. 2013;45:487–95. doi: 10.1002/lsm.22173. [DOI] [PubMed] [Google Scholar]
- 8.Afifi, L. et al. Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg Med doi:10.1002/lsm.22512 (2016). [DOI] [PubMed]
- 9.Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014;46:144–51. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avci P, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff LD, et al. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg. 2004;22:241–247. doi: 10.1089/1549541041438623. [DOI] [PubMed] [Google Scholar]
- 12.Pfaff S, Liebmann J, Born M, Merk HF, von Felbert V. Prospective Randomized Long-Term Study on the Efficacy and Safety of UV-Free Blue Light for Treating Mild Psoriasis Vulgaris. Dermatology. 2015;231:24–34. doi: 10.1159/000430495. [DOI] [PubMed] [Google Scholar]
- 13.Weinstabl, A., Hoff-Lesch, S., Merk, H. F. & von Felbert, V. Prospective Randomized Study on the Efficacy of Blue Light in the Treatment of Psoriasis Vulgaris. Karger Dermatology223 (2011). [DOI] [PubMed]
- 14.Metelitsa AI, Green JB. Home-use laser and light devices for the skin: an update. Semin. Cutan. Med. Surg. 2011;30:144–7. doi: 10.1016/j.sder.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006;5:215–237. doi: 10.1039/B504573H. [DOI] [PubMed] [Google Scholar]
- 16.Moan J, Nielsen KP, Juzeniene A. Immediate pigment darkening: its evolutionary roles may include protection against folate photosensitization. FASEB J. 2012;26:971–975. doi: 10.1096/fj.11-195859. [DOI] [PubMed] [Google Scholar]
- 17.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed. Laser Ther. 2005;23:355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 18.Opländer C, et al. Mechanism and biological relevance of blue-light (420-453 nm)-induced nonenzymatic nitric oxide generation from photolabile nitric oxide derivates in human skin in vitro and in vivo. Free Radic. Biol. Med. 2013;65:1363–1377. doi: 10.1016/j.freeradbiomed.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Huang Y-Y, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Invest. Dermatol. 2010;130:259–269. doi: 10.1038/jid.2009.194. [DOI] [PubMed] [Google Scholar]
- 21.Denda M, Fuziwara S. Visible radiation affects epidermal permeability barrier recovery: selective effects of red and blue light. J. Invest. Dermatol. 2008;128:1335–1336. doi: 10.1038/sj.jid.5701168. [DOI] [PubMed] [Google Scholar]
- 22.Kim H-JJ, et al. Violet Light Down-Regulates the Expression of Specific Differentiation Markers through Rhodopsin in Normal Human Epidermal Keratinocytes. PLoS One. 2013;8:1–10. doi: 10.1371/annotation/c8b2e360-b78a-4c2f-a1a3-c53325f18211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker A, et al. Gene expression profiling reveals aryl hydrocarbon receptor as a possible target for photobiomodulation when using blue light. Sci. Rep. 2016;6:33847. doi: 10.1038/srep33847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mignon C, et al. Photobiomodulation of distinct lineages of human dermal fibroblasts: a rational approach towards the selection of effective light parameters for skin rejuvenation and wound healing. Proc. SPIE. 2016;9695:969508–969516. doi: 10.1117/12.2208574. [DOI] [Google Scholar]
- 25.Seo M-D, Kang T-J, Lee C-H, Lee A-Y, Noh M-S. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol. Ther. 2012;20:171–176. doi: 10.4062/biomolther.2012.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker M. Reproducibility: Respect your cells! Nature. 2016;537:433–435. doi: 10.1038/537433a. [DOI] [PubMed] [Google Scholar]
- 27.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–81. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driskell RR, et al. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–9. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Sorrell JM, Caplan AI. Fibroblast heterogeneity: more than skin deep. J Cell Sci. 2004;117:667–675. doi: 10.1242/jcs.01005. [DOI] [PubMed] [Google Scholar]
- 30.Janson D, Saintigny G, Mahé C, El Ghalbzouri A, Ghalbzouri A. El. Papillary fibroblasts differentiate into reticular fibroblasts after prolonged in vitro culture. Exp. Dermatol. 2013;22:48–53. doi: 10.1111/exd.12069. [DOI] [PubMed] [Google Scholar]
- 31.Janson DG, Saintigny G, van Adrichem A, Mahé C, El Ghalbzouri A. Different gene expression patterns in human papillary and reticular fibroblasts. J Invest Dermatol. 2012;132:2565–2572. doi: 10.1038/jid.2012.192. [DOI] [PubMed] [Google Scholar]
- 32.Karu TI. Cellular and Molecular Mechanisms of Photobiomodulation (Low-Power Laser Therapy) IEEE J. Sel. Top. Quantum Electron. 2014;20:143–148. doi: 10.1109/JSTQE.2013.2273411. [DOI] [Google Scholar]
- 33.Upton JH, et al. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Invest. Dermatol. 2015;135:1244–52. doi: 10.1038/jid.2015.28. [DOI] [PubMed] [Google Scholar]
- 34.Wang W. Oxygen partial pressure in outer layers of skin: simulation using three-dimensional multilayered models. Microcirculation. 2005;12:195–207. doi: 10.1080/10739680590905062. [DOI] [PubMed] [Google Scholar]
- 35.Stoppiglia LF, Nogueira TA, Leite AR, Carneiro EM, Boschero AC. Protective effect of d-glucose, l-leucine and fetal calf serum against oxidative stress in neonatal pancreatic islets. Biochim. Biophys. Acta - Mol. Basis Dis. 2002;1588:113–118. doi: 10.1016/S0925-4439(02)00154-0. [DOI] [PubMed] [Google Scholar]
- 36.Ling Z, Hannaert JC, Pipeleers D. Effect of nutrients, hormones and serum on survival of rat islet beta cells in culture. Diabetologia. 1994;37:15–21. doi: 10.1007/BF00428772. [DOI] [PubMed] [Google Scholar]
- 37.Rieder CL, Maiato H. Stuck in Division or Passing through: What Happens When Cells Cannot Satisfy the Spindle Assembly Checkpoint. Dev. Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Chan K-S, Koh C-G, Li H-Y. Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis. 2012;3:e411. doi: 10.1038/cddis.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohara M, Fujikura T, Fujiwara H. Augmentation of the inhibitory effect of blue light on the growth of B16 melanoma cells by riboflavin. Int J Oncol. 2003;22:1291–1295. [PubMed] [Google Scholar]
- 40.Olsen RKJ, et al. Riboflavin-Responsive and -Non-responsive Mutations in FAD Synthase Cause Multiple Acyl-CoA Dehydrogenase and Combined Respiratory-Chain Deficiency. Am. J. Hum. Genet. 2016;98:1130–45. doi: 10.1016/j.ajhg.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azzam EI, Jay-Gerin J-P, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayanan PK, LaRue KE, Goodwin EH, Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiat. Res. 1999;152:57–63. doi: 10.2307/3580049. [DOI] [PubMed] [Google Scholar]
- 43.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat. Rev. Cancer. 2009;9:351–60. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klammer H, Mladenov E, Li F, Iliakis G. Bystander effects as manifestation of intercellular communication of DNA damage and of the cellular oxidative status. Cancer Lett. 2015;356:58–71. doi: 10.1016/j.canlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Ramanathan, N. L. A new weighting system for mean surface temperature of the human body. J. Appl. Physiol. 19 (1964). [DOI] [PubMed]
- 46.Olesen, B. W. Thermal comfort. Tech. Rev (1982).
- 47.Anders J, et al. Light Supports Neurite Outgrowth of Human Neural Progenitor Cells. In Vitro: The Role of P2Y Receptors. 2008;14:118–125. [Google Scholar]
- 48.Oplander C, et al. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B. 2011;103:118–125. doi: 10.1016/j.jphotobiol.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Anders J, et al. The Combination of Light and Stem Cell Therapies: A Novel Approach in Regenerative Medicine. Am. Inst. Phys. 2010;3:3–10. [Google Scholar]
- 50.Holanda VM, Chavantes MC, Wu X, Anders JJ. The mechanistic basis for photobiomodulation therapy of neuropathic pain by near infrared laser light. Lasers Surg. Med. 2017 doi: 10.1002/lsm.22628. [DOI] [PubMed] [Google Scholar]
- 51.McFarland KL, Glaser K, Hahn JM, Boyce ST, Supp DM. Culture medium and cell density impact gene expression in normal skin and abnormal scar-derived fibroblasts. J. Burn Care Res. 2011;32:498–508. doi: 10.1097/BCR.0b013e3182223cb1. [DOI] [PubMed] [Google Scholar]
- 52.van Tonder A, et al. Limitations of the 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes. 2015;8:47. doi: 10.1186/s13104-015-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhang SZ, Lipsky MM, Trump BF, Hsu IC. Neutral red (NR) assay for cell viability and xenobiotic-induced cytotoxicity in primary cultures of human and rat hepatocytes. Cell Biol. Toxicol. 1990;6:219–34. doi: 10.1007/BF00249595. [DOI] [PubMed] [Google Scholar]
- 55.[Editorial]. Reality check on reproducibility. Nature533, 437–437 (2016). [DOI] [PubMed]


