Abstract
The objectives of this observational study were to document ovarian and endocrine responses associated with the treatment of cystic ovarian follicles (COFs) in dairy cows, using gonadotropin releasing hormone (GnRH) and prostaglandin F2α(PGF) with or without exogenous progesterone. A secondary objective was to determine pregnancy establishment following synchronization of ovulation and timed insemination in cows diagnosed with COFs. In trial I, 18 Holstein cows diagnosed with COFs received 2 injections of 100 μg GnRH, 9 d apart, with 25 mg PGF given 7 d after the 1st GnRH. A new follicle developed in all 18 cows after the 1st GnRH, and 83% of cows ovulated following the 2nd GnRH. Cows were inseminated 16 h after the 2nd GnRH. Of the 17 cows available for pregnancy diagnosis, 7 were confirmed pregnant. In trial II, 8 cows with COFs received GnRH and an intravaginal progesterone device (CIDR) concurrently, then PGF 7 d later. The CIDR was removed 2 d after PGF administration. Plasma estradiol concentrations declined following CIDR insertion. In all cows, a new follicle developed following GnRH treatment; estradiol-surge and estrus occurred spontaneously after CIDR-removal. Seven of 8 cows ovulated the new follicle. In dairy cows diagnosed with COFs, treatment with GnRH followed by PGF 7 d later, with or without exogenous progesterone, resulted in the recruitment of a healthy new follicle; synchronization of ovulation and timed insemination resulted in a 41% pregnancy rate.
Abstract
Résumé — Réponse ovarienne et endocrinienne associées au traitement des kystes folliculaires ovariens chez la vache laitière par la gonadolibérine et la prostaglandine F2α , avec ou sans progesterone exogène. Les objectifs de cette étude étaient de vérifier les réponses ovarienne et endocrinienne à la suite du traitement de kystes folliculaires ovariens (KFO) chez des vaches laitières par la gonadoliberine (GnRH) et la prostaglandine F2α, avec ou sans progestérone exogène. L’objectif secondaire était de déterminer la présence de gestation à la suite de la synchronisation de l’ovulation et de l’insémination programmée chez les vaches avec un diagnostic de KFO. Dans l’expérience 1, 18 vaches Holstein porteuses d’un diagnostic de KFO ont reçu 2 injections de 100 μg de GnRH, à 9 jours d’intervalle, ainsi que 25 mg de PGF administrée 7 jours après la première injection de GnRH. Un nouveau follicule s’est développé chez chacune des 18 vaches après la première administration de GnRH et 83 % des vaches ont ovulé après la 2ième administration. Les vaches ont été inséminées 16 h après la 2ième administration de GnRH. Sur les 17 vaches disponibles pour le diagnostic de gestation, 7 ont été confirmées gestantes. Dans l’expérience II, 8 vaches avec KFO ont reçu simultanément de la GnRH ainsi qu’un dispositif intravaginal contenant de la progestérone (CIDR), puis de la PGF 7 jours plus tard. Le CIDR a été enlevé 2 jours après l’administration de PGF. Les concentrations plasmatiques d’estradiol ont décliné à la suite de l’insertion du CIRD. Chez toutes les vaches, un nouveau follicule s’est développé à la suite du traitement à la GnRH; une poussée d’estradiol ainsi que l’œstrus sont survenus spontanément après le retrait du CIDR. Sept des huit vaches ont ovulé à partir de ce nouveau follicule. Chez la vache laitière porteuse d’un diagnostic de KFO, le traitement par GnRH suivi de PGF 7 jours plus tard, avec ou sans progestérone exogène, assure le développement d’un nouveau follicule sain; la synchronisation de l’ovulation et l’insémination programmée ont produit un taux de gestation de 41 %.
(Traduit par Docteur André Blouin)
Introduction
Cystic ovarian follicles (COFs) occur in dairy cows at a prevalence rate of 10% to 13% (1) and the condition is a major cause of infertility and economic loss to the dairy industry. Though no single cause could be attributed to COFs, high milk production, season, stress, and negative energy status were all considered predisposing factors (2–4). A physiologic mechanism responsible for the development of a COF may be the absence of the preovulatory surge of gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH). This phenomenon may be the result of hypothalamic insensitivity to the estradiol (E2) surge caused by inadequate exposure to progesterone (P4) (5). More recently, an association between intermediate (0.1 to 1.0 ng/mL) concentrations of circulating P4 and the formation of COFs has been reported (6).
Regardless of the etiology of COFs, treating cows diagnosed with the condition is a frustrating experience for both veterinarians and dairy farmers. It is often believed that the physical presence of a COF is detrimental to fertility, and some practitioners may resort to manual rupture of the cystic structure, because this is still a documented form of treatment (3). Preparations of GnRH, human chorionic gonadotropin (hCG), LH, and P4 are frequently used to treat COFs (7–13), but treatment outcomes are highly variable. Recent reports indicate that the protocol for synchronizing ovulation (2 injections of GnRH given 9 d apart, with prostaglandin F2α (PGF) given 7 d after the 1st GnRH), commonly referred to as ovsynch, followed by timed artif icial insemination (AI) performed 16 to 20 h after the 2nd GnRH treatment yields pregnancy rates of about 25% in cows with COFs (14,15). Though these studies reported pregnancy rates, ovarian responses to the sequential treatments with GnRH, PGF, and GnRH were not fully documented.
In a preliminary study (in Florida), 6 Holstein cows, diagnosed by a veterinarian as having at least 1 COF, were assigned to receive 100 μg of GnRH (Cystorelin; Merial USA, Duluth, Georgia, USA), followed 7 d later by PGF, 25 mg of dinoprost tromethamine (Lutalyse; Pharmacia Animal Health, Kalamazoo, Michigan, USA). Two days after receiving PGF, cows received a 2nd GnRH treatment (100 μg). Diagnosis of a COF was based on the palpation per rectum of an abnormally large (≥ 20 mm) follicle and no corpus luteum (CL), verified by transrectal ultrasonography (Aloka 500V; Aloka, Tokyo, Japan) with a 7.5 MHz linear-array transducer. Ovaries were scanned on alternate days, starting at the 1st GnRH treatment (day 0), until at least day 22. Even though none of the COFs ovulated, another existing follicle ovulated following the 1st GnRH injection in 4 cows. A new follicle developed in all cows. Ovulation of the new follicle occurred in all 6 cows within 48 h of the 2nd GnRH injection, and the development of a CL was confirmed in all cases.
The primary objectives of the present observational study were to expand on the preliminary study by documenting ovarian and endocrine responses in dairy cows with COFs: a) when a standard ovsynch protocol was used, or b) when a coordinated sequence of treatments with GnRH and PGF was used in the presence of an intravaginal P4-releasing device. A secondary objective was to determine pregnancy establishment following ovsynch and timed AI in cows diagnosed with COFs.
Materials and methods
Two trials were conducted in lactating dairy cows diagnosed with 1 or more spontaneously-developed COFs. Trial I involved cows from herds in Alberta and Florida, USA; trial II was conducted entirely in Florida. Housing and management of cows in both trials were in accordance with Canadian Council on Animal Care guidelines (16).
Trial I
Eighteen Holstein cows in Alberta, diagnosed with COFs, received 100 μg of GnRH (Fertiline; Vetoquinol NA, Lavaltrie, Quebec) 25 mg of PGF 7 d later and a 2nd GnRH after 2 d and were artificially inseminated approximately 16 h after the 2nd GnRH treatment, without estrus detection. Ovaries were scanned at the time of each treatment, at 2 d after AI (to confirm ovulation), and also at 7 d after AI (to confirm the presence of a CL). Pregnancy was diagnosed by ultrasonography 32 to 40 d after AI. Visualization of an embryo with a beating heart conf irmed a viable pregnancy. Ovarian responses were determined by ultrasonography in all 18 cows and the diagnosis of pregnancy was conducted in 17 of the 18 cows. Blood samples were collected by coccygeal venipuncture at the time of the 1st GnRH injection (day -10), at PGF injection (day -3), at AI (day 0), and at 7 d after AI (day 7). Samples were kept in ice and plasma was separated by centrifugation within 6 h and stored at −20°C until assayed for P4 by radioimmunoassay (RIA). A solid-phase RIA kit (Coat-a-Count; Diagnostic Products, Los Angeles, California, USA) was used to determine P4 concentrations in plasma and all samples were analyzed in 1 assay. The intraassay coefficient of variation was 7.6%.
Cows with at least 1 large (≥ 20 mm) follicular structure, no detectable signs of luteinization or CL, and low P4 concentrations (< 1 ng/mL at day -10) were categorized, retrospectively, as having a follicular cyst (FC). Cows with at least 1 large follicular structure and visible signs of luteinization but no detectable CL, or those with P4 > 1 ng/mL in the absence of a detectable CL, were categorized as having a luteinized cyst (LC). Cows with at least 1 large follicular structure and a distinct CL on either ovary, with P4 > 1 ng/mL were categorized as having persistent cyst (PC).
The mixed model of statistical analysis system (17) was used to perform a repeated measures design analysis of variance (18) for P4. The main effects in the model included type of cyst (FC, LC, PC), time of sampling (days -10, -3, 0, and 7) as a repeated effect, and the interactions of type of cyst and time of sampling. Cow nested within type was used as the error term. The variance-covariance matrix chosen for the statistical analysis was based on an iterative process wherein the best fit was based on Schwarz’s Bayesian criterion (19). The Kenward-Roger method was used to determine denominator degrees of freedom (20). When the main effect or type by time interaction was significant, means separation procedures were carried out using the Tukey procedure. Data on COF diameter were analyzed by least-squares analysis of variance (17) adjusted for repeated measures. Differences were considered significant at P ≤ 0.05.
Trial II
Eight Holstein cows, diagnosed with COFs (as described in trial I), were used. Ovarian structures were monitored daily by ultrasonic-imaging during a 10- to 14-day pretreatment period. At the end of the pretreatment period, cows received 8 μg of the GnRH-agonist, buserelin acetate (Receptal; Hoechst AG, Frankfurt, Germany), concurrent with the intravaginal placement of a P4- releasing device (CIDR, [1.9 g]; Carter Holt-Harvey Plastic Products Group, Hamilton, New Zealand). Seven days later, cows received 25 mg of PGF. At the end of the 9-day treatment period, the CIDR device was removed and cows were observed for estrus, twice daily. Tail chalk (All-weather Paintstick; LA-CO Industries, Chicago, Illinois, USA) was used to aid in estrus detection, as described previously (21). Ovarian dynamics were monitored daily by ultrasonography until the onset of estrus and then continued for 1 complete estrous cycle. Blood samples were obtained daily, by coccygeal venipuncture, to determine the concentrations of P4 and E2 and the E2: P4 ratio in peripheral plasma. Samples were handled and stored as in trial I. Concentrations of P4 and E2 in plasma were determined by RIA, as described by Knickerbocker et al (22) and Badinga et al (23), respectively. Intraassay coefficients of variation for P4 and E2 were 7.2% and 8.2%, respectively. Least squares means pertaining to E2, P4, and E2:P4 ratios of the pretreatment period were estimated within the COF category by least-squares analysis of variance (17), adjusted for repeated measures. The same approach was used to determine the diameter of the COF during the pretreatment period.
Cows with at least 1 large (≥ 20 mm) follicular structure, no luteinization or CL, and a high E2:P4 ratio during the pretreatment period were categorized, retrospectively, as having an FC. Cows with 1 large follicular structure, no CL, and a low E2:P4 ratio were considered to have an LC. Cows with a COF, a detectable CL, and a low E2:P4 ratio during the pretreatment period were categorized as having a PC.
Results
Trial I
Of the 18 cows, 10, 8, and 0, were FC, PC, and LC, respectively. The type of COF did not influence (P = 0.19) P4 concentrations, but the time of sampling (P < 0.01) and the interactions between type of COF and time of sampling (P < 0.01) were significant. The P4 profiles of FC and PC cows are presented in Figure 1. Ovarian responses to the ovsynch treatment and pregnancy outcome are presented in Table 1.
Figure 1.

Progesterone profiles of cows categorized as having either a follicular cyst (FC) or a persistent cyst (PC) in trial I at the time of 1st gonadotropin releasing hormone (GnRH) treatment (day -10), at prostaglandin F2α (PGF) (day -3), at artificial insemination (AI) (day 0), and 7 d after AI (day 7).
Table 1.
Observations based on ultrasonography, including pregnancy outcome, in cows with follicular cysts (FC) and those with persistent cysts (PC) (trial I)
| Observation | Cows with FC | Cows with PC | Both combined |
|---|---|---|---|
| Number of cows | 10 | 8 | 18 |
| Mean diameter of cystic structure (mm) | 32.3, sχ̄ = 1.5 | 36.0, sχ̄ = 3.1 | 33.9, sχ̄ = 1.5 |
| Ovulation of cyst after 1st GnRH | 0 | 0 | 0 |
| Ovulation of an existing follicle after 1st GnRH | 5/10 (50%) | 3/8 (38%) | 8/18 (44%) |
| Cows that developed a new follicle after 1st GnRH | 10/10 (100%) | 8/8 (100%) | 18/18 (100%) |
| Cows that ovulated new follicle after 2nd GnRH | 8/10 (80%) | 7/8 (88%) | 15/18 (83%) |
| Cows in which new CL detected | 8/8 (100%) | 7/7 (100%) | 15/15 (100%) |
| Cows pregnant 32 d after AI | 5/9 (56%) | 2/8 (25%) | 7/17 (41%) |
| Cyst detectable 32 d after AI | 6/9 (67%) | 5/8 63%) | 11/17 (65%) |
FC — follicular cyst (CL absent); PC — persistent cyst (CL present); sχ̄— standard error of means; GnRH — gonadotropin releasing hormone; AI — artificial insemination
Though none of the COFs ovulated, partial luteinization, characterized by thickening of the follicular wall, was observed in several cases. Two cows (an FC and a PC) were unique in that complete luteinization occurred, and by 7 d after GnRH treatment, the luteinized structures resembled a large CL with a central cavity. Eight of 18 cows (44%) ovulated a follicle other than the COF in response to the 1st GnRH treatment. A new dominant follicle developed following the 1st GnRH treatment in all 18 cows, 83% of the new follicles ovulated after the 2nd GnRH treatment, and the presence of a new CL was confirmed 7 d after AI. Ultrasonographic images of ovarian responses to sequential treatments with GnRH, PGF, and GnRH in a PC cow are presented in Figure 2. Of the 18 cows that were inseminated, 17 were available for pregnancy diagnosis and 7 (41%) were confirmed pregnant. In 11 (65%) of the 17 cows, the COF was still detectable 32 to 40 d after the ovsynch treatment ended.
Figure 2.

Ovarian ultrasonographic images of 1 cow during the 10-day treatment protocol in trial I. Panels 1, 2, 3, and 4 display key ovarian structures at 1st gonadotropin releasing hormone (GnRH), prostaglandin F2α (PGF), 2nd GnRH, and 7 d after insemination, respectively. A cystic follicle > 35 mm diameter was present on the right ovary at all observations (1b, 2b, 3b, 4b). This cow, reported in standing estrus 48 to 60 h prior to the first observation (day -10; 1st GnRH), had a corpus hemorrhagicuma on its left ovary (see black arrows, 1a). At the 2nd examination 7 d later (day -3; PGF given), the corpus luteum (CL) was clearly defined (2a) and a 10-mm follicle was also present on the same ovary (follicle not in picture). At the time of the 2nd GnRH treatment, the dominant follicle (F) had reached 13 mm in diameter and the CL was regressing (RCL, 3a). The dominant follicle ovulated following the 2nd GnRH treatment and developed into a well-defined CL by 7 d after artificial insemination (4a). Signs of luteinization (white arrows, 4b) were visible within the cystic follicle at the time.
a Even though the apparently dense echotexture likens this image to that of a regressing CL, this was the result of an improper printer setting.
Trial II
Of the 8 cows in this trial, 5 were determined to have an FC, based on a high E2:P4 ratio and the absence of any luteal tissue; and 1 cow had an LC (low E2:P4 ratio, no CL). The remaining 2 cows had a large cyst-like structure alongside a CL with a low E2:P4 ratio and were categorized as having a PC (Table 2). Ultrasonographic observations of the ovarian structures and their response to treatment are presented in Table 3. Though none of the COFs ovulated in response to GnRH treatment, 5 of the 8 cows ovulated another existing follicle, following GnRH treatment. As observed in trial I, all cows developed a new follicle in response to GnRH treatment (mean day of follicle emergence was 3.6, sχ̄= 0.3 after GnRH treatment). Seven of 8 cows ovulated the newly recruited follicle following CIDR-removal and developed a CL that was detected by ultrasonography. The P4 profile and CL development representing 1 cow are shown in Figure 3. The mean interval to ovulation after CIDR removal was 3.0, sχ̄= 0.3 d. Length of the luteal phase following spontaneous ovulation was 16.4, sχ̄= 2.5 d, with an average concentration of plasma P4 for the luteal phase of 4.4, sχ̄= 0.3 ng/mL. Follicular turnover during the luteal phase was clearly documented in 7 of the 8 cows, with a 3-wave pattern in 3 cows and a 2-wave pattern in 4 cows. The cow that failed to ovulate formed a new FC. Following a new 10-day preliminary period, the treatment sequence was repeated on this cow, except that a 2nd GnRH injection was given 2 d after removal of the CIDR. This cow ovulated a newly developed follicle 1 d after the 2nd GnRH injection and formed a 28-mm CL with a 15-mm lumen. After a 20-day interval with 2 intervening follicular waves, a spontaneous ovulation occurred, which was preceded by a standing estrus with clear mucous discharge.
Table 2.
Least squares means of plasma concentrations of estradiol, progesterone, and estradiol:progesterone ratio in the pretreatment period in cows determined to have different types of ovarian cysts (trial II)
| Observation | Cows with FC | Cows with LC | Cows with PC |
|---|---|---|---|
| Number of cows | 5 | 1 | 2 |
| Estradiol (pg/mL) | 25.7, sχ̄ = 2.5 | 3.8, sχ̄ = 1.1 | 3.9, sχ̄ = 0.9 |
| Progesterone (ng/mL) | 0.4, sχ̄ = 0.0 | 3.5, sχ̄ = 0.7 | 4.2, sχ̄ = 0.7 |
| Estradiol:progesterone ratio | 270.5, sχ̄ = 77.0 | 3.4, sχ̄ = 1.9 | 6.6, sχ̄ = 4.1 |
FC — follicular cyst (CL absent); LC — luteinized cyst (CL absent); PC — persistent cyst (CL present); sχ̄— standard error of means
Table 3.
Observations based on ovarian ultrasonography in cows with follicular cysts (FC), luteinized cysts (LC), and persistent cysts (PC) (trial II)
| Observation | Cows with FC | Cows with LC | Cows with PC | All cows combined |
|---|---|---|---|---|
| Number of cows | 5 | 1 | 2 | 8 |
| Mean diameter of cystic structure (mm) | 25.2, sχ̄ = 0.6 | 22.4, sχ̄ = 0.3 | 27.7, sχ̄ = 1.5 | 25.1 |
| Ovulation of cyst after GnRH | 0 | 0 | 0 | 0/8 |
| Ovulation of an existing follicle after GnRH | 3/5 (60%) | 0/1 (0%) | 2/2 (100%) | 5/8 (63%) |
| Cows that developed new follicle after GnRH | 5/5 (100%) | 1/1 (100%) | 2/2 (100%) | 8/8 (100%) |
| Cows that ovulated new follicle after CIDR removal | 5/5 (100%) | 0/1 (0%) | 2/2 (100%) | 7/8 (88%) |
| Cows in which new CL detected after CIDR removal | 5/5 (100%) | 0/1 (0%) | 2/2 (100%) | 7/8 (88%) |
FC — follicular cyst (CL absent); LC — luteinized cyst (CL absent); PC — persistent cyst (CL present); sχ̄— standard error of means; GnRH — gonadotropin releasing hormone; CIDR — controlled internal drug (progesterone) release device
Figure 3.

Plasma progesterone (P4) profile typical of 1 cow categorized as having a follicular cyst (FC) in trial II. Note that P4 remained low during the 10-day pretreatment period. Concentrations of P4 increased and remained elevated following treatments with gonadotropin releasing hormone (GnRH) and controlled internal drug release (CIDR) device and declined rapidly following CIDR removal after 9 d. Concentrations of P4 increased again following ovulation and corpus luteum (CL) formation. Diameter of the CL is plotted on the Y-2 axis. Spontaneous estrus occurred after a 3-week period.
In the 5 cows with an FC, mean E2 concentrations declined (P < 0.01) rapidly following CIDR insertion and GnRH injection, decreasing from 23 pg/mL during the pretreatment phase to 6 pg/mL during the treatment phase. A proestrus E2-surge occurred in all 8 cows consequent to PGF injection and CIDR removal, with a mean plasma E2 concentration of 9.9 pg/mL 1 d prior to estrus. The E2 profile and follicular activity during the luteal phase of 1 cow (the same cow as in Figure 3) are presented in Figure 4. A clear suppression in plasma E2 occurred during the treatment period (Figure 4), when concurrent concentrations of P4 were elevated due to CIDR insertion and induction of a CL following GnRH (Figure 3). Coupled with the recruitment of a new dominant follicle was a proestrus rise in E2 that decreased precipitously following onset of estrus and impending ovulation (Figure 4). A normal cycle was evident based on the presence of a new CL and luteal phase P4 concentrations (Figure 3), as well as 3 follicular waves leading to a rise in plasma E2 and the occurrence of estrus 21 d after the 1st synchronized estrus (Figure 4).
Figure 4.

Plasma estradiol (E2) profile typical of 1 cow categorized as having a follicular cyst (FC) in trial II. Estradiol declined following controlled internal drug release (CIDR) device insertion and gonadotropin releasing hormone (GnRH) injection, and began to rise after prostaglandin F2α (PGF) injection and peaked after CIDR removal in association with the onset of estrus. Follicular activity ensued and 3 follicular waves (DF1, DF2, DF3) were recorded. The 3rd dominant follicle (DF3) ovulated following a spontaneous estrus.
A decrease (P < 0.01) in COF size was observed in all cows. The mean cumulative size of the COFs was 37.1, sχ̄= 1.3 mm in the pretreatment period versus 32.5, sχ̄= 1.4 mm during the treatment period; this declined further to 19.1, sχ̄= 0.8 mm, during the posttreatment period. Decrease in the size of 2 COFs of 1 cow (the same cow as in Figures 3 and 4) is presented in Figure 5.
Figure 5.
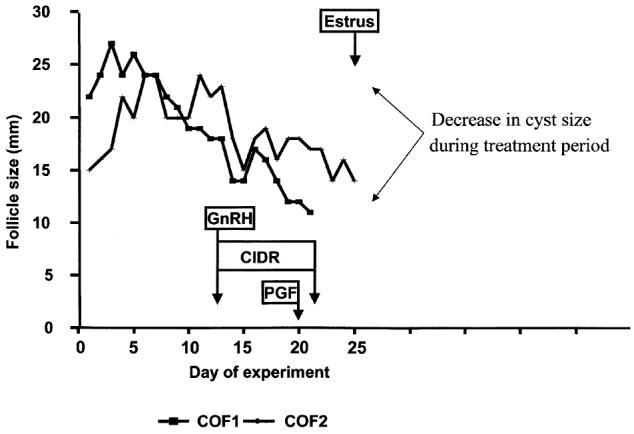
Size (diameter) of 2 cystic follicles of 1 cow shown relative to the pretreatment, treatment, and posttreatment periods in trial II. In this cow, a 2nd cystic ovarian follicle (COF2) appeared to be replacing the 1st COF (COF1) until intervention occurred with the gonadotropin releasing hormone (GnRH) and controlled internal drug release device treatment. The COFs had a notable decline in size following treatment.
Discussion
This observational study in which 2 different protocols were used documented ovarian responses in cows diagnosed with COFs. Even though none of the cows ovulated a COF, 50% of the cows ovulated a follicle other than the COF that was present at the time of GnRH injection. Ovulation of a COF in response to GnRH treatment does not usually occur and has not been observed in other studies (1,14,24). However, luteinization of the COF following GnRH-treatment has been reported (1,24). In the present study, the COFs in 2 cows underwent complete luteinization after the 1st GnRH treatment. Both cases of complete luteinization occurred in cows in trial I. Unlike in trial II, there was no pretreatment observation period for cows in trial I. Therefore, there is room to speculate that both COFs that underwent complete luteinization in response to the 1st GnRH treatment were relatively new and highly responsive to the GnRHinduced LH surge.
Development of a new follicle following GnRH treatment occurred in all cows in both trials; this response is well documented for normal cycling cows that are treated with the ovsynch protocol (25). The effectiveness of the ovsynch and timed AI protocol in lactating dairy cows diagnosed with ovarian cysts has been reported by Fricke and Wiltbank (14) and Bartolome et al (15), with pregnancy rates of 26.9% (calculated based on a reported conception rate of 36.8%) and 23.6%, respectively. Ovarian responses to the ovsynch treatment were either partially recorded or not recorded in these reports. By using a protocol similar to that used by the above authors, we have determined that all cows developed a new follicle in response to the 1st GnRH treatment and that a majority of these newly recruited follicles subsequently ovulated. In the cows that were subjected to ovsynch and timed AI, a 41% pregnancy rate was achieved in the present study. In a majority of the cows that were bred following the ovsynch protocol, the COF remained detectable for up to 40 d after AI with no apparent detrimental effect on pregnancy. The persistence of COFs as benign structures for prolonged periods has been reported previously (1,26), and it is highly unlikely that these COFs have any negative influence on new follicular development or pregnancy establishment.
Regardless of the type of ovarian cyst (FC, LC, or PC), a new follicle developed following GnRH treatment in 100% of the cows examined. In a high percentage of cases, the newly developed follicle ovulated either in response to a 2nd GnRH treatment (83%, trial I) or spontaneously (88%, trial II) upon P4 withdrawal. Cows with COFs are known to have high plasma LH concentrations (1) and increased LH pulse frequency (11). Increased LH concentrations favor the development and persistence of large ovarian follicles (27), whereas treatment with exogenous P4 induces atresia of persistent follicles (28,29). The latter approach has been used successfully to reduce LH pulse frequency and induce turnover of COFs in cattle by placing intravaginal P4-releasing devices for either 9- (11) or 14- (12) day periods. As observed in this and previous studies (11,12), the COFs declined in size following insertion of the P4 device. Associated with the insertion of a CIDR device and GnRH injection were an increase in P4 and a concomitant decline in circulating E2 concentrations. Upon removal of the CIDR device after 9 d, E2 concentrations increased rapidly and in trial II all cows came into estrus within 3 d, and 7 of 8 cows ovulated spontaneously. Follicular activity resembling normal follicular wave patterns was observed during the posttreatment period. Estrus and ovulation occurred spontaneously again after a 3-week period.
Based on our findings and those of others, we recommend that treatment of COFs must focus on altering the endocrine milieu, such that it allows recruitment of a new follicle (by using GnRH) and induces the turnover of the COF (by using a P4 device), preferably within an ovsynch-type protocol. The potential importance of completing the full ovsynch protocol in the treatment of COFs is emphasized by the ability of GnRH given after CIDR withdrawal to induce ovulation of a newly recruited follicle in an FC cow that failed to ovulate spontaneously after CIDR withdrawal in a previous instance. Furthermore, the observations that a) 100% of the cystic cows developed a new follicle following GnRH treatment, b) 41% of the inseminated cows conceived following ovsynch and timed AI, and c) in many of these cows, the COF persisted benignly with no apparent detrimental influence on the establishment or maintenance of pregnancy strengthen the argument to adopt this treatment approach to enhance the chances for conception in cows diagnosed with COFs.
In summary, administration of GnRH (day 0), followed by PGF (day 7), with or without exogenous progesterone (for 9 d), resulted in the recruitment of a healthy new ovarian follicle in cows diagnosed with COFs. The newly recruited follicle ovulated in a majority of the cases, either in response to a 2nd GnRH treatment or spontaneously following CIDR-removal. Ovsynch followed by timed AI resulted in a 41% pregnancy rate. Contrary to common belief, the physical presence of a COF did not interfere with new follicular growth or pregnancy establishment in Holstein cows. The ovsynch and timed AI protocol is an effective method of establishing pregnancies in dairy cows diagnosed with COFs. The incorporation of an intravaginal P4-releasing device, such as CIDR, into the ovsynch and timed AI protocol is recommended as an additional strategy to increase the chances of resolving the COF. Studies are warranted to determine if the inclusion of a CIDR device into the ovsynch protocol would enhance pregnancy rates in cows diagnosed with COFs.
Acknowledgments
Thanks to Drs. Steve Radostits, Leduc Veterinary Clinic; Pavol Zalkovic, University of Alberta; and producerparticipants for their cooperation and assistance during this investigation. CVJ
References
- 1.Garverick HA. Ovarian follicular dynamics and endocrine profiles in cows with ovarian follicular cysts. In: Howard JL, Smith RA, eds. Current Veterinary Therapy, Food Animal Practice. Philadelphia: WB Saunders, 1999:577–580.
- 2.Farin PW, Estill CT. Infertility due to abnormalities of the ovaries in cattle. Vet Clin North Am Food Anim Pract. 1993;9:291–308. doi: 10.1016/s0749-0720(15)30647-2. [DOI] [PubMed] [Google Scholar]
- 3.Seguin BE. Follicular cystic ovary disease: overview. In: Aiello SE, Mays A, eds. The Merck Veterinary Manual. Whitehouse Station: Merck and Company, 1998:1005–1007.
- 4.López-Gatius F, Santolaria P, Yániz J, Fenech M, López-Béjar M. Risk factors for postpartum ovarian cysts and their spontaneous recovery or persistence in lactating dairy cows. Theriogenology. 2002;58:1623–1632. doi: 10.1016/s0093-691x(02)01046-4. [DOI] [PubMed] [Google Scholar]
- 5.Gümen A, Wiltbank MC. An alteration in the hypothalamic action of estradiol due to lack of progesterone exposure can cause follicular cysts in cattle. Biol Reprod. 2002;66:1689–1695. doi: 10.1095/biolreprod66.6.1689. [DOI] [PubMed] [Google Scholar]
- 6.Hatler TB, Hayes SH, Laranja da Fonseca LF, Silvia WJ. Relationship between endogenous progesterone and follicular dynamics in lactating dairy cows with ovarian follicular cysts. Biol Reprod. 2003;69:218–223. doi: 10.1095/biolreprod.102.012179. [DOI] [PubMed] [Google Scholar]
- 7.Garverick HA. Ovarian follicular cysts in dairy cows. J Dairy Sci. 1997;80:995–1004. doi: 10.3168/jds.S0022-0302(97)76025-9. [DOI] [PubMed] [Google Scholar]
- 8.Osawa T, Nakao T, Kimura M, et al. Fertirelin and buserelin compared by LH release, milk progesterone and susbsequent reproductive performance in dairy cows treated for follicular cysts. Theriogenology. 1995;44:835–847. doi: 10.1016/0093-691x(95)00269-e. [DOI] [PubMed] [Google Scholar]
- 9.Peter AT. Infertility due to abnormalities of the ovaries. In: Youngquist RS, ed. Current Therapy in Large Animal Theriogenology. Philadelphia: WB Saunders, 1998:349–354.
- 10.Thatcher WW, Drost M, Savio JD, et al. New clinical uses of GnRH and its analogues in cattle. Anim Reprod Sci. 1993;33:27–49. [Google Scholar]
- 11.Calder MD, Salfen BE, Bao B, Youngquist RS, Garverick HA. Administration of progesterone to cows with ovarian follicular cysts results in a reduction in mean LH and LH pulse frequency and initiates ovulatory follicular growth. J Anim Sci. 1999;77:3037–3042. doi: 10.2527/1999.77113037x. [DOI] [PubMed] [Google Scholar]
- 12.Todoroki J, Yamakuchi H, Mizoshita K, et al. Restoring ovulation in beef donor cows with ovarian cysts by progesterone-releasing intravaginal silastic devices. Theriogenology. 2001;55:1919–1932. doi: 10.1016/s0093-691x(01)00533-7. [DOI] [PubMed] [Google Scholar]
- 13.Tebble JE, O’Donnell MJ, Dobson H. Ultrasound diagnosis and treatment outcome of cystic ovaries in cattle. Vet Rec. 2001;148:411–413. doi: 10.1136/vr.148.13.411. [DOI] [PubMed] [Google Scholar]
- 14.Fricke PM, Wiltbank MC. Effect of milk production on the incidence of double ovulation in dairy cows. Theriogenology. 1999;52:1133–1143. doi: 10.1016/S0093-691X(99)00205-8. [DOI] [PubMed] [Google Scholar]
- 15.Bartolome JA, Archbald LF, Morresey P, et al. Comparison of synchronization of ovulation and induction of estrus as therapeutic strategies for bovine ovarian cysts in the dairy cow. Theriogenology. 2000;53:815–825. doi: 10.1016/S0093-691X(99)00276-9. [DOI] [PubMed] [Google Scholar]
- 16.Olfert DE, Cross BM, McWilliam AA, eds. Guide to the care and use of experimental animals. 2nd ed. vol 1. Ottawa: Canadian Council on Animal Care, 1993:31–34.
- 17.Statistical Analysis System (SAS). SAS Online Doc, version 8. Cary, North Carolina: SAS Institute. 1988.
- 18.Little RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 20.Kenward MG, Roger JH. Small sample inference for fixed effects from the restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 21.Schmitt EJ-P, Drost M, Diaz T, Roomes C, Thatcher WW. Effect of a gonadotropin-releasing hormone agonist on follicle recruitment and pregnancy rate in cattle. J Anim Sci. 1996;74:154–161. doi: 10.2527/1996.741154x. [DOI] [PubMed] [Google Scholar]
- 22.Knickerbocker JJ, Thatcher WW, Bazer FW, et al. Proteins secreted by day 16 to 18 conceptuses extend corpus luteum functioning in cows. J Reprod Fertil. 1986;77:381–391. doi: 10.1530/jrf.0.0770381. [DOI] [PubMed] [Google Scholar]
- 23.Badinga L, Driancourt MA, Savio JD, et al. Endocrine and ovarian responses associated with the first wave dominant follicle in cattle. Biol Reprod. 1992;47:871–883. doi: 10.1095/biolreprod47.5.871. [DOI] [PubMed] [Google Scholar]
- 24.Wiltbank MC, Gümen A, Sartori R. Physiological classification of anovulatory conditions in cattle. Theriogenology. 2002;57:21–52. doi: 10.1016/s0093-691x(01)00656-2. [DOI] [PubMed] [Google Scholar]
- 25.Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995;44:915–923. doi: 10.1016/0093-691x(95)00279-h. [DOI] [PubMed] [Google Scholar]
- 26.Noble KM, Tebble JE, Harvey D, Dobson H. Ultrasonography and hormone profiles of persistent ovarian follicles (cysts) induced with low doses of progesterone in cattle. J Reprod Fertil. 2000;120:361–366. [PubMed] [Google Scholar]
- 27.Taft R, Ahmad N, Inskeep EK. Exogenous pulses of luteinizing hormone cause persistence of the largest bovine ovarian follicle. J Anim Sci. 1996;74:2985–2991. doi: 10.2527/1996.74122985x. [DOI] [PubMed] [Google Scholar]
- 28.Manikkam M, Rajamahendran R. Progesterone-induced atresia of the proestrous dominant follicle in the bovine ovary: Changes in diameter, insulin-like growth factor system, aromatase activity, steroid hormones, and apoptotic index. Biol Reprod. 1997;57:580–587. doi: 10.1095/biolreprod57.3.580. [DOI] [PubMed] [Google Scholar]
- 29.McDowell CM, Anderson LH, Kinder JE, Day ML. Duration of treatment with progesterone and regression of persistent ovarian follicles in cattle. J Anim Sci. 1998;76:850–855. doi: 10.2527/1998.763850x. [DOI] [PubMed] [Google Scholar]


