Abstract
NKX6.3 plays an important role in gastric epithelial differentiation and also acts as a gastric tumor suppressor. The specific aim of this study was to determine whether NKX6.3 contributes to gastric mucosal barrier function by regulating reactive oxygen species (ROS) production. NKX6.3 reduced ROS production and regulated expression of anti-oxidant genes, including Hace1. In addition, NKX6.3 reduced DNMT1 expression and activity by down-regulating NF-kB family gene transcription. Silencing of Hace1 recovered ROS production, whereas knock-down of DNMT1 and NF-kB reduced ROS production and induced Hace1 expression by hypomethylating its promoter region. In addition, NKX6.3 inhibited CagA effects on cell growth, ROS production, and NF-kB and DNMT1 activity. In gastric mucosae and cancers, NKX6.3 and Hace1 expression was significantly reduced. The NKX6.3 expression was positively correlated with Hace1 and Nrf2 genes, but negatively correlated with DNMT1. Hypermethylation of Hace1 gene was observed only in gastric mucosae with H. pylori, atrophy and intestinal metaplasia. Thus, these results suggest that NKX6.3 inhibits ROS production by inducing the expression of Hace1 via down-regulating NF-kB and DNMT1 activity in gastric epithelial cells.
Introduction
Gastric cancer has a high incidence in Asia and is a leading cause of cancer death in the region1. Cellular damage from various agents and chronic infection with H. pylori most likely influence the risk of gastric carcinogenesis through inflammation-induced reactive oxygen species (ROS) and reactive nitrogen species (RNS) leading to DNA damage in gastric epithelial cells and precancerous cascade including atrophic gastritis, intestinal metaplasia and dysplasia2–4.
The ROS, including superoxide anion radical (•O2 −), hydrogen peroxide (H2O2), and hydroxyl radical (•OH), are highly reactive, diffusible, and ubiquitous molecules generated as inevitable by-products of aerobic respiration and metabolism5. In stomach, H. pylori infection induces a strong inflammatory host response, leading to the generation of several ROS and RNS via neutrophils and macrophages6. Imbalance between ROS production and the capacity for detoxification induces an alteration of gene expression7, increased cell death and proliferation, and induction of DNA mutation8. Notably, cancer cells augment intracellular ROS, which contributes to tumorigenesis and cancer progression by promoting genomic instability through increased DNA damage and reduced mismatch repair9. However, as excessive levels of ROS stress can induce cancer cell cycle arrest and apoptosis, cancer cells also increase expression of anti-oxidant proteins to detoxify from ROS and anti-apoptotic proteins10. Thus, manipulating the unique redox regulatory mechanisms of cancer cells might be an effective strategy to eliminate these cells11.
Previously, we and others have reported that the novel transcription factor, NKX6.312, is a key factor for gastric differentiation and prevention of intestinal metaplasia and acts as a tumor suppressor for gastric cancer13–16. ROS is reportedly involved in the development of precancerous gastritis and gastric cancer, hence, we hypothesized that NKX6.3 may protect the gastric mucosal epithelia from harmful ROS.
Herein, we examined the effects of NKX6.3 on ROS production and Hace1, Nrf2, mnSOD, catalase, GSH, Nqo1, and Ho-1 expression in mock and NKX6.3 stable AGS and MKN1 cells. In addition, the expression of NKX6.3, Hace1, Nrf2, and DNMT1 was compared between non-neoplastic gastric mucosae and gastric cancers. Overall, we found that NKX6.3 significantly decreased ROS production and regulated the expression of ROS-related genes, including Hace1, by suppressing DNMT1 and NF-kB activity. These results suggest that NKX6.3 plays an important role in gastric epithelial protection and gastric cancer prevention by regulating the ROS levels.
Results
NKX6.3 attenuates ROS production by regulating ROS-responsive genes
To determine whether NKX6.3 contributes to ROS production, we performed in vitro ROS analysis in AGSMock, MKN1Mock, AGSNKX6.3 and MKN1NKX6.3 cells using DCFDA staining assay. Stable NKX6.3 expression in AGS and MKN1 cells showed reduced cellular ROS levels in a time-dependent manner, as compared to mock stable AGS and MKN1 cells (Fig. 1A–C). To further confirm these initial observations, we measured the expression levels of ROS-related genes, including Hace1, Nox1, Noxa1 and Nrf2, and oxidative stress-responsive genes, including catalase, mnSOD, GSH, Nqo1 and Ho-1 by real-time RT-PCR and western blotting. Interestingly, NKX6.3 induced the expression of Hace1, Nrf2, catalase, and mnSOD, while decreasing the expression of Nox1 and Noxa1 proteins. In addition, it also reduced the expression of GSH, Nqo1 and Ho-1 at the mRNA level (Fig. 1D and E). To further support our results, we recapitulated NKX6.3, Hace1 and Nrf2 gene expression patterns from large cohorts of gastric cancer patients available from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (accession numbers GSE27342). Interestingly, NKX6.3 expression was positively correlated with Hace1 and Nrf2 expression in gastric cancer cohort (Supplementary Figure S1A). In addition, Hace1 and Nrf2 expression showed a positive correlation (Supplementary Figure S1A). Next, we aimed to determine whether the anti-oxidant activity of NKX6.3 is dependent on Hace1 expression. In AGS and MKN1 cells, treatment with siHace1 partially recovered ROS production (Fig. 2A) and markedly reduced Nrf2 expression (Fig. 2B). Also, Hace1 silencing in NKX6.3 stable cells decreased GSH, Nqo1 and Ho-1 mRNA expression (Fig. 2C) and showed moderate ablation of NKX6.3-induced cell growth inhibition (Fig. 2D). Thus, it is likely that NKX6.3 attenuates ROS production by Hace1-dependent or –independent regulation of ROS-mediated gene expression.
Figure 1.
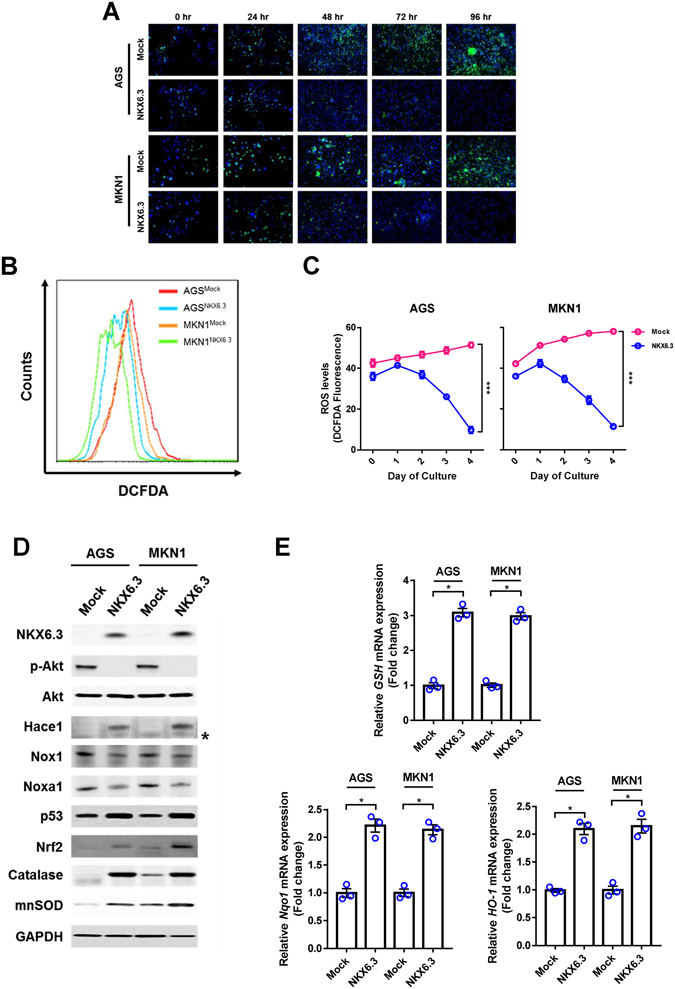
NKX6.3 reduces ROS levels by regulating ROS-responsive genes. (A–C) Measurement of ROS using Fluorescence microscopy and FACS analysis by DCFDA staining. Stable expression of NKX6.3 in AGS and MKN1 cells showed reduced levels of ROS in a time-dependent manner, compared to mock stable AGS and MKN1 cells. Results are represented as mean ± SEM from three independent experiments. (D) NKX6.3 significantly induced the expression of Hace1, Nrf2, catalase and mnSOD, and reduced the expression of Nox1 and Noxa1 proteins in western blot assay. (E) In real-time PCR analysis, NKX6.3 induced GSH, Nqo-1 and Ho-1 mRNA transcript. Results are represented as mean ± SEM from three independent experiments. Each dot plot represents the result from the individual experiment. ***P < 0.0001, *P < 0.05, based on the student’s t-test.
Figure 2.
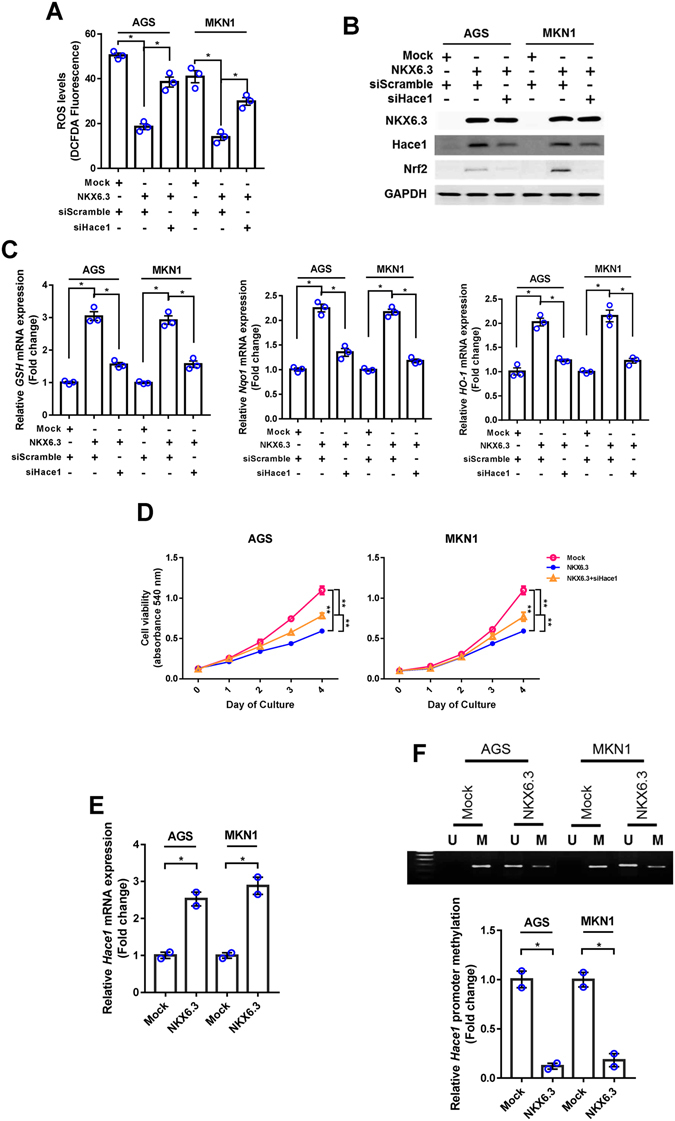
NKX6.3 increases Hace1 expression by inducing demethylation of Hace1 promoter. (A) In DCFDA staining analysis, knock-down of Hace1 partially recovered ROS production in NKX6.3 stable cells. Results are represented as mean ± SEM from three independent experiments. (B) In NKX6.3 stable cells, knock-down of Hace1 reduced Nrf2 expression in western blot analysis. (C) In real-time PCR analysis, Hace1 silencing resulted in decreased GSH, Nqo1 and Ho-1 mRNA expression in NKX6.3 stable cells. Results are represented as mean ± SEM from three independent experiments. (D) NKX6.3 stable cells showed time-dependent inhibition of cell viability, but knock-down of Hace1 in NKX6.3 stable cells showed recovery of cell viability. Results are represented as mean ± SEM from three independent experiments. (E) NKX6.3 induced the expression of Hace1 mRNA transcript in AGS and MKN1 cells in real-time PCR. Results are represented as mean ± SEM from three independent experiments. (F) Hypermethylation of CpG island at Hace1 promoter was found in mock stable AGS and MKN1 cells, whereas NKX6.3 expression induced demethylation of Hace1 promoter in MSP and real-time qPCR. Results are represented as mean ± SEM from three independent experiments. Each dot plot represents the result from the individual experiment. **P < 0.01, *P < 0.05, based on the student’s t-test.
NKX6.3 induces expression of Hace1-HECT E3 ligase
The Hace1-HECT E3 ligase is a tumor suppressor that directly regulates ROS production, and its reduced expression due to promoter hypermethylation is frequently observed in several cancers17, 18. Since NKX6.3 induced re-expression of Hace1 protein (Fig. 1D), we hypothesized that NKX6.3 functions as a hypomethylating agent. Expectedly, expression of Hace1 mRNA was markedly increased in NKX6.3 stable transfectants (Fig. 2E). Notably, hypermethylation of CpG islands on Hace1 promoter was observed in mock stable AGS and MKN1 cells, whereas de-methylation on Hace1 promoter was detected in NKX6.3 stable transfectants (Fig. 2F). Next, we evaluated the regulatory role of NKX6.3 in DNMT1 expression. As shown in Fig. 3A, NKX6.3 significantly down-regulated expression of DNMT1 mRNA and protein in AGSNKX6.3 and MKN1NKX6.3 cells. Knockdown of NF-kB, a transcription factor for DNMT119, with siNF-kB markedly decreased DNMT1 expression at the mRNA and protein levels (Fig. 3B). ChIP assay results indicated that p50 binding to the DNMT1 gene promoter was significantly inhibited by NKX6.3, comparable with the effect of siNF-kB (Fig. 3C). In addition, NKX6.3 expression as well as NF-kB silencing significantly decreased DNMT1 activity (Fig. 3D). Knock-down of DNMT1 with shDNMT1 increased Hace1 mRNA and protein expression (Fig. 3E) and induced de-methylation of Hace1 gene in AGS and MKN1 cells (Fig. 3F). These findings collectively suggest that NKX6.3 induces Hace1 expression by suppressing DNMT1 activity.
Figure 3.
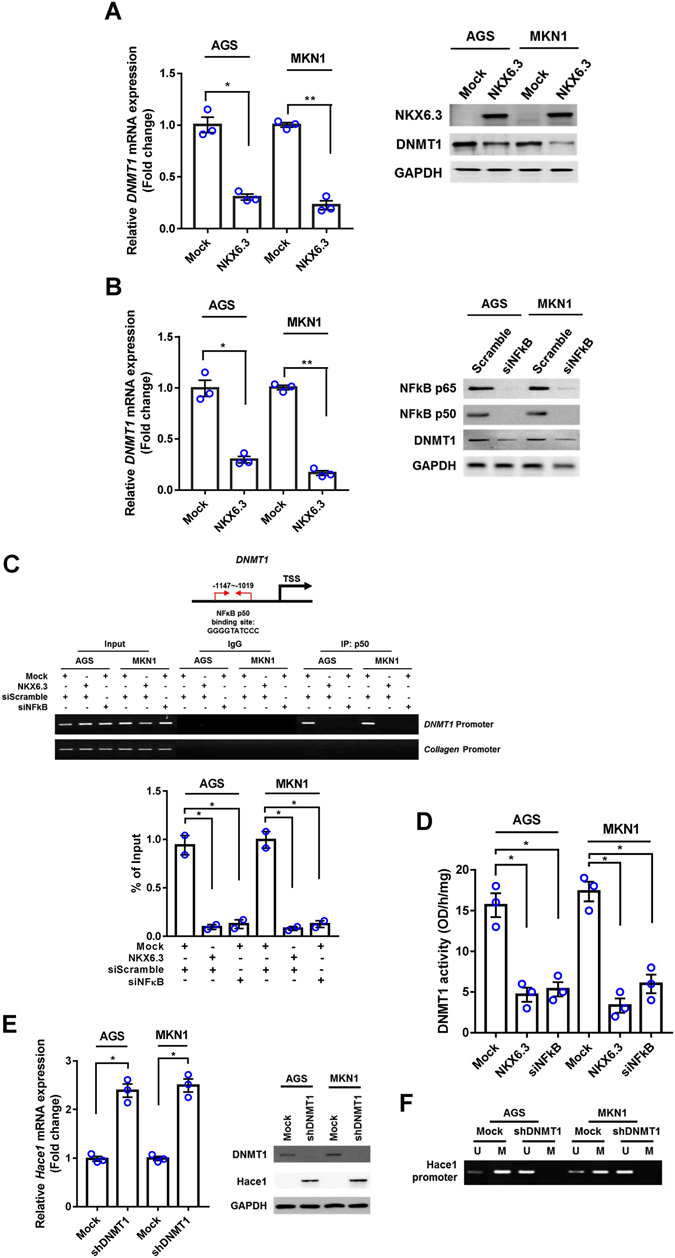
NKX6.3 inhibits the expression and activity of DNMT1. (A) NKX6.3 reduced DNMT1 mRNA and protein expression in AGS and MKN1 cells. (B) Inactivation of NF-kB significantly decreased DNMT1 mRNA and protein expression in AGS and MKN1 cells. (C) In ChIP assay, NKX6.3 inhibited p50 binding to the promoter region of DNMT1 gene and its inhibitory effect was very similar to that of siNF-kB. Collagen gene was used as a negative control and bands were seen in the input but not in the IgG and p50 precipitated genomic DNA. (D) NKX6.3 expression and NF-kB silencing significantly reduced DNMT1 activity. (E) Inactivation of DNMT1 with shDNMT1 induced Hace1 mRNA and protein expression. (F) DNMT1 silencing induced de-methylation of Hace1 promoter. All results are represented as mean ± SEM from three independent experiments. Each dot plot represents the result from the individual experiment. **P < 0.01, *P < 0.05, based on the student’s t-test.
NKX6.3 down-regulates NF-kB expression and activity
Next, we investigated whether NKX6.3 regulates NF-kB activity. Interestingly, NKX6.3 markedly down-regulated NF-kB p65 and p50 at the mRNA and protein levels (Fig. 4A–C). We performed ChIP assay, followed by PCR and real-time QPCR, to identify NKX6.3 binding activity within promoter sequences of the NF-kB p65 and p50 genes in AGSMock, MKN1Mock, AGSNKX6.3 and MKN1NKX6.3 cells. We defined regions upstream of the NF-kB p65 (between −1872 to −1662 bp) and p50 (between −3640 to −3371 bp) genes, overlapping with the transcription start site (TSS) designated 0 bp with 6 and 5 NKX6.3 candidate binding sites (TAAT), respectively. NKX6.3 showed binding activity in these promoter regions of p65 and p50 in AGS and MKN1 cells (Fig. 4D and E). To further confirm that NKX6.3 down-regulates NF-kB expression, we analyzed NF-kB downstream target genes, including interleukin (IL)-6, IL-1β, and TNF-α. As expected, stable NKX6.3 expression markedly reduced IL-6, IL-8 and TNF-α mRNA expression (Supplementary Figure S2), indicating that NKX6.3 inhibits DNMT1 activity by down-regulating NF-kB activity at the transcriptional level.
Figure 4.
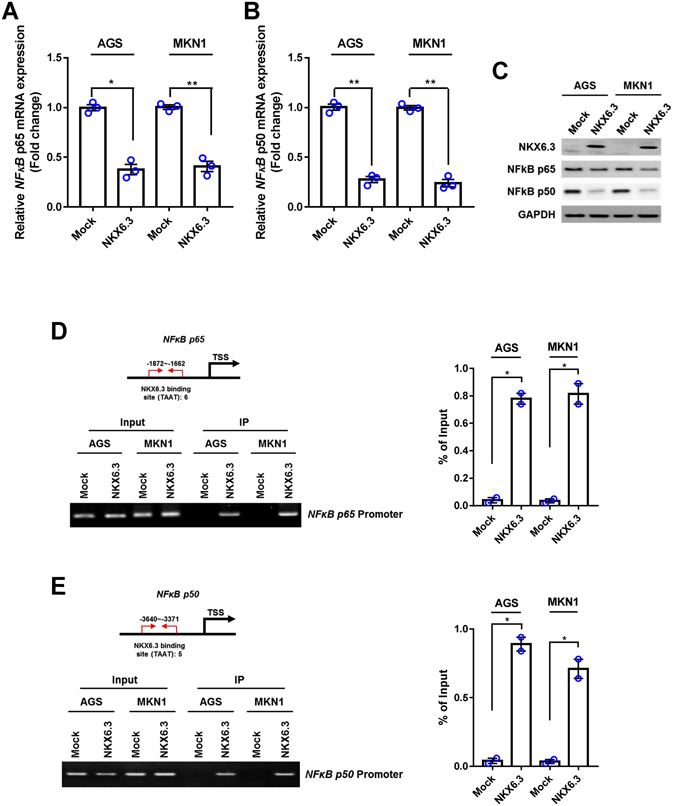
NKX6.3 negatively regulates NF-kB expression and activity. (A) NKX6.3 decreased the expression of NF-kB subunit p65 mRNA transcript in real-time PCR analysis. Results are represented as mean ± SEM from three independent experiments. (B) NKX6.3 reduced NF-kB subunit p50 mRNA expression in AGS and MKN1 cells. Results are represented as mean ± SEM from three independent experiments. (C) In AGS and MKN1 cells, NKX6.3 reduced expression of NF-kB p65 and p50 proteins in western blot analysis. (D and E) ChIP and ChIP-qPCR analyses of NKX6.3 binding to the promoters of NF-kB p65 (D) and p50 (E) genes. The results are represented as mean ± SEM from two independent experiments. Each dot plot represents the result from the individual experiment. **P < 0.01, * P < 0.05, based on the student’s t-test.
Hace1 regulates NF-kB expression and activity
Next, we determined the regulatory role of Hace1 in NF-kB expression. As shown in Figure 5A–D, silencing of Hace1 with siHace1 increased expression of p65, p50 and DNMT1 at the mRNA and protein levels in NKX6.3 stable transfectants. In addition, DNMT1 activity was also increased in siHace1 transfected AGSNKX6.3 and MKN1NKX6.3 cells (Fig. 5E), suggesting that Hace1 is involved in NF-kB inactivation.
Figure 5.
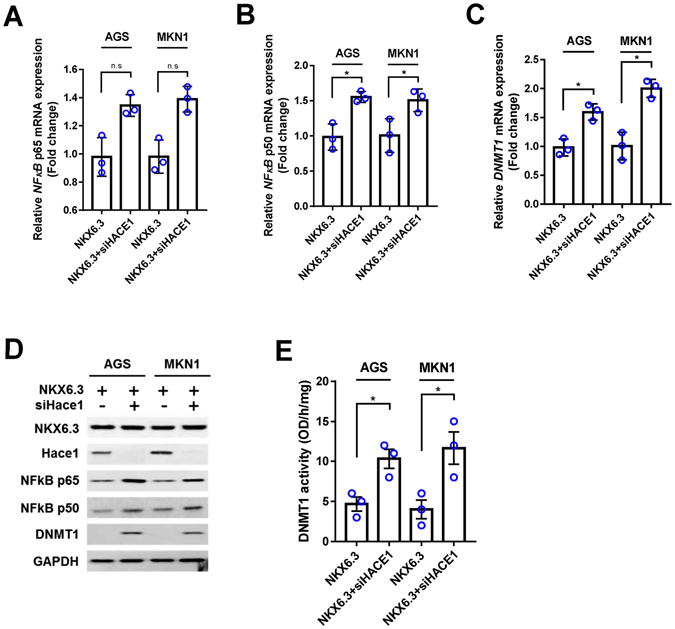
Knock-down of Hace1 induces NF-kB expression and activity. (A–C) Knock-down of Hace1 in NKX6.3 stable cells induced expression of NF-kB p65 (A), p50 (B) and DNMT1 (C) mRNA transcript in real-time PCR analysis. Results are represented as mean ± SEM from three independent experiments. (D) In NKX6.3 stable cells, silencing of Hace1 with siHace1 increased NF-kB p65, p50 and DNMT1 protein expression. (E) Knock-down of Hace1 induced DNMT1 activity in NKX6.3 stable cells. Results are represented as mean ± SEM from three independent experiments. Each dot plot represents the result from the individual experiment. *P < 0.05, based on the student’s t-test.
H. pylori CagA is an important factor for ROS production
Here, we examined the effects of H. pylori CagA on ROS production and expression of ROS-related genes. Expectedly, CagA significantly increased cell growth and ROS production in AGS and MKN1 cells, whereas NKX6.3 ablated the effects of CagA (Fig. 6A–C). To further confirm that CagA induces ROS production, we examined ROS levels in AGS cells by using the strain of H. pylori with or without CagA. As shown in Figure 6D, H. pylori with CagA significantly increased ROS production, but H. pylori without CagA did not affect ROS levels in AGS cells. Additionally, CagA increased the expression of NF-kB p65, p50 and DNMT1 proteins and induced loss of Hace1 expression, whereas NKX6.3 markedly inhibited CagA effects on protein expression (Fig. 6E). Furthermore, NKX6.3 suppressed CagA-induced DNMT1 activity and hypermethylation of Hace1 gene in AGSNKX6.3 and MKN1NKX6.3 cells (Fig. 6F,G). Our data suggest that NKX6.3 inactivates stimulatory effects of CagA on cell growth, ROS production, NF-kB signaling pathway, and DNMT1 activity in gastric epithelial cells.
Figure 6.
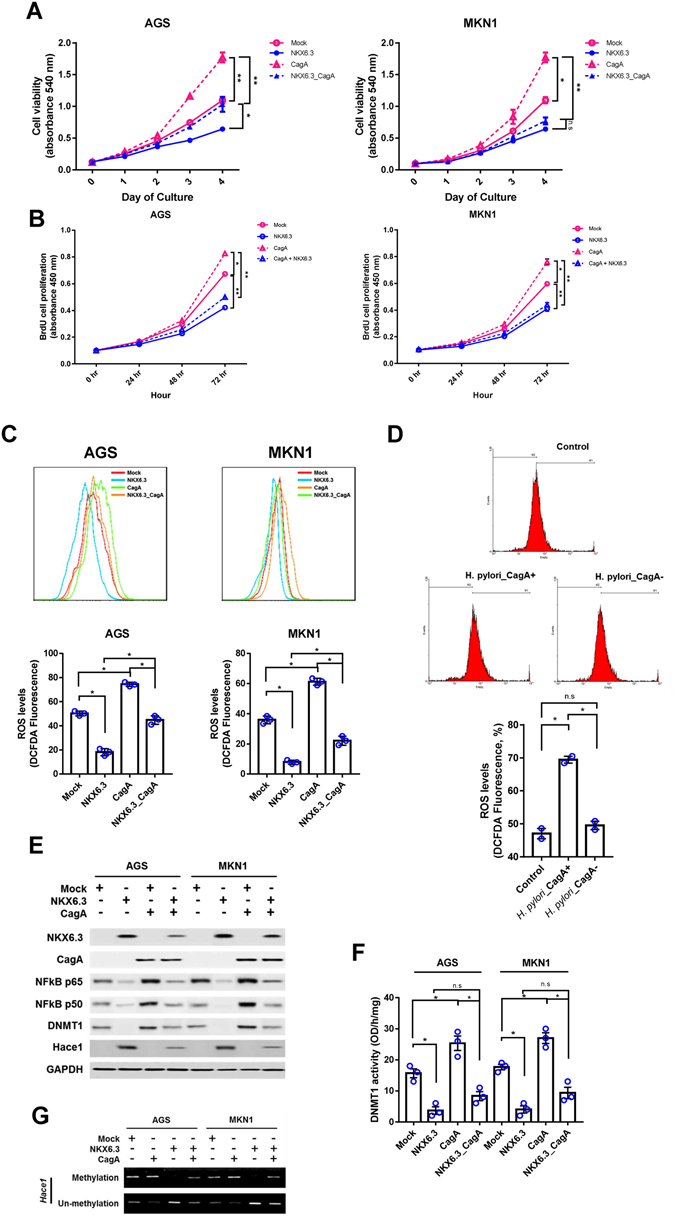
NKX6.3 suppresses the effects of H. pylori CagA on cell growth, ROS production, NF-kB signaling pathway and DNMT1 activity. (A and B) CagA induced a time-dependent increase in cell viability (A) and cell proliferation (B), but the expression of NKX6.3 inhibited the stimulatory effects of CagA on cell viability and proliferation. Results are represented as mean ± SEM from three independent experiments. (C) NKX6.3 abrogated CagA-induced ROS production in NKX6.3 stable cells. (D) CagA increased the expression of NF-kB p65, p50 and DNMT1 proteins and induced loss of Hace1 expression, but NKX6.3 markedly inhibited the CagA effects on expression of these proteins. (E) CagA enhanced DNMT1 activity in AGS and MKN1 cells, but NKX6.3 suppressed the CagA-induced DNMT1 activity. Results are represented as mean ± SEM from three independent experiments. (F) NKX6.3 inhibited CagA-induced hypermethylation of Hace1 gene. Each dot plot represents the result from the individual experiment. **P < 0.005, *P < 0.05, based on the student’s t-test.
Expression of NKX6.3, Hace1, and DNMT1 is closely associated in gastric mucosae and cancers
In 65 gastric cancer tissues, DNMT1 expression was significantly increased, whereas Hace1 expression was significantly reduced (Fig. 7A and B). NKX6.3 and Hace1 expression were positively correlated, while DNMT1 expression was inversely correlated with NKX6.3 and Hace1 expression in gastric cancer tissues (Fig. 7C–E). Consistently, NKX6.3, Hace1, and Nrf2 expression was reduced and showed positive correlation in the large cohorts of gastric cancer patients available from the NCBI GEO database (accession numbers GSE27342; Supplementary Figure S1).
Figure 7.
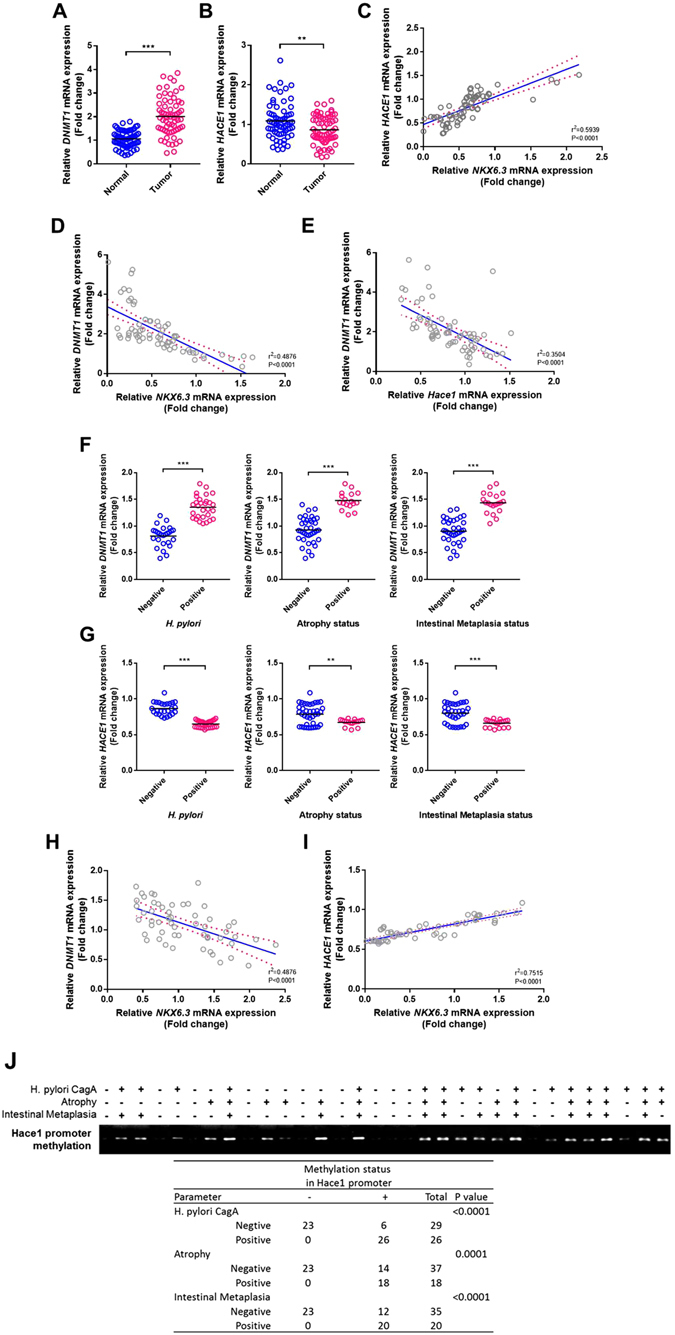
NKX6.3 is correlated with DNMT1 and Hace1 in gastric mucosae and gastric cancer tissues. (A and B) The relative mRNA expression levels of DNMT1 (A) and Hace1 (B) in noncancerous (Normal) and gastric cancer (Tumor) tissues are illustrated by scatterplot. The median expression level of each group is indicated by horizontal line. (C–E) A positive correlation of NKX6.3 with Hace1 and inverse correlation of DNMT1 expression with NKX6.3 and Hace1 expression. (F and G) Expression levels of DNMT1 (F) and Hace1 (G) in 55 gastric mucosae with H. pylori infection, atrophy and intestinal metaplasia. (H and I) A positive correlation between NKX6.3 and Hace1 expression and inverse correlation between DNMT1 and NKX6.3 expression in non-neoplastic gastric mucosae. (J) Methylation status of Hace1 promoter region examined by MSP. Hypermethylation of Hace1 gene was observed only in the gastric mucosae with H. pylori infection, atrophy, and/or intestinal metaplasia. ***P < 0.001, **P < 0.005, based on the two-way ANOVA, linear progression and Chi-square tests.
In 55 non-neoplastic gastric mucosae, DNMT1 and Hace1 expression was increased and reduced, respectively, in gastric mucosae with H. pylori infection, atrophy and intestinal metaplasia (Fig. 7F and G). Non-neoplastic gastric mucosae also showed positive correlation between NKX6.3 and Hace1 expression and inverse correlation between DNMT1 and NKX6.3 expression (Fig. 7H and I). The methylation status of Hace1 promoter region was examined by MSP to confirm that reduced Hace1 mRNA expression in gastric mucosae is caused by hypermethylation of Hace1. Interestingly, Hace1 hypermethylation was observed only in the gastric mucosae with H. pylori infection, atrophy, and/or intestinal metaplasia (Fig. 7J), suggesting that NF-kB-induced DNMT1 expression caused by NKX6.3 inactivation may reduce Hace1 expression by hypermethylating the promoter region of Hace1 in the gastric mucosa.
Discussion
Gastric cancers develop as a consequence of chronic inflammation from persistent mucosal or epithelial cell colonization by microorganisms, including H. pylori 20. Chronic inflammatory cells such as macrophages/monocytes, lymphocytes, and plasma cells are present in the gastric mucosa of chronic gastritis and lead to the generation of several ROS and RNS21. Persistent ROS can damage cellular DNA, RNA, and proteins by chemical reactions, which subsequently cause proto-oncogene activation, oncogene/tumor suppressor gene mutations, and chromosomal aberrations22, 23. In stomach, H. pylori and ROS collaborate in the gastric epithelium to activate the NF-κB and AP-1 transcription factors that up-regulate the expression of chemokines, including IL-824, 25, and in turn, NF-kB-dependent genes play a major role in regulating the cellular ROS levels26. Recently, we and others reported that NKX6.3 acts as a master regulator in gastric differentiation and proliferation12–14. Since the gastric mucosal barrier may protect the host from potentially harmful agents to maintain cell survival, we focused on the role of NKX6.3 in the protection of the gastric mucosal epithelia from harmful ROS.
It is well known that Hace1 functions as an important component of the cellular ROS detoxification and anti-oxidative stress responses by facilitating optimal activation of Nrf227. Here, we showed that NKX6.3 reduced intracellular ROS and modulated the expression of ROS-related genes (Fig. 1). Of these, NKX6.3 induced expression of Hace1 and Nrf2 proteins in gastric cancer cells (Fig. 1D) and showed positive correlation with Hace1 and Nrf2 in the gastric cancer cohort (Supplementary Figure S1). In addition, Hace1 silencing with siHace1 recovered ROS production and reduced Nrf2, GSH, Nqo and Ho-1 expression (Fig. 2A–C). Furthermore, knock-down of Hace1 ablated NKX6.3-induced cell growth inhibitory activity (Fig. 2D). Taken together, these results suggest that NKX6.3 plays an important role in suppression of ROS production by regulating expression of ROS-related genes, especially Hace1.
NKX6.3 increased Hace1 expression and was positively correlated with Hace1, suggestive of direct regulation of Hace1 expression. Since Hace1 expression is significantly reduced by promoter hypermethylation in most cancer patients17, we investigated whether DNMT1 regulates Hace1 expression in gastric epithelial cells. NF-kB reportedly binds to one possible NF-kB binding element in the DNMT1 promoter and DNMT1 mediates NF-kB-dependent p16 gene promoter hypermethylation19. Here, we showed that NKX6.3 induced Hace1 promoter demethylation and increased its expression (Fig. 2E and F) by down-regulating DNMT1 expression via inhibiting p50 binding in the promoter region of DNMT1 gene (Fig. 3C). In addition, the effect of NKX6.3 on DNMT1 activity was comparable with that of siNF-kB treatment (Fig. 3D). Furthermore, DNMT1 silencing with siDNMT1 led to demethylation of Hace1 and increased its expression at the mRNA and protein levels (Fig. 3E and F). These results suggest that NKX6.3 induces Hace1 expression by inhibiting DNMT1 expression and activity.
Next, we investigated whether NKX6.3 regulates NF-kB, which is constitutively activated in most cancers28. NF-κB consists of a family of transcription factors, including p65 (RelA), p50 (RelB), c-Rel, p105/p50 (NF-κB1), and p100/p52 (NF-κB2), which play critical roles in inflammation, immunity, cell proliferation and survival29. The amino-terminus of the Rel homology domain in p50 homo- and p50/p65 heterodimers mediates specific DNA binding to the NF-κB consensus sequence present in regulatory elements of NF-κB target genes30–32. NKX6.3 significantly reduced p65 and p50 expression at the mRNA and protein levels (Fig. 4A–C). In particular, the promoter regions of p65 and p50 have 6 and 5 NKX6.3 candidate binding sites, respectively (Fig. 4D), and the binding activity of NKX6.3 to these regions was confirmed in ChIP analysis (Fig. 4E). In addition, NKX6.3 markedly reduced the expression of NF-κB target genes including IL-6, IL-8 and TNF- α (Supplementary Figure S2). In addition, silencing of Hace1 with siHace1 increased the expression of p65, p50 and DNMT1 and activity of DNMT1 (Fig. 5). Thus, these data indicate that NKX6.3 and Hace1 negatively control NF-kB expression and activity.
It has been reported that H. pylori induces the production of ROS and DNA damage in gastric epithelial cells and frequently causes chromosomal aberrations33, 34. Previously, we showed that H. pylori CagA increased the expression of NF-kB proteins and ROS levels in gastric cancer cells35. In the current study, CagA significantly increased cell growth and ROS production in AGSMock and MKN1Mock cells, whereas NKX6.3 ablated these CagA effects by down-regulating NF-kB p65, p50 and DNMT1 and up-regulating Hace1 expression (Fig. 6A–F). These findings suggest that NKX6.3 may counteract CagA-induced NF-kB activity in gastric epithelial cells.
Damage to cellular components results in increased mutations and altered functions of important proteins in premalignant tissues, thereby contributing to the multi-stage carcinogenesis36. Chronic inflammation of gastric mucosa triggers a pathway of chronic gastritis, atrophy, intestinal metaplasia, dysplasia, which finally progress to gastric cancer37. H. pylori, especially CagA strains, is considered as the most important risk factor of atrophy and intestinal metaplasia38, 39. In gastric cancer tissue, NKX6.3 and Hace1 expression showed a positive correlation, while DNMT1 expression was inversely correlated with NKX6.3 and Hace1 (Fig. 7A–E). The NCBI GEO database also showed reduced NKX6.3, Hace1 and Nrf2 expression and a positive correlation between these genes (Supplementary Figure S1). In non-neoplastic gastric mucosae with H. pylori infection, atrophy and intestinal metaplasia, DNMT1 mRNA was increased, whereas Hace1 mRNA was reduced. (Fig. 7F and G). NKX6.3 expression was positively and inversely correlated with Hace1 and DNMT1, respectively (Figure H and I). Interestingly, Hace1 hypermethylation was observed only in the gastric mucosae with H. pyori infection, atrophy, and/or intestinal metaplasia (Fig. 7J). Thus, NKX6.3 inactivation in gastric mucosa may increase activity of NF-kB and DNMT1 and reduce Hace1 expression, subsequently progressing to atrophy, intestinal metaplasisa and cancer.
In conclusion, NKX6.3 induced Hace1 expression by suppressing DNMT1 expression and activity via down-regulating the NF-kB signaling pathway. In addition, NKX6.3 ablated CagA effects on cell proliferation, ROS production, and activities of DNMT1 and NF-kB signaling pathway. Overall, we conclude that NKX6.3 protects gastric mucosal epithelia by regulating harmful ROS production. Modulation of NKX6.3 anti-oxidant activity could contribute to the prevention of precancerous changes in gastric mucosa and gastric cancer.
Materials and Methods
Cell culture and generation of NKX6.3 stable cells
AGS and MKN1 gastric cancer cell lines were cultured at 37 °C in 5% CO2 in RPMI-1640 medium (Lonza, Basel, Switzerland) with 10% heat-inactivated fetal bovine serum (FBS). NKX6.3 and CagA cDNAs were cloned into the pcDNA3.1 expression vectors (Invitrogen, Carlsbad, CA, USA) and siHACE1, siDNMT1, and siNF-kB were cloned into pSilencer neo vectors (Invitrogen, Carlsbad, CA, USA). We generated stable NKX6.3 transfectants of AGS and MKN1 cells, AGSNKX6.3 and MKN1NKX6.3, stably expressing human NKX6.3, as well as mock transfectants, AGSMock and MKN1Mock cells, as described previously13. AGSNKX6.3 and MKN1NKX6.3 cells were transfected in 60 mm-diameter dishes with the expression plasmids (2 μg total DNA) using Lipofectamine Plus transfection reagent (Invitrogen) according to the manufacturer’s recommendations. Stable expression of NKX6.3 was confirmed in AGSNKX6.3 and MKN1NKX6.3 cells by western blot analysis.
Gastric mucosa and cancer tissue specimens
A total of 65 patients with sporadic gastric cancer who underwent a gastrectomy at the Chonnam National University Hwasun Hospital were enrolled. In addition, a total of 55 non-neoplastic frozen gastric mucosa remote (>5 cm) from gastric cancer were included in this study. Adjacent gastric mucosal tissues to each frozen specimen were also fixed in formalin and stained with hematoxylin-eosin. Informed consent was provided according to the Declaration of Helsinki. Written informed consent was obtained from all subjects. The study was approved by the Institutional Review Board of The Catholic University of Korea, College of Medicine (MC15SISI0015). There was no evidence of familial cancer in any of the patients.
Histological assessment of 55 non-neoplastic gastric mucosae was performed independently by two pathologists. According to the updated Sydney system40, 41, gastritis was determined by polymorphonuclear leukocyte infiltration, mononuclear cell infiltration, glandular atrophy and intestinal metaplasia, as previously described14. Infection with a CagA-positive strain of H. pylori was determined by the presence of CagA protein in 55 gastric mucosa tissue samples using western blot analysis, as described previously35.
Measurement of cell viability and proliferation
Cell viability was determined in AGS and MKN1 gastric cancer cells after treatment with siHace1 or H. pylori CagA transfection. To assess cell viability, a MTT [3-(4,5 dimethylthiazol-2-yl)−2,5-diphenyltetrazoliumbromide] assay was performed at 24, 48, 72, and 96 hrs following transfection with siHace1 and CagA in mock, AGSMock and MKN1Mock cells, and NKX6.3 stable transfectants, AGSNKX6.3 and MKN1NKX6.3 cells. Absorbance was measured at 540 nm using a spectrophotometer and viability was expressed relative to the mock control.
For cell proliferation assay, a BrdU incorporation assay was performed at 24, 48, 72, and 96 hrs following transfection with CagA in mock and NKX6.3 stable cells using the BrdU cell proliferation assay kit (Millipore, Billerica, MA, USA), according to the manufacturer’s instruction. Absorbance was measured using a spectrophotometer at 450 nm and proliferation was expressed relative to the mock control.
Expression of ROS-related genes in gastric cancer cell lines and tissues
Expression of NKX6.3, Hace1, and DNMT1 was examined in 65 frozen gastric cancers, corresponding non-cancerous gastric mucosal tissues and 2 gastric cancer cell lines by real-time RT-PCR and Western blot analysis. After quantification of mRNA extracted from cancer tissues and non-cancerous gastric mucosae, cDNA was synthesized using the reverse transcription kit from Roche Molecular System (Roche, Mannheim, Germany), according to the manufacturer’s protocol. For QPCR, 50 ng cDNA was amplified using Fullvelocity SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA, USA) and 20 pmol/μl of each primer (forward and reverse) using Stratagene Mx 3000 P QPCR system, according techniques previously published39. NKX6.3, Hace1, and DNMT1 mRNAs were quantified by SYBR Green Q-PCR and normalized to mRNA of the β-actin. Sequences of the primers are described in Supplementary Table S1. Data are reported as relative quantities according to an internal calibrator using the 2−△△CT method42. All samples were tested in duplicate, and the mean values were used for quantification. In addition, the relation between expression levels of ROS-related genes and gastritis parameters, such as atrophy, intestinal metaplasia and H. pylori infection was also examined in 55 non-neoplastic gastric mucosae.
To investigate whether ablation of NKX6.3 is associated with ROS production, the expression of NF-kB, Hace1, DNMT1, GSH, Nqo1, and Ho-1 mRNA were analyzed using real-time RT-PCR in AGSMock, MKN1Mock, AGSNKX6.3, and MKN1NKX6.3 cells. The effects of Hace1 silencing with siHace1 on the expression of ROS-related genes were also examined. To further confirm that NKX6.3 regulates NF-kB activity, we analyzed mRNA transcript expression of IL-6, IL-8, and TNF-α, which are downstream targets of the NF-kB43. Each mRNA was quantified by SYBR Green QPCR and normalized to mRNA of the housekeeping gene, β-actin. The primer sequences are described in Supplementary Table S1.
For Western blot analysis, the samples were ground to very fine powder in liquid nitrogen using a pestle and mortar, suspended in an ice-cold Nonidet P-40 lysis buffer supplemented with a 1x protease inhibitor mix (Roche). Cell lysates were separated on 10% polyacrylamide gel and blotted onto a Hybond-PVDF transfer membrane (Amersham), which had been subsequently probed with specific antibodies, and then incubated with anti-mouse IgG conjugated with horseradish peroxidase. The list of antibodies is described in Supplementary Table S2. The protein bands were detected using enhanced chemiluminescence Western blotting detection reagents (Amersham).
Methylation status of Hace1
Methylation analysis was carried out in 55 frozen non-neoplastic gastric mucosae and 2 gastric cancer cell lines after transfection with NKX6.3. Methylation status of the promoter region of the Hace1 gene was determined using sodium bisulfite treatment of the DNA followed methylation specific PCR (MSP), as described in the literature with minor modifications17. 5 μl of the bisulfite-modified DNA was subjected to MSP using two sets of primers for methylated and unmethylated Hace1. The primer sequences are described in Supplementary Table S1. PCR was performed in a total volume of 30 μl, containing 5 μl of the template DNA, 0.5 μM of each primer, 0.2 μM of each dNTP, 1.5 mM MgCI2, 0.4 unit of Ampli Taq gold polymerase (Perkin-Elmer) and 3 μl of 10X buffer. The reaction solution was initially denatured for 1 min at 95 °C. Amplification was carried out for 40 cycles of 30 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C, followed by a final 5 min extension at 72 °C. Each PCR product was loaded directly onto 2% agarose gels, stained with ethidium bromide and visualized under UV illumination.
Generation of CagA gene deleted H. pylori strains
The isogenic mutant H. pylori 26695 (∆cagA::aphA), in which most of the cagA gene was replaced by a aphA (kanamycin resistant gene from pIP1433) cassette, was made using PCR products generated with primers “kanF” (5′-GATAAACCCAGCGAACCAT) and “aphAR” (5′-GTACTAAAACAATTCATCCAGTAA) (1402 bp; aphA kanamycin resistance cassette); “cagA F1” (5′-ATCGTTGATAAGAACGATAGGG)and “cagA R2” (5′-ATGGTTCGCTGGGTTTATCATTGATTGCTTCTTTGACATCGGTACCAAGCGACCCAAATAG) (552 bp, upstream of deleted cagA segment); “cagA F5” (5′TTACTGGATGAATTGTTTTAGTACATCAAATAGCAAGTGGTTTGGGAATGACCTACTTAACAAAATCATG-) and “cagA R6” (5′-ATTGCTATTAATGCGTGTGTGG) (425 bp; downstream of deleted cagA segment). Natural transformation was carried out by adding 7 μl of purified PCR product containing this ΔcagA::aphA allele to a lawn of cells (wild type H. pylori 26695) growing exponentially on nonselective medium, and re-streaking the population on selective medium (containing 15 μg/ml of kanamycin) after 6–8 hrs or overnight incubation to obtain transformant colonies. PCR tests and sequencing of representative kanr transformants demonstrated expected replacement of cagA by aphA in each case.
Reactive oxygen species (ROS) analysis
ROS levels were determined in mock or NKX6.3 stable AGS and MKN1 cells using 2′-7′-dichlorodihydrofluorescein diacetate (DCFDA). The AGS and MKN1 cells were incubated with 10 uM DCFDA at 37 °C for 20 min and rinsed twice with cold PBS, then trypsinized and subjected to FACScan flow cytometer. To determine the effects of H. pylori CagA on ROS production, the ROS levels were examined in H. pylori with/without CagA- infected AGS cells at 12 hrs as well as in mock and NKX6.3 stable AGS and MKN1 cells at 72 hrs after transfection with H. pylori CagA. DCF fluorescence was measured by FACS analysis and intensity was plotted against the number of cells. Additionally, cells were incubated with 10 uM DCFDA at 37 °C for 20 min and quickly washed with cold PBS, and photos of representative fluorescent fields were taken under an inverted microscope.
Chromatin immunoprecipitation (ChIP) analysis
For assessing the NKX6.3 binding activity in the promoter region of NF-kB p65 and p50, ChIP assays were performed using the Thermo Scientific Pierce Agarose ChIP kit (Thermo Scientific Pierce, Rockford, IL, USA), as previously described13. Briefly, AGSMock, MKN1Mock, AGSNKX6.3 and MKN1NKX6.3 cells were cultured in a 10-cm dish for 4 days. The cells were fixed with 1% formaldehyde in PBS for 10 min, washed twice with ice-cold PBS and re-suspended in lysis buffer. Nuclei were recovered by centrifugation and MNase digestion was carried out at 37 °C for 15 min. Nuclei were lysed and the extracts were immunoprecipitated with 4 µg of antibody against NKX6.3 at 4 °C overnight. Normal rabbit IgG was used as the negative control. Protein-bound DNA was recovered using affinity chromatography purification columns according to the manufacturer’s protocol (Thermo Scientific), and 5 µl of lysed nuclei were also purified under the same procedure and used as input. DNA amplification was performed by PCR using specific primers for the promoters of NF-kB p65, p50 and DNMT1 genes, as described in Supplementary Table S1. Amplification products were separated on a 2% agarose gel.
Measurement of DNMT1 activity
The DNMT1 activity was analyzed using the DNMT1 activity assay kit (Abcam) according to manufacturer’s instructions. Briefly, AGS and MKN1 cells were collected and suspended in PBS. After centrifugation, the pellet was lysed in lysis buffer (10 mM Tris-Hcl pH 7.5, 10 mM NaCl, 2 mM MgCl2) containing protease inhibitor mixture (Complete; Roche Molecular Biochemicals). Then, 6 ul of 20% NP-40 was added and the mixture was incubated for 10 min at 4 °C and centrifuged for 5 min at 3000 rpm. The supernatant was collected and the pellet containing the nuclei was resuspended in 50 μl of extraction buffer (20 mM Hepes pH 7.9, 420 mM NaCl, 1.5 mM Mgcl2, 0.2 mM EDTA and 10% glycerol) followed by incubation for 30 min at 4 °C and collection of the nuclear extract by centrifugation. All reactions were carried out in triplicate.
Statistical analysis
Student’s t-test was used to analyze the effects of NKX6.3 on cellular ROS levels, cell viability and mRNA expression changes. Two-way ANOVA test was used to analyze the expression of DNMT1 and HACE1 in normal gastric mucosae and gastric cancer tissues. Linear progression test was used to analyze correlations between DNMT1, HACE1 and NKX6.3 expression levels in normal gastric mucosae and gastric cancer tissues. Chi-square test was used to analyze correlations between H. pylori CagA, atrophy, intestinal metaplasia and methylation status of the HACE1 promoter. Data are expressed as means ± S.D. from at least three independent experiments. A P-value less than 0.05 was considered to be the limit of statistical significance. All experiments were performed in triplicate to verify the reproducibility of the findings.
Electronic supplementary material
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2015R1A2A2A01002531, 2014R1A1A2058693). We thank Dr. Seong Yeob Ryu, Department of Gastroenterologic Surgery, Chonnam National University Hwasun Hospital, 160, Ilsim-ri, Hwasun-eup, Hwasun-gun, Jeollanam-do, 519-809, Korea, for providing the gastric cancer samples with clinical information.
Author Contributions
J.H.Y. and W.S.P. Conceptualization, J.H.Y. Investigation, J.H.Y., and O.K. Formal analysis, J.H.Y. and W.S.P. Writing-Original Draft, S.W.N. and J.Y.L. Writing-Review & Editing, J.H.Y. and W.S.P. Funding Acquisition.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02901-y
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60. doi: 10.1016/S0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- 4.Correa P, Piazuelo MB. The Gastric Precancerous Cascade. J Clin Exp Pathol. 2013;3:147. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myatt SS, Brosens JJ, Lam EWF. Sense and sensitivity: FOXO and ROS in cancer development and treatment. Antioxid Redox Signal. 2011;14:675–687. doi: 10.1089/ars.2010.3383. [DOI] [PubMed] [Google Scholar]
- 6.Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439–450. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 7.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 8.Toyokuni S. Novel aspects of oxidative stress-associated carcinogenesis. Antioxid Redox Signal. 2006;8:1373–1377. doi: 10.1089/ars.2006.8.1373. [DOI] [PubMed] [Google Scholar]
- 9.Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann N Y Acad Sci. 2006;1067:182–190. doi: 10.1196/annals.1354.022. [DOI] [PubMed] [Google Scholar]
- 10.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JH, et al. NKX6.3 Is a Transcription Factor for Wnt/β-catenin and Rho-GTPase Signaling-Related Genes to Suppress Gastric Cancer Progression. EBioMedicine. 2016;9:97–109. doi: 10.1016/j.ebiom.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon JH, et al. NKX6.3 induces gastric differentiation and inhibits gastric carcinogenesis. Oncotarget. 2015;6:28425–28439. doi: 10.18632/oncotarget.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon JH, et al. Inactivation of NKX6.3 in stomach leads to abnormal expression of CDX2 and SOX2 required for gastric-to-intestinal transdifferentiation. Mod Pathol. 2016;29:194–208. doi: 10.1038/modpathol.2015.150. [DOI] [PubMed] [Google Scholar]
- 15.Choi MY, et al. Requirement of the tissue-restricted homeodomain transcription factor Nkx6.3 in differentiation of gastrin-producing G cells in the stomach antrum. Mol Cell Biol. 2008;28:3208–3218. doi: 10.1128/MCB.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alanentalo T, et al. Cloning and analysis of Nkx6.3 during CNS and gastrointestinal development. Gene Expr Patterns. 2006;6:162–170. doi: 10.1016/j.modgep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, et al. The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat Med. 2007;13:1060–1069. doi: 10.1038/nm1621. [DOI] [PubMed] [Google Scholar]
- 18.Daugaard M, et al. Hace1 controls ROS generation of vertebrate Rac1-dependent NADPH oxidase complexes. Nat Commun. 2013;4:2180. doi: 10.1038/ncomms3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong J, Li D, Wands J, Souza R, Cao W. Role of NADPH oxidase NOX5-S, NF-κB, and DNMT1 in acid-induced p16 hypermethylation in Barrett’s cells. Am J Physiol Cell Physiol. 2013;305:C1069–1079. doi: 10.1152/ajpcell.00080.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowe SE. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr Opin Gastroenterol. 2005;21:32–38. [PubMed] [Google Scholar]
- 23.Du MQ, Carmichael PL, Phillips DH. Induction of activating mutations in the human c-Ha-ras-1 proto-oncogene by oxygen free radicals. Mol Carcinog. 1994;11:170–175. doi: 10.1002/mc.2940110308. [DOI] [PubMed] [Google Scholar]
- 24.Crowe SE, et al. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 25.Keates S, Hitti YS, Upton M, Kelly CP. Helicobacter pylori infection activates NF-kappa B in gastric epithelial cells. Gastroenterology. 1997;113:1099–1109. doi: 10.1053/gast.1997.v113.pm9322504. [DOI] [PubMed] [Google Scholar]
- 26.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotblat B, et al. HACE1 reduces oxidative stress and mutant Huntingtin toxicity by promoting the NRF2 response. Proc Natl Acad Sci USA. 2014;111:3032–3037. doi: 10.1073/pnas.1314421111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu T, Stark GR. NF-κB: Regulation by Methylation. Cancer Res. 2015;75:3692–3695. doi: 10.1158/0008-5472.CAN-15-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh G, van Duyne G, Ghosh S, Sigler PB. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature. 1995;373:303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 31.Müller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature. 1995;373:311–317. doi: 10.1038/373311a0. [DOI] [PubMed] [Google Scholar]
- 32.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410–413. doi: 10.1038/34356. [DOI] [PubMed] [Google Scholar]
- 33.Obst B, Wagner S, Sewing KF, Beil W. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis. 2000;21:1111–1115. doi: 10.1093/carcin/21.5.111. [DOI] [PubMed] [Google Scholar]
- 34.Wu CW, et al. Clinical implications of chromosomal abnormalities in gastric adenocarcinomas. Genes Chromosomes Cancer. 2002;35:219–231. doi: 10.1002/gcc.10106. [DOI] [PubMed] [Google Scholar]
- 35.Yoon JH, et al. Gastrokine 1 inhibits the carcinogenic potentials of Helicobacter pylori CagA. Carcinogenesis. 2014;35:2619–2629. doi: 10.1093/carcin/bgu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohshima H, Tazawa H, Sylla BS, Sawa T. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat Res. 2005;591:110–122. doi: 10.1016/j.mrfmmm.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol. 1988;83:504–509. [PubMed] [Google Scholar]
- 38.Ohata H, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–143. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 39.Nogueira C, et al. Helicobacter pylori genotypes may determine gastric histopathology. Am J Pathol. 2001;158:647–654. doi: 10.1016/S0002-9440(10)64006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Yoon JH, et al. Inactivation of the Gastrokine 1 Gene in Gastric Adenomas and Carcinomas. J Pathol. 2011;223:618–625. doi: 10.1002/path.2838. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


