Abstract
Brain tissue from a case of bovine spongiform encephalopathy (BSE) from Alberta was subjected to a Western immunoblotting technique to ascertain the molecular profile of any disease-specific, abnormal prion protein, that is, prion protein that is protease-resistant (PrPres). This technique can discriminate between isolates from BSE, ovine scrapie, and sheep experimentally infected with BSE. Isolates of brain tissue from the BSE case in Alberta, 3 farmed elk with chronic wasting disease (CWD) from different parts of Saskatchewan, and 1 farmed white-tailed deer with CWD from Edmonton, Alberta, were examined alongside isolates of brain tissue from BSE, ovine scrapie, and sheep experimentally infected with BSE from the United Kingdom (UK). The molecular weights of PrPres and the cross reactions to 2 specific monoclonal antibodies (mAbs) were determined for each sample. The BSE isolates from Canada and the UK had very similar PrPres molecular weights and reacted with only 1 of the 2 mAbs. The PrPres isolated from both elk and white-tailed deer with CWD had a higher molecular weight profile than did the corresponding PrPres from the scrapie and BSE isolates. The PrPres from CWD cases cross reacted with both mAbs, a property shared with PrPres in isolates from scrapie but not with PrPres isolates from BSE or sheep experimentally infected with BSE. The results from this study seem to confirm that the PrPres isolated from the BSE case in Alberta has similar molecular properties to the PrPres isolated from a BSE case in the UK, and that it differs in its molecular and immunological characteristics from the CWD and scrapie cases studied.
Abstract
Résumé — Le premier cas canadien d’encéphalopathie spongiforme bovine (ESB) indigene possède les caractéristiques moléculaires d’une protéine de prion similaire à celle de l’ESB du Royaume-Uni mais différent de celle de la maladie débilitante chronique du wapiti et du cerf en captivité. Des tissus nerveux provenant du cas d’encéphalopathie spongiforme bovine (ESB) en Alberta ont été soumis à un test d’immunobuvardage de Western pour établir le profil moléculaire de toute protéine de prion anormale spécifique de la maladie et résistante aux protéases (PrPres).
Cette technique permet de distinguer les isolats d’ESB de ceux de la tremblante du mouton ainsi que ceux de moutons infectés expérimentalement d’ESB.
Des tissus cérébraux du cas d’ESB albertain, de 3 wapitis d’élevage de différentes régions de la Saskatchewan et d’un cerf de Virginie d’Edmonton, Alberta, atteints tous les quatre de maladie débilitante chronique (MDC) ont été examinés en parallèle avec des tissus nerveux de bovins positifs pour l’ESB, de moutons atteints de tremblante et de moutons infectés expérimentalement par l’agent de l’ESB provenant tous du Royaume-Uni (RU).
Les poids moléculaires des PrPres et les réactions croisées envers deux anticorps monoclonaux spécifiques (Acm) ont été déterminés pour chaque échantillon.
Les tissus nerveux de bovins positifs pour l’ESB, qu’ils proviennent du Canada ou du RU avaient des poids moléculaires similaires et ont réagit avec un seul des deux Acm.
Les PrPres isolées des tissus nerveux de wapitis et de cerfs de Virginie positifs pour la MDC avaient des poids moléculaires plus élevés que ceux des PrPres correspondantes dans la Tremblante et l’ESB. La PrPres isolée des cas de MDC montre une réaction croisée avec les deux Acm, une propriété partagée avec la PrPres obtenue à partir de cas de Tremblante mais pas avec la PrPres des cas d’ESB ou celle isolée du tissu nerveux des moutons infectés par l’agent de l’ESB. Les résultats de cette etude semblent confirmer que la PrPres isolée du cas d’ESB de l’Alberta a des propriétés moléculaires semblables à celles de la PrPres isolée de cas d’ESB du RU et qu’elle diffère dans ses caractéristiques moléculaires et immunologiques de celle des cas de MDC et de Tremblante étudiés.
(Traduit par les auteurs)
Introduction
The transmissible spongiform encephalopathies (TSEs) affect both humans and animals and are fatal neuro-degenerative diseases. The human forms of the disease include Creutzfeldt-Jakob disease (CJD) and its variant (vCJD) (1). Animal forms, relevant to this study, are scrapie, which is a naturally occurring TSE of sheep and goats, chronic wasting disease (CWD) in cervids, and bovine spongiform encephalopathy (BSE). Scrapie has been recognized in Europe since at least the mid 1700s. Chronic wasting disease was once thought to be enzootic in free ranging mule deer, Rocky Mountain elk, and white-tailed deer in North America. It has since emerged as an important concern in the farmed cervid industry, being first recognized in Canada in 1996 (2). Bovine spongiform encephalopathy was first identified in 1986 in cattle within the United Kingdom (UK) (3).
Although now in decline in the UK, more than 180 000 cases of BSE had been confirmed up to the end of 2003. Most European countries have discovered affected animals by means of systematic epidemiological surveillance and, more recently, by immunological testing of the brains of slaughtered cattle. Varying numbers of indigenous cases in 21 countries, other than the UK, have been recorded (4) and regular updates of global BSE figures are available at the Office International des Épizooties (5). In 1993, BSE was diagnosed in 1 cow that had been imported into Canada in 1987 from the UK as a 5-month-old calf (6). This 1st indigenous case of BSE in Canada, detected in May 2003 as part of the Canadian BSE surveillance program, came from a commercial cow-calf operation in northern Alberta. The 8-year-old, black Angus cow was emaciated, a downer at the abattoir, and condemned because of emaciation and pneumonia.
Unlike the transmission of scrapie, there is little evidence for either direct horizontal or vertical transmission of BSE in cattle (7). Since the introduction of a UK-wide ban on the use of ruminant-derived protein in cattle feed in 1988, the prevalence of BSE in cattle has steadily declined in the UK (8). However, it was considered likely by the UK Spongiform Encephalopathy Advisory Committee that, during the 1980s, BSE-contaminated meat-and-bone meal was also fed to sheep, though probably to a lesser extent than to cattle, which raises concerns that the agent of BSE may have been transmitted to the UK national sheep flock. It has also been reported that genetically susceptible sheep, experimentally exposed to oral challenge with as little as 0.5 g of BSE brain material, contracted infection and developed clinical disease (9).
Until recently, the search for BSE in the UK national flock had been restricted to time-consuming assessments of incubation periods and brain lesion profiles in experimentally infected mice (10). Thus, there was a clear requirement for more rapid bench tests that would discriminate between BSE and scrapie in sheep. The Western immunoblotting technique employed in this study was developed specifically for this purpose (11).
An abnormal form of the prion protein (PrP) (12), termed PrPSc (13,14), is the most common disease-marker for the TSEs. This PrPSc, thought to be derived from a normal cell membrane glycoprotein (PrPc), is highly expressed in the central nervous system in diseased animals and humans. Whereas PrPc (molecular weight 33 to 35 kDa) is completely digested after proteinase K enzyme treatment, PrPSc is partially resistant to the enzyme with 62 amino acids being cleaved, leaving a core fragment of some 141 amino acids that are protease resistant (PrPres) and have a molecular weight of 27 to 30 kDa.
One way to detect the PrPres is by the application of polyacrylamide gel electrophoresis after protease digestion (15), followed by Western immunoblotting (16). With the use of these techniques, 3 disease-characteristic protein bands of PrPres, namely, the diglycosylated (upper band), monoglycosylated (middle band), and unglycosylated (lower band) forms of the protein, can be separated and then detected by antibodies produced against PrP (14). For glycoform analysis, the combined magnitude of signals from all 3 protein bands is defined as 100% and the contribution of each band is calculated as a percentage of the whole. To obtain a comparison of ratio between samples, the percentage signal from the diglycosylated band is plotted against the percentage signal obtained for the monoglycosylated band and shown as positions on a scattergram. In fact, the similarities in the glycoform ratios and molecular weights obtained have provided evidence of a bovine origin for vCJD human infections by showing that glycoform ratios and molecular weights of PrPres derived from sporadic or iatrogenic forms of CJD are quite different from those of vCJD and BSE (17,18).
The technique takes advantage of the fact that some antibodies bind to an epitope in the N-terminal region of PrPres and show a higher affinity for scrapie PrPres than for BSE PrPres, probably due to differences in amino acid sequence or proteinase K sensitivity within the N-terminal region (11,19). Discrimination between BSE and scrapie can thus be achieved by testing samples in parallel, using 2 specific monoclonal antibodies (mAbs). One mAb is raised to an amino acid sequence of the proteinase resistant core (a region conserved in both species) and the other is raised to an amino acid sequence of the N-terminal, which, after enzyme digestion, is conserved in scrapie derived PrPres, but not in BSE derived PrPres, and only partially in PrPres from sheep experimentally infected with BSE.
The Western immunoblots obtained from the tissues used for this present study were compared by using these molecular weight differences and the differential cross-reaction to the 2 mAbs as criteria for discrimination. In this way, molecular similarities or differences for PrPres among the BSE isolate from Alberta, the CWD isolates, and UK derived BSE and scrapie isolates can be compared.
Materials and methods
Animals and tissues
Brain tissue (caudal medulla) from the Alberta BSE isolate, 3 previously confirmed cases of CWD from farmed elk from different parts of Saskatchewan, and 1 farmed white-tailed deer from the outskirts of Edmonton, Alberta, was processed and examined along-side samples from isolates of BSE, natural ovine scrapie, and sheep experimentally infected with BSE derived from the UK.
The initial diagnosis of BSE in the cow in Alberta was made by the Alberta Provincial Food Safety Division following histopathological examination and immunohistochemical (IHC) testing of the obex of the medulla; paraffin blocks and remaining frozen brain tissues were forwarded to the Canadian BSE reference laboratory in Winnipeg for confirmation by IHC. A 2nd confirmatory opinion was obtained from the British BSE reference laboratory at the Veterinary Laboratories Agency (VLA) Weybridge, England.
Brain tissue (caudal medulla) from the UK BSE case (positive control) and ovine scrapie case were obtained through normal routine submissions of sheep and cattle brain to the VLA and were from clinically-suspect cases that were positive by statutory tests (histopathological examination, immunohistochemical staining, and the Prionics-Check Western immunoblotting test kit [Prionics Ag, Zurich, Switzerland]) for the detection of PrPres. The ovine scrapie sample was from a female Suffolk cross breed with a PrPARQ/ARQ genotype.
The experimental BSE in sheep sample came from previously homogenized and frozen brain tissue from a Cheviot with a PrPAHQ/AHQ genotype. This was a primary experimental infection by oral dose of brain tissue from an inoculum prepared from the brains of cattle clinically affected with BSE and positive by statutory tests.
Three negative controls were used: brain tissue from a farmed elk that was negative for CWD on previous testing, brain tissue from a bovine animal that was negative in the UK, and brain tissue from a sheep that was negative in the UK. Additional controls and standards loaded on the gel were biotinylated molecular weight markers and a bovine recombinant PrP (Prionics Ag) control.
Western immunoblotting technique
The Western immunoblotting technique that was used has been published previously and is known as the VLA hybrid technique (11); it is based on modifications to the Prionics-Check Western immunoblotting technique (20) and incorporates a centrifugation step to clarify the resultant immunoblots (17). The Prionics-Check technique has been evaluated by the European Commission (21) and is used globally as a screening test for the diagnosis of BSE. Previous results with the VLA hybrid technique have shown that sheep scrapie PrPres shows higher molecular weight protein bands than does cattle BSE PrPres, particularly for the unglycosylated protein band (11). The 2 mAbs used for discriminatory purposes are the mAb 6H4 (Prionics Ag), which is raised against the core amino acid sequence 144–152 of bovine PrPc (22), and the mAb P4, (BioPharm, Darmstadt, Germany), which is raised against the amino acid sequence 89–104 of ovine PrPc, near the N-terminal region (23). Sheep scrapie PrPres is detected by both these mAbs, whereas cattle BSE PrPres is detected only by the mAb 6H4.
Results
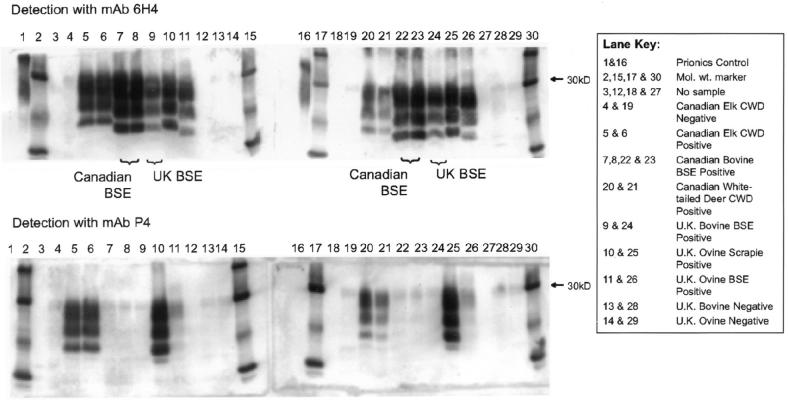
The immunoblots obtained with mAb 6H4 and mAb P4 are shown in Figure 1. The samples from the Canadian case of BSE and the 3 elk and 1 white-tailed deer were all detected by the mAb 6H4. The UK BSE, ovine scrapie, and the ovine experimental BSE positive samples were also detected by this antibody. Only the UK ovine scrapie positive control, the 3 elk, and the 1 white-tailed deer samples were immunoreactive with the mAb P4. Neither the UK BSE positive control nor the Canadian BSE sample were detected with the mAb P4. There was some residual faint staining for the ovine experimental BSE sample; this had previously been observed as a characteristic of samples from experimental BSE in sheep (11). The bovine PrP recombinant protein (Prionics Ag) was not detected with the mAb P4, again a previous observation (11).
Figure 1.
Representative Western immunoblots obtained by the Veterinary Laboratories Agency hybrid technique to compare the molecular profiles for the Canadian bovine spongiform encephalopathy (BSE) case, United Kingdom cattle BSE and sheep scrapie cases, and elk and white-tailed deer with chronic wasting disease (CWD). The top row of blots were detected with the mAb 6H4 and the bottom row with the mAb P4. Negative controls were processed in 1 lane and positive samples in duplicate lanes.
The unglycosylated protein band of the elk and white-tailed deer samples had a higher molecular weight than that of any of the other samples. The blots obtained for the Canadian BSE sample were very similar to those of the UK BSE positive control in terms of molecular weight. The ovine experimental BSE sample gave the lowest molecular weight for the unglycosylated band of all the samples tested. The molecular weights of the unglycosylated band obtained for the elk and white-tailed deer samples were higher than that found for the UK ovine scrapie sample.
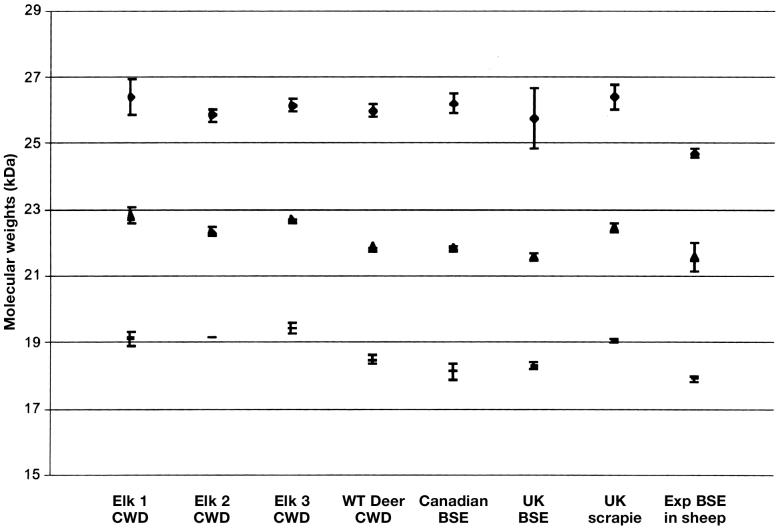
Molecular weights and standard deviations from the mean for all samples obtained with the mAb 6H4 are shown in Figure 2.
Figure 2.
Mean molecular weights of di-glycosylated, mono-glycosylated, and un-glycosylated protein bands of the Canadian bovine spongiform encephalopathy (BSE) sample, chronic wasting disease (CWD) samples, and United Kingdom controls (BSE positive, scrapie positive and experimental BSE in sheep) as detected with the mAb 6H4. Number of blots measured to provide standard error bars.
Canadian cases:
— Canadian BSE case = 8
— Three elk CWD + ve cases = 2 of each
— One white-tailed deer case = 2
UK controls:
— BSE positive (Bov + ve) = 4
— Scapie positive (Ov + ve) = 4
— Experimental BSE in sheep (Exp BSE in sheep) = 4
Discussion
The results from this study seem to confirm that the PrPres for the BSE case in Canada and the UK BSE positive control have similar molecular properties on Western immunoblotting and that they differ in their molecular and immunological characteristics from the CWD and scrapie cases studied here. The Canadian BSE isolate showed very similar molecular properties to the UK BSE isolate with regard to molecular weights of PrPres, immunoreactivity with the mAb 6H4, and a lack of immunoreaction with the mAb P4. The CWD samples had a different molecular weight profile to scrapie and BSE, with band molecular weights being higher than both, irrespective of whether they were derived from elk or white-tailed deer. However, more samples would be required for a full statistical analysis to determine whether this higher molecular weight is maintained for all CWD isolates from different geographical locations. This need for further comparisons of CWD isolates is highlighted in a report that describes the analysis of glycoform patterns from CWD-affected deer and elk, scrapie-affected sheep, scrapie-affected cattle, and BSE-affected cattle (24). This analysis failed to identify patterns capable of reliably distinguishing these TSEs, and showed PrPres patterns that sometimes differed between individual animals, suggesting infection by different or multiple CWD strains in some species. However, the general similarity of PrPres glycoform patterns in scrapie-affected sheep and CWD-affected cervids seemed to support the hypothesis that CWD arose from sheep scrapie, as did in vitro analyses (25). In many geographical areas, sheep, deer, and elk share pastures and ranges. If scrapie-affected sheep were present in these situations, cross-species transmission might have occurred.
In this study, the sheep scrapie and elk and white-tailed deer CWD samples were immunoreactive with both mAb 6H4 and mAb P4. Given that the CWD cases gave a high molecular weight profile and immunoreacted with both antibodies, it is considered that the samples tested had similar, but not identical, molecular properties to those found for sheep scrapie, but distinct from those for BSE. The molecular weight differences between CWD samples and sheep scrapie could be due to variation in the genetics of individual animals or to possible strain differences. Our observations are that, with this test, UK sheep scrapie gives a uniform molecular weight profile, regardless of breed, geographical area, and genotype, suggesting that strain variation would be the more likely hypothesis.
Previously, glycoform ratio analysis was also used to aid in discriminating between cases of experimental BSE in sheep and scrapie in sheep (11). However, there is considerable variation in signal from individual samples on different gels, which leads to large standard deviation measurements in the glycoform ratio analyses. The glycoform ratios obtained for natural scrapie cases and bovine BSE cases overlap, whereas ratios for experimental BSE in sheep are distinct from those for natural sheep scrapie (11). In our follow-up studies on the reproducibility of the VLA hybrid test, it was clear that glycoform ratio analysis was a less useful tool than molecular weight for discriminating between bovine BSE and scrapie. Therefore, glycoform ratios are not reported for this study.
Although CWD has been established as being endemic in free ranging Rocky Mountain elk and white-tailed deer in north central Colorado and south eastern Wyoming (26), surveillance has revealed its presence also in farmed cervids. Three cases of CWD were diagnosed in Canada, the 1st in 1978 by histopathological examination of tissues from a mule deer that had died at the Toronto Zoo in 1974 (Ian Barker, personal communication). In 1996 and 1998, CWD was confirmed in farmed elk in Saskatchewan. In both instances a single elk was diagnosed with CWD. Follow-up investigations included the destruction and testing of exposed elk. In 2000, the Canadian Food Inspection Agency (CFIA) confirmed CWD in another elk, on the same premises as the elk found to be infected in 1998. The CFIA quarantined the premises, quarantined all velvet produced on the premises in the preceding 3 y, and quarantined the farm of origin (source herd) of the infected elk. The CFIA also took immediate action to trace the movement of all elk from this farm. As of November 2003, 8740 farmed cervids (elk and deer) in the provinces of Saskatchewan and Alberta have been destroyed. All cervids (7598), except very young animals, were tested, 232 of which were infected, 31 of them considered to have clinical disease. Since 1996, these animals have come from 41 infected premises in Saskatchewan and 2 premises in Alberta.
With regard to the risk to human health, scientific evidence indicates that vCJD cases originated from BSE (10,17,18). In contrast, scrapie has never been associated with human disease. Although it is not clear whether CWD can be transmitted to humans who hunt and eat affected animals, in vitro studies provide evidence of a molecular barrier, which may limit the susceptibility of humans, cattle, and sheep to CWD (25). Also, the results in this present study do not suggest any molecular similarities between CWD and BSE. However, little is known about natural cross-species transmission and, even though there is the theoretical possibility and the experimental evidence that BSE could be transmitted to sheep, it is unknown whether any eventual natural BSE infection would have propagated within particular sheep flocks in the UK and spread in similar ways to scrapie, or whether the sheep BSE agent would be pathogenic for humans. Several tests, including the technique used in this study, are presently being evaluated in a preliminary European Union trial for their potential to discriminate between scrapie and BSE in sheep. Essentially, discrimination by means of proteinase K digestion and 2 different antibodies is utilized in all of these tests, with the exception of the conformation-dependent immunoassay (27), which relies on conformational changes in the absence of protease enzymes. By using this latter technique, which simultaneously measures specific antibody binding to denatured and native forms of the prion protein, 8 different prion strains passaged in hamsters were distinguished; by plotting the denatured and native protein ratio as a function of the PrPres concentration, it was found that each of the 8 isolates occupied a unique position.
In the UK, it is considered likely that BSE was derived from scrapie-infected tissue being included in the meat-and- bone meal rations fed to cattle (28). The scientific evidence so far favors this hypothesis, but other theories of origin include a spontaneous genetic mutation in cattle or infection via another mammalian species (4). Irrespective of the origin of BSE, it is generally accepted that the BSE epidemic in the UK was primarily driven by the feeding of BSE-contaminated meat-and-bone meal to cattle (28). The finding of a single confirmed case of BSE in Canada leaves room for conjecture that the case did arise spontaneously, perhaps (by analogy with CJD) as a result of a somatic protein misfolding event or a germline mutation. Recent publication of DNA sequence data from the BSE-affected animal’s prion coding sequence (29) and a subsequent finding from an animal exported to the United States argues definitively against spontaneous occurrence.
This study has provided molecular comparisons of PrPres from a limited number of samples from different species of specific relevance to Canadian and North American interests. However, the use of the developed differentiation technique in this context also provides a framework against which profiles of index cases of BSE from countries so far free from the disease, but which may acquire it, can be compared with existing BSE cases in affected countries and other natural or experimental animal TSEs.
Acknowledgments
The authors thank Danny Matthews, James Hope, and Otto Windl at the VLA and Brian Evans and Brian Peart at the CFIA for their valued comments on the manuscript. This work was funded as part of the Veterinary Laboratories Agency’s transmissible spongiform encephalopathy, European Community Reference Laboratory activities. CVJ
Footnotes
Funded as part of the Veterinary Laboratories Agency’s transmissible spongiform encephalopathy, European Community Reference Laboratory activities.
This report has not been peer reviewed.
References
- 1.Will RG, Ironside JW, Zeidler M, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Food Inspection Agency (CFIA) Chronic wasting disease (CWD) of deer and elk. CFIA fact sheet 2001. Available from http://www.inspection.gc.ca. Last accessed November 2002.
- 3.Wells GAH, Scott AC, Johnson CT, et al. A novel progressive spongiform encephalopathy in cattle. Vet Rec. 1987;121:419–420. doi: 10.1136/vr.121.18.419. [DOI] [PubMed] [Google Scholar]
- 4.Brown P. Transmissible spongiform encephalopathy as a zoonotic disease. International Life Sciences Institute (ILSI) Europe Report, Brussels, Belgium: 2003.
- 5.Office International des Épizooties [homepage on the Internet]. Available from http://www.oie.int. Last accessed February 2004.
- 6.Chen S, Charlton KM, Balachandran A, et al. Bovine spongiform encephalopathy identified in a cow imported to Canada from the United Kingdom — A case report. Can Vet J. 1996;37:38–39. [PMC free article] [PubMed] [Google Scholar]
- 7.Wrathall AE, Brown KFD, Bellerby P, et al. Studies on embryo transfer from cattle clinically affected with bovine spongiform encephalopathy (BSE) Vet Rec. 2002;12:365–378. doi: 10.1136/vr.150.12.365. [DOI] [PubMed] [Google Scholar]
- 8.Stevensen MA, Wilesmith JW, Ryan JBM, et al. Temporal aspects of the epidemic of bovine spongiform encephalopathy in Great Britain: individual animal-associated risk factors in disease. Vet Rec. 2000;147:349–354. doi: 10.1136/vr.147.13.349. [DOI] [PubMed] [Google Scholar]
- 9.Foster JD, Hope J, Fraser H. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet Rec. 1993;133:339–341. doi: 10.1136/vr.133.14.339. [DOI] [PubMed] [Google Scholar]
- 10.Bruce ME, Will RG, Ironside JW, et al. Transmission to mice indicate that “new variant” CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 11.Stack MJ, Chaplin MJ, Clark J. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol (Berl) 2002;104:279–286. doi: 10.1007/s00401-002-0556-2. [DOI] [PubMed] [Google Scholar]
- 12.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 13.Chesebro B, Race R, Wherly K, et al. Identification of scrapie prion protein specific mRNA in scrapie-infected and uninfected brain. Nature. 1985;315:331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- 14.Oesch B, Westaway D, Walchli M, et al. A cellular gene encodes scrapie PrP 27–30 protein. Cell. 1985;40:735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci, USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collinge J, Sidle KCL, Meads J, et al. Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 18.Hill AF, Desbruslais M, Joiner S, et al. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 19.Thuring CMA, Erkins HF, Jacobs JG, et al. Discrimination between scrapie and bovine spongiform encephalopathy in sheep by molecular size, immunoreactivity and glycoprofile of prion protein. J Clin Microbiol. 2004;42:972–980. doi: 10.1128/JCM.42.3.972-980.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaller O, Fatzer R, Stack MJ, et al. Validation of a Western immunoblotting procedure for bovine PrPSc detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE) Acta Neuropathol (Berl) 1999;98:437–443. doi: 10.1007/s004010051106. [DOI] [PubMed] [Google Scholar]
- 21.Moynagh J, Schimmel H. Tests for BSE evaluated. Nature. 1999;400:105. doi: 10.1038/21981. [DOI] [PubMed] [Google Scholar]
- 22.Korth C, Stierli B, Streit P, et al. Prion (PrPSc) — specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 23.Harmeyer S, Pfaff E, Groschup MH. Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J Gen Virol. 1998;79:937–945. doi: 10.1099/0022-1317-79-4-937. [DOI] [PubMed] [Google Scholar]
- 24.Race RE, Raines A, Baron TGM, Miller MW, Jenny A, Williams ES. Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent-infected deer, elk sheep and cattle. J Virol. 2002;76:12365–12368. doi: 10.1128/JVI.76.23.12365-12368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raymond GJ, Bossers A, Raymond LD, et al. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 2000;19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech Off Int Epiz. 2002;21:305–316. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- 27.Safar J, Wille H, Itri V, et al. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 28.Wilesmith JW, Ryan JBM, Atkinson MJ. Bovine spongiform encephalopathy: Epidemiological studies on the origin. Vet Rec. 1991;128:199–203. doi: 10.1136/vr.128.9.199. [DOI] [PubMed] [Google Scholar]
- 29.Coulthart MB, Mogk R, Rancourt JM, Godal DL, Czub S. Prion protein gene sequence of Canada’s first non-imported case of bovine spongiform encephalopathy (BSE) Genome. 2003;46:1005–1009. doi: 10.1139/g03-124. [DOI] [PubMed] [Google Scholar]