Abstract
Oncolytic viruses (OV) represent a promising strategy to augment the spectrum of cancer therapeutics. For efficacy, they rely on two general mechanisms: tumor-specific infection/cell-killing, followed by subsequent activation of the host’s adaptive immune response. Numerous OV genera have been utilized in clinical trials, ultimately culminating in the 2015 Food and Drug Administration approval of a genetically engineered herpes virus, Talminogene laherparepvec (T-VEC). It is generally accepted that OV as monotherapy have only modest clinical efficacy. However, due to their ability to elicit specific antitumor immune responses, they are prime candidates to be paired with other immune-modulating strategies in order to optimize therapeutic efficacy. Synergistic strategies to enhance the efficacy of OV include augmenting the host antitumor response through the insertion of therapeutic transgenes such as GM-CSF, utilization of the prime-boost strategy, and combining OV with immune-modulatory drugs such as cyclophosphamide, sunitinib, and immune checkpoint inhibitors. This review provides an overview of these immune-based strategies to improve the clinical efficacy of oncolytic virotherapy.
Keywords: oncolytic virus, immune therapy, GM-CSF, prime boost, cyclophosphamide, immune checkpoint, sunitinib
Introduction
Despite the introduction of molecular interrogation and personalized medicine strategies for both the diagnosis and treatment of cancer over the past decade, the burden of this disease is still large. In 2016, an estimated 600,000 individuals died from cancer in the USA alone (1). Thus, while there is more efficacy in cancer treatment than ever before, there is still a significant potential for improvement.
Until recently, the myriad of genetic and epigenetic alterations that exist among cancer cells provided a seemingly insurmountable therapeutic challenge. How could one specific drug target all the machinery that the cancer cell uses to grow? Additionally, tumor heterogeneity and resistance mechanisms allow growth of cancer cells under the selective pressures of both the tumor microenvironment and attempted treatments (2). Thus, the answer to these treatment barriers may be in the ability to harness the potential of an equally diverse entity—the human immune system. One unique class of cancer therapeutics that utilizes the immune system is oncolytic viruses (OV).
The recognition that viral infection could play a role in the treatment of cancer first came to light over one hundred years ago (3). Only recently, though, has there been an increasing interest in the field, culminating in the US Food and Drug Administration (FDA) approval of a modified herpes simplex virus (HSV) for use in metastatic melanoma (4). There are numerous other clinical trials of OV currently ongoing (Table 1).
Table 1.
Selected ongoing clinical trials using oncolytic viruses.
| Virus | Name | Mods/effect | Tumor | Phase | Route | Combination | Trial ID |
|---|---|---|---|---|---|---|---|
| Adenovirus | DNX-2401 | Enhance viral tumor entry: Δ24-RGD insertion | Glioma, gliosarcoma | I | IT | IFN-γ | NCT02197169 |
| II | IT | Pembrolizumab | NCT02798406 | ||||
| Glioma | I | IT | Temozolomide | NCT01956734 | |||
| VCN-01 | Enhance intratumoral distribution: PH20 hyaluronidase insertion | Pancreas | I | IT | Gemcitabine + Abraxane | NCT02045589 | |
| Solid tumors | I | IV | Gemcitabine + Abraxane | NCT02045602 | |||
| Colo-Ad1 | Increase tumor specificity: Chimeric Ad11/3 group B | Ovarian | I/II | IP | – | NCT02028117 | |
| Solid tumors | I | IV | Nivolumab | NCT02636036 | |||
| I/II | IV | – | NCT02028442 | ||||
| AdV-tk | Increased tumor sensitivity to drug: TK insertion | MPE | I | IPl | – | NCT01997190 | |
| Pediatric (brain) | I | IT | RT + Valcyclovir | NCT00634231 | |||
| Pancreas | I/II | IT | Gemcitabine + RT + mFOLFIRINOX | NCT02446093 | |||
| Prostate | II/III | IT | Valcyclovir | NCT02768363 | |||
| III | IT | RT + Valcyclovir | NCT01436968 | ||||
| Oncos-102 | Enhance viral tumor entry and immune activation: Δ24-RGD-GM-CSF insertion | Melanoma | I | IT | CPA + Pembrolizumab | NCT03003676 | |
| Mesothelioma | II | IPl | Carboplatin/Paclitaxel + CPA | NCT02879669 | |||
| Solid tumors | I | IP | Durvalumab | NCT02963831 | |||
| CG0070 | Immune activation: GM-CSF insertion and E3 deletion | Bladder | III | Intravesicular | – | NCT02365818 | |
| Coxsackie | CVA21 | None | Lung (NSLC) | I | IV | Pembrolizumab | NCT02824965 |
| Melanoma | I | IT | Ipilimumab | NCT02307149 | |||
| IT | Pembrolizumab | NCT02043665 | |||||
| Solid tumors | I | IV | Pembrolizumab | NCT02043665 | |||
| Herpes simplex | Talminogene laherparepvec | Decreased virulence and prolong viral replication: ICP34.5 deletion, US11 deletion, GM-CSF insertion | Breast | I/II | IT | Paclitaxel | NCT02779855 |
| II | IT | – | NCT02658812 | ||||
| H/N | I | IT | Pembrolizumab | NCT02626000 | |||
| HCC, Liver Mets | I | IT | – | NCT02509507 | |||
| Lymphoma | II | IT | Nivolumab | NCT02978625 | |||
| Melanoma | I/II | IT | Ipilimumab | NCT01740297 | |||
| II | IT | RT | NCT02819843 | ||||
| – | NCT02366195 | ||||||
| – | NCT02211131 | ||||||
| Pembrolizumab | NCT02965716 | ||||||
| III | IT | – | NCT02297529 | ||||
| Pembrolizumab | NCT02263508 | ||||||
| Pediatric | I | IT | – | NCT02756845 | |||
| Sarcoma | I/II | IT | RT | NCT02453191 | |||
| II | IT | RT | NCT02923778 | ||||
| HF-10 | Decreased virulence: UL56 deletion, single partial UL52 | Melanoma | II | IT | Ipilimumab | NCT02272855 | |
| Solid tumors | I | IT | – | NCT02428036 | |||
| HSV1716 | Decreased virulence: ICP34.5 deletion | Mesothelioma | I/II | IPl | – | NCT01721018 | |
| Pediatric | I | IT/IV | – | NCT00931931 | |||
| G207 | Decreased virulence: ICP34.5 deletion, UL39 disruption | Pediatric (brain) | I | IT | RT | NCT02457845 | |
| Maraba | MG1 | Tumor antigen to enhance antitumor immune activity: MAGE-A3 | Lung (NSCLC) | I/II | IM | AdMA3 Vaccine + Pembrolizumab | NCT02879760 |
| Solid tumors | I/II | IM | AdMA3 vaccine | NCT02285816 | |||
| Reovirus | Reolysin | None | Bladder | I | IT | Gemcitabine + Cisplatin | NCT02723838 |
| Breast | II | IV | Paclitaxel | NCT01656538 | |||
| Colorectal | I | IV | FOLFIRI + Bevacizumab | NCT01274624 | |||
| II | IV | FOLFOX + Bevacizumab | NCT01622543 | ||||
| Myeloma | I | IV | Bortezomib + Dexamethasone | NCT02514382 | |||
| Lenalidomide or Pomalidomide | NCT03015922 | ||||||
| Pancreas | I | IV | Pembrolizumab + Chemo | NCT02620423 | |||
| II | IV | Carboplatin + Paclitaxel | NCT01280058 | ||||
| Pediatric (brain) | I | IV | GM-CSF | NCT02444546 | |||
| Solid tumors | II | IV | Paclitaxel | NCT01199263 | |||
| Vaccinia | GL-ONC1 | Increased tumor sensitivity to drug and reduced virulence: TK disruption, hemagglutinin disruption, F14.5L disruption | MPE | I | IPl | – | NCT01766739 |
| Ovarian | I | IP | – | NCT02759588 | |||
| Solid tumors | I | IV | Eculizumab | NCT02714374 | |||
| JX-594 | Immune activation and increased tumor sensitivity to drug: GM-CSF insertion, TK disruption | Breast, sarcoma | I/II | IV | CPA | NCT02630368 | |
| HCC | III | IT | Sorafenib | NCT02562755 | |||
| Solid tumors | I | IT | Ipilimumab | NCT02977156 | |||
| PROSTVAC | Tumor antigen to enhance antitumor immune activity: PSA, LFA-3, ICAM-1, B7.1 additions | Prostate | I/II | SC | Nivolumab and/or Ipilimumab | NCT02933255 | |
| II | SC | – | NCT02326805 | ||||
| – | NCT02649439 | ||||||
| – | NCT02772562 | ||||||
| Ipilimumab | NCT02506114 | ||||||
| Docetaxel + Prednisone | NCT01145508 | ||||||
| Docetaxel | NCT02649855 | ||||||
| Flutamide | NCT00450463 | ||||||
| – | NCT02153918 | ||||||
| Enzalutamide | NCT01867333 | ||||||
| NCT01875250 | |||||||
| III | SC | GM-CSF | NCT01322490 | ||||
| Vesicular stomatitis | VSV-IFNβ-NIS | Increased tumor specificity and enhanced sensitivity to radiotherapy: IFN-β + NIS | Hematologic malignancy | I | IV | – | NCT03017820 |
| Solid tumors | I | IV | – | NCT02923466 |
CPA, cyclophosphamide; IM, intramuscular; IP, intraperitoneal; IPl, intrapleural; IT, intratumoral; IV, intravenous; MPE, malignant pleural effusion; SC, subcutaneous; RT, radiotherapy.
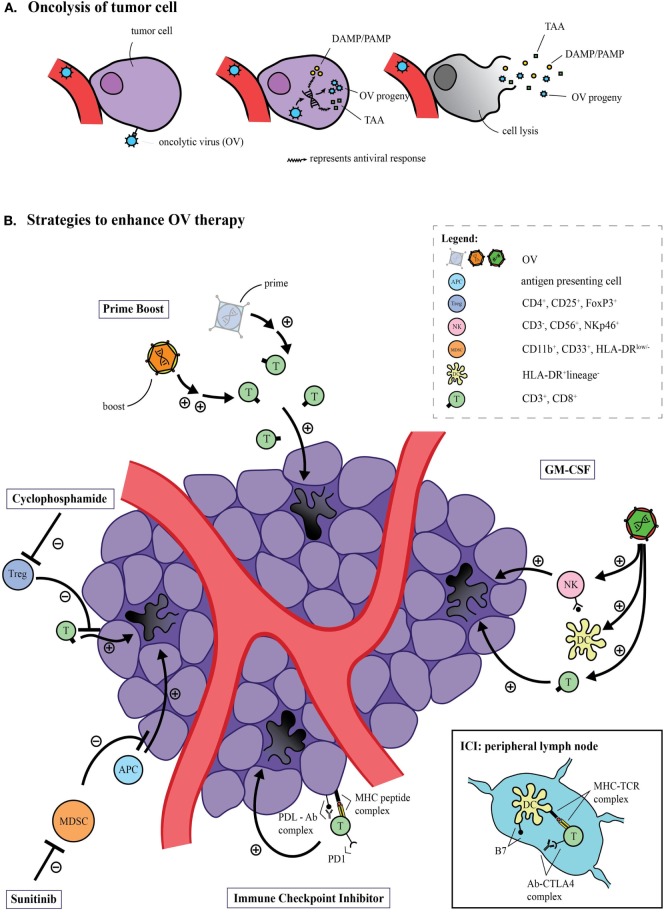
OV therapy is based on the finding that certain viruses selectively replicate within cancer cells. Initially, OV therapy was thought to exert its primary anticancer effect through direct tumor oncolysis (apoptosis/autophagy). However, almost 20 years ago, findings by Mastrangelo and colleagues (5) demonstrated that, in fact, another mechanism may be at play with oncolytic virotherapy. Not only did primary tumors decrease in size when injected with an oncolytic vaccinia virus (VV), but non-injected tumors did as well (5). Their findings suggested that OV have the potential to induce systemic antitumor immunity. It is now commonly accepted that exposure of tumor neoantigens after OV-induced oncolysis (Figure 1A) can activate both the innate and adaptive arms of the host immune system and direct them specifically toward areas of tumor burden. It is currently unclear to what extent each of these mechanisms contributes to the overall success of clinical efficacy in an individual OV.
Figure 1.
Oncolytic virus (OV)-mediated tumor cell lysis. (A) OV can specifically infect cancer cells, and subsequent replication can induce oncolysis. The release of tumor antigens has the potential to activate a systemic antitumor immune response. (B) The immune response induced by OV can be improved through several strategies. The prime-boost approach utilizes one priming viral platform carrying tumor-specific antigens, while a second platform—usually an OV—carrying the same antigens boosts the resultant antitumor immune response. The insertion of transgenes, such as GM-CSF, can facilitate antigen presentation on the surface of dendritic cells, and thus augment an antitumor response by recruiting natural killer (NK) cells and inducing tumor-specific cytotoxic T-cells. Immune checkpoint inhibitors can function both at the level of the tumor, targeting the programmed cell death protein 1 (PD-1) axis or peripherally at the level of the lymph nodes by targeting the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) axis. Both approaches ultimately improve the antitumor response. Immunomodulatory drugs such as sunitinib and cyclophosphamide can augment the antitumor immune response of OV by inhibiting immunosuppressive populations, such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), respectively.
Interestingly, there has been only modest success in the introduction of OV to the clinical arena as monotherapies (6, 7). The explanation for these modest results is likely multifactorial, including host antiviral mechanisms limiting effective viral dissemination, development of tumor resistance to key oncogenic signaling pathways typically exploited by OV, and a host of immunosuppressive regulatory factors within the tumor microenvironment. Current clinical approaches utilizing OV seek to enhance their efficacy with complimentary immunotherapeutic strategies (Figure 1B).
As the field of OV is in the midst of renewed excitement and optimism, we seek herein to provide an overview of the most frequently utilized immune-based strategies to improve the clinical efficacy of oncolytic virotherapy and review the available evidence for doing so.
Manipulating OV for Clinical Benefit
The Hallmark Transgene: GM-CSF
Early in the process of bringing OV into the clinical setting, it was realized that certain viral candidates could be genetically modified to reduce virulence and/or be armed with therapeutic transgenes to augment oncolytic activity with local gene delivery. Transgenes to enhance therapeutic benefit of OV are quite varied and include inflammatory cytokines, proteases that degrade the tumor microenvironment, antiangiogenic proteins, prodrug-converting enzymes, and proapoptotic genes (8). In general, the trend in the OV field is to enhance candidate viruses in such a way that their ability to induce antitumor immunity is optimized. No transgene has been utilized as frequently or with as much success as GM-CSF.
Ever since the antitumor effects of GM-CSF were first appreciated by Dranoff and colleagues (9), it has held particular interest as a therapeutic adjuvant in immune-based cancer treatments. Based on its effects in cytokine-transduced cancer cell vaccines such as Sipuleucel-T for prostate cancer, it has become an attractive OV therapeutic transgene. By promoting monocyte-to-dendritic cell (DC) differentiation, GM-CSF facilitates antigen presentation on the surface of DCs following viral-induced oncolysis (10). This ultimately leads to a more robust antitumor immune response by recruiting natural killer (NK) cells and inducing tumor-specific cytotoxic T-cells (11).
To date, GM-CSF has been used with success in OV platforms such as HSV (4, 12), VV (13, 14), and adenovirus (AdV) (15, 16). Of these, HSV and VV have arguably served as the most efficacious platforms. A phase III randomized clinical trial comparing HSV-1 with a GM-CSF Transgene Talminogene laherparepvec (T-VEC) vs. GM-CSF alone in advanced melanoma led to the first FDA approval of an OV. Of 436 patients randomized, 295 were in the T-VEC group and 141 in the GM-CSF arm. The objective response rate (ORR) was 26.4% for T-VEC, including 10.8% with a complete clinical response, vs. 5.7% for GM-CSF alone. Despite not quite reaching statistical significance, those in the T-VEC arm achieved an overall survival of 23.3 vs. 18.9 months in the GM-CSF group, thus demonstrating a meaningful trend toward improved survival (4).
The utility and efficacy of T-VEC are currently being explored across a variety of cancer types with phase II clinical trials open in breast (NCT02658812), lymphoma (NCT02978625), and sarcoma (NCT02923778). Additionally, another randomized phase III trial in melanoma is open exploring the value of adding T-VEC to the programmed cell death protein 1 (PD-1) inhibitor, pembrolizumab, for treatment of unresected melanoma (NCT02263508).
Furthermore, an oncolytic VV has been programmed with a GM-CSF insertion (JX-594) and has been the subject of much clinical investigation. Early-phase I/II trials have been completed with JX-594 in colorectal cancer (17), melanoma (18), pediatric malignancy (19), and non-specific solid tumors (14). The greatest clinical promise, however, has been seen with JX-594 in hepatocellular carcinoma (HCC). A phase II dose-finding trial demonstrated significant survival benefit with high doses (14.1 months) compared to low doses (6.7 months) of JX-594 (20). Furthermore, it was found that objective tumor responses were present in both injected- and non-injected tumors, indicating a possible element of systemic antitumor immunity. Studies of this OV in a preclinical setting have demonstrated that tumor oncolysis is mediated by antibodies in a complement-dependent nature (21), likely related to its ability to increase the release of specific tumor neoantigens/epitopes to the systemic circulation. Further exploration of its efficacy in HCC is currently ongoing, with a phase III trial open for recruitment (NCT02562755) with or without with the VEGFR tyrosine kinase inhibitor Sorafenib.
It is important to consider that despite the clinical promise of OV expressing a GM-CSF transgene, the underlying mechanisms mediating antitumor activity are both poorly understood and subject to controversy. There are little data surrounding the specific mechanistic contributions of GM-CSF to the success of the OV previously mentioned. Moreover, despite the recognition that GM-CSF has a certain level of antitumor potency, it is also intricately linked to the modulation (increase) of immunosuppressive myeloid-derived suppressor cells (MDSCs) (22). Specifically, not only has GM-CSF been shown to increase MDSC numbers in transplantable tumor models (23) but it has also been implicated as the main factor driving MDSC generation in these models (24). Thus, further study is needed to determine the best use of GM-CSF with OV in order to maximize its antitumor effects, while minimizing its recruitment and proliferation of immunosuppressive MDSCs.
“Boosting” OV Efficacy: The Prime-Boost Strategy
Based on the success of traditional vaccinations to combat virally induced disease, vaccinating patients with tumor antigens has been a therapeutic approach of interest in cancer, although has only demonstrated modest success to date. Eliciting a successful systemic immune response against tumor antigens requires the breaking of tolerance that typically prevents host antitumor immunity. One answer may be to utilize viral delivery platforms. One problem with this approach lies in that the use of viral vectors may induce a competitive immune response against the viral antigens, rather than the tumor antigens of interest (25). A solution is to utilize the emerging heterologous “prime-boost” approach. For example, tumor-specific antigens can be encoded into the backbone of one viral platform to prime the immune system before being introduced to a second viral platform carrying the same antigens that upregulates, or boosts, the resultant antitumor immune response.
Classic viral vaccine vectors are non-replicating and therefore do not have oncolytic properties. However, the prime-boost strategy with non-OV has still seen demonstrable clinical applicability. PROSTVAC, which is utilized in prostate cancer, is the prototypical example. Despite not utilizing an OV platform, ongoing clinical trials of PROSTVAC are highlighted in Table 1, as success of this platform to date demonstrates the power of the prime-boost strategy in viral-based cancer vaccination.
There are two members of the Rhabdoviridae family that have been investigated for use as OV, both belonging to the Vesiculovirus genus—vesicular stomatitis virus (VSV) and Maraba virus. These enveloped ssRNA viruses were first noted to have oncolytic potential in 2000 when VSV was demonstrated to induce tumor regression in a mouse xenograft model of melanoma (26). VSV is a promising oncolytic agent due to its reasonable safety profile and lack of preexisting neutralizing antibodies in humans—problems that have been encountered with other OV platforms. It has been demonstrated that VSV can be utilized effectively as a cancer vaccine, with increased capacity as part of a heterologous prime-boost strategy (27, 28). In a murine model of melanoma, VSV vaccine not only induced upregulation of tumor-specific immunity but also decreased adaptive antiviral immunity leading to an increase in the overall survival of treated animals (27). Following the early preclinical success of VSV, other mammalian cell-trophic rhabdovirus family members were screened for oncolytic capacity (29). From this study, Maraba virus was identified as having the broadest oncotropism, which could be further enhanced with the induction of two-point mutations (L123W in M and Q242R in G). In a direct comparison to a similarly mutated VSV in a murine model of metastatic colorectal cancer, this Maraba virus (MG1) induced total tumor clearance in 100% of treated animals, as compared to 30% in VSV (29). Later studies specifically investigating a Maraba MG1 expressing a melanoma antigen demonstrated its inability to prime an adaptive immune response but significant capacity as a boosting vector. In a syngeneic murine model of melanoma, utilizing Maraba MG1 had dramatic effects leading to significantly extended median survival and complete remission of 20% of animals treated (30). Preclinical promise has allowed Maraba MG1 to move into early-phase clinical trials, with two currently ongoing (NCT02879760, NCT02285816). Both trials utilize a non-replicating AdV vector for priming with MG1 as the boost. Results are not yet available.
Synergistic Strategies with OV and Immune-Modulatory Drugs
Cyclophosphamide (CPA)
Cyclophosphamide is a commonly used anticancer agent that non-specifically causes DNA alkylation and induces apoptotic cell death. Additionally, CPA can modulate the immune system through its ability to kill proliferating NK cells, T cells, and B cells with relatively low clinical doses (31). Thus, CPA has been investigated for a synergistic effect along with OV and has demonstrated improved tumor destruction in preclinical models of reovirus (RV) (32, 33), VV (34), measles (35), and AdV (36). Specifically, in a murine model of melanoma, preconditioning with CPA led to an increased intratumoral viral level of oncolytic RV and led to enhanced antitumor efficacy (32). Additionally, one study demonstrated that CPA treatment in conjunction with OV therapy leads to control of the host antiviral response, a problem that can dampen effective OV proliferation, especially in viral platforms that are ubiquitous in humans (37). Furthermore, CPA can potentiate OV replication by suppressing local innate immune cells (38) and depleting regulatory T cells (Tregs), thus enhancing antitumor activity of cytotoxic T-cells (11). Recently, a number of early-phase clinical trials investigating OV synergy with CPA have been completed in oncolytic AdV (solid tumors) (15), oncolytic RV (pediatric tumors, solid tumors) (39, 40), and oncolytic Seneca Valley Virus (neuroendocrine tumors) (41). These trials, however, did not examine the role of CPA specifically in advancing the efficacy of the OV platforms. Furthermore, two current early-phase clinical trials utilizing CPA and an AdV platform are being conducted (NCT00634231, NCT02879669) as well as one trial utilizing CPA and an oncolytic VV (NCT02630368). The general landscape of cancer immune therapies, however, is gravitating toward more tumor-specific therapies. As such, other immune-modulatory agents are being explored, and CPA’s role as a synergistic treatment strategy to compliment OV therapy is diminishing.
Immune Checkpoint Inhibitors (ICIs)
Immune checkpoint inhibitors function as immune suppression antagonists. Normally crucial for the maintenance of self-tolerance, immune checkpoint proteins can be overexpressed by tumors as a way to evade detection by the host immune system (42). The first immune checkpoint to be targeted for therapeutic benefit was cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), but superior clinical outcomes, broader clinical applications, and more favorable safety profiles have led PD-1 and its cognate ligand (PD-L1) inhibition to be the new vogue. Importantly, PD-1/PD-L1 inhibition can be combined with CTLA-4 antagonists. PD-L1 expression specifically is induced on activated T cells following a stimulatory signal from IFN-γ (43). CTLA-4 acts at the level of the draining lymph node for T cell priming. Conversely, the PD-1/PD-L1 pathway only inhibits activated T cells, which attenuates the potential for loss of self-tolerance. Since many tumors overexpress PD-L1 (44), they can escape recognition by tumor-infiltrating lymphocytes. Inhibiting this pathway effectively “removes the brakes” on the normal immune response. The impressive success of PD-1/PD-L1 inhibition as monotherapy in phase III clinical trials of melanoma (45), non-small cell lung cancer (46), renal cell carcinoma (RCC) (47), and urothelial carcinoma (48) has led to FDA approval for clinical use. One crucial problem with ICI is that despite their profound efficacy in responding patients, the majority of patients are non-responders (49, 50). This can possibly be explained by the lack of active tumor-specific T cells in the tumor microenvironment. As OV therapy can induce antitumor adaptive immunity, it seems as though ICI and OV could be a perfect therapeutic match.
Preclinical success marrying ICI with OV therapy has been encouraging. Specifically, a study conducted by Zamarin and colleagues (51) demonstrated the potential for combining CTLA-4 inhibition with an oncolytic Newcastle disease virus in a murine model of melanoma. They found that OV therapy alone triggered a systemic antitumor immune response, but accumulated T cells overexpressed CTLA-4, leading to an immunosuppressive tumor microenvironment and diminished treatment efficacy. Adding in CTLA-4 inhibition, however, improved the antitumor response, leading to increased long-term survival of dually treated animals. This response was dependent on NK cells, CD8+ T cells, and type I interferon (51). Although still ongoing, one clinical trial (NCT01740297) utilizing T-VEC and CTLA-4 blockade has promising interim results; ORR has been found in 41% of treated patients and complete responses in 24%. Given that T-VEC monotherapy has a reported ORR of 26% and a complete response rate of 10.8% (4), the combination therapy with CTLA-4 blockade seems to be an improvement. Additionally, a preclinical study in a murine model of melanoma utilizing an oncolytic RV in combination with PD-1 inhibition demonstrated promising results (52). This group found that combination treatment significantly enhances survival compared to either monotherapy. The enhanced survival was tied to increased activity of NK cells, reduced Tregs, and increased CD8+ antitumor responses (52). Between PD-1 inhibitors nivolumab and pembrolizumab, PD-Ll inhibitor durvalumab, and CTLA-4 inhibitor ipilimumab, there are currently 19 clinical trials ongoing that combine ICI and OV (Table 1). Results from these trials are eagerly anticipated in order to assess the value of combining these two immune-based treatment modalities.
Sunitinib
Sunitinib is a multi-tyrosine kinase inhibitor (VEGFR, PDGFR, c-kit, flt3, RET, CSF-1R) that has FDA approval for use in RCC and gastrointestinal stromal tumors. Its primary antitumor effect is through inhibition of VEGFR, leading to a reduced capacity for tumor angiogenesis (53). It is also now understood that sunitinib also has a role in indirectly inhibiting tumor growth through the promotion of antitumor immune responses (54–56). For example, immunosuppressive immune cell populations such as Tregs and MDSC are decreased with sunitinib treatment (54, 55). Its role as an immunotherapeutic adjuvant makes it a suitable candidate for combination with OV. Interestingly, it has been demonstrated that sunitinib can lead to the enhancement of viral replication through targeting innate immune pathways of viral resistance such as double-stranded RNA protein Kinase R (PKR) and RNase (57). The timing of sunitinib administration seems to be of importance, as administering it prior to and during oncolytic RV therapy allowed for the preconditioning of the tumor microenvironment to facilitate a maximal OV-induced antitumor response (58). Although no clinical trials have been initiated utilizing sunitinib and OV, one preclinical study seems to suggest potential for this combination in the treatment of RCC. Sunitinib and an oncolytic RV were found to significantly decrease tumor burden and significantly increase lifespan in a murine model of RCC (59). This therapeutic effect could be explained by their finding that this treatment combination increased the presence of tumor-specific CD8+ T cells and decreased accumulation of both MDSCs and Tregs. Additionally, dually treated mice had protective immunity upon tumor rechallenge. In the same study, Lawson and colleagues (59) also demonstrated similar results in a murine model of squamous cell lung carcinoma, thus highlighting the possible broad application of this treatment strategy. Furthermore, sunitinib combination with an oncolytic VSV led to the elimination of prostate, breast, and kidney malignant tumors in mice (60). Additionally, the antiangiogenic effects of sunitinib can be augmented by the utilization of an oncolytic VV, leading to reduction of tumor growth in murine models of cancer (61). Hopefully, the preclinical success of sunitinib and OV will be replicated in clinical trials once they are initiated.
Other Strategies to Enhance OV
Although the focus of this review has been necessarily limited to a handful of combinatorial immunotherapeutic strategies to enhance OV therapy, there are a number of other exciting approaches under preclinical investigation. For example, the combination of adoptive T cell therapy with OV has shown preclinical promise and efforts are underway to bring this strategy to clinical investigation (62, 63). Additionally, a number of different OV platforms are being utilized in combination with inhibitors of histone deacetylases (HDACIs) [reviewed in Ref. (64)]. Although the mechanisms underpinning their tumor tropism are not fully understood (65), HDACIs led to immunogenic cell death of cancer cells thus potentially enhancing antitumor immune responses in synergy with OV (66, 67).
Finally, a transgene-modified oncolytic AdV, NG-348 (PsiOxus Therapeutics), has been recently designed in hopes that it will drive T-cell immune responses within the tumor microenvironment independent of tumor-specific antigens. When two transgenes, a membrane anchored full-length human CD80 and a membrane anchored antibody fragment for the T-cell receptor, are expressed together on the surface of NG-348-infected tumor cells they provide both the T-cell receptor and costimulatory signal required to activate tumor-infiltrating T-cells (68). This strategy mimics that of CAR-T therapies but does not require autologous cell processing or tumor-specific antigens. Furthermore, since the expression of the encoded transgenes is encoded by the endogenous viral major late promoter, their expression is limited to the surface of cells permissive to viral infection—i.e. tumor cells. It is hoped that preclinical testing of NG-348 will ultimately support clinical application.
Concluding Remarks
Oncolytic viruses represent a promising immunotherapeutic approach to the treatment of cancer. Although clinical trials have demonstrated that their use as a monotherapy is likely insufficient for meaningful efficacy in the clinical arena, it has become clear that the ability for OV to induce a systemic antitumor immune response is intricately linked to their potential for success. Therefore, combining OV with other immunotherapies seems to represent the approach with the most promise. As numerous clinical trials are underway across multiple OV platforms utilizing different immunotherapies for treatment synergy, time will ultimately unveil the potential for OV as a future standard treatment option for our patients with cancer.
Author Contributions
DM is the primary author of this manuscript. AW designed and produced the included figure. All authors assisted in the conception of this review, acquisition of relevant literature, and editing the manuscript. All authors gave approval of the final version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The authors received no funding to assist with the preparation of this manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin (2016) 66:7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis (2003) 20:237–50. 10.1023/A:1022939318102 [DOI] [PubMed] [Google Scholar]
- 3.Dock G. The influence of complicating diseases upon leukaemia. Am J Med Sci (1904) 127:563–92. 10.1097/00000441-190412740-00001 [DOI] [Google Scholar]
- 4.Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol (2015) 33:2780–8. 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 5.Mastrangelo MJ, Maguire HC, Eisenlohr LC, Laughlin CE, Monken CE, McCue PA, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther (1999) 6:409–22. 10.1038/sj.cgt.7700066 [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Kratzke RA. Oncolytic virus therapy for cancer: the first wave of translational clinical trials. Transl Res (2013) 161:355–64. 10.1016/j.trsl.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Pol J, Buqué A, Aranda F, Bloy N, Cremer I, Eggermont A, et al. Trial watch-oncolytic viruses and cancer therapy. Oncoimmunology (2016) 5:e1117740. 10.1080/2162402X.2015.1117740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampath P, Thorne SH. Arming viruses in multi-mechanistic oncolytic viral therapy: current research and future developments, with emphasis on poxviruses. Oncolytic Virother (2014) 3:1–9. 10.2147/OV.S36703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A (1993) 90:3539–43. 10.1073/pnas.90.8.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer (2014) 14:559–67. 10.1038/nrc3770 [DOI] [PubMed] [Google Scholar]
- 11.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer (2013) 12(1):103. 10.1186/1476-4598-12-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu BL, Robinson M, Han Z-Q, Branston RH, English C, Reay P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther (2003) 10:292–303. 10.1038/sj.gt.3301885 [DOI] [PubMed] [Google Scholar]
- 13.Park B-H, Hwang T, Liu T-C, Sze DY, Kim J-S, Kwon H-C, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol (2008) 9:533–42. 10.1016/S1470-2045(08)70107-4 [DOI] [PubMed] [Google Scholar]
- 14.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQM, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature (2011) 477:99–102. 10.1038/nature10358 [DOI] [PubMed] [Google Scholar]
- 15.Ranki T, Pesonen S, Hemminki A, Partanen K, Kairemo K, Alanko T, et al. Phase I study with ONCOS-102 for the treatment of solid tumors – an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer (2016) 4:17. 10.1186/s40425-016-0121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res (2010) 70:4297–309. 10.1158/0008-5472.CAN-09-3567 [DOI] [PubMed] [Google Scholar]
- 17.Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, et al. Phase 1b trial of biweekly intravenous Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther (2015) 23:1532–40. 10.1038/mt.2015.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang T-H, Moon A, Burke J, Ribas A, Stephenson J, Breitbach CJ, et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther (2011) 19:1913–22. 10.1038/mt.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cripe TP, Ngo MC, Geller JI, Louis CU, Currier MA, Racadio JM, et al. Phase 1 study of intratumoral Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus, in pediatric cancer patients. Mol Ther (2015) 23:602–8. 10.1038/mt.2014.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med (2013) 19:329–36. 10.1038/nm.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MK, Breitbach CJ, Moon A, Heo J, Lee YK, Cho M, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med (2013) 5:185ra63. 10.1126/scitranslmed.3005361 [DOI] [PubMed] [Google Scholar]
- 22.Fernández A, Oliver L, Alvarez R, Fernández LE, Lee KP, Mesa C. Adjuvants and myeloid-derived suppressor cells: enemies or allies in therapeutic cancer vaccination. Hum Vaccin Immunother (2014) 10:3251–60. 10.4161/hv.29847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res (2004) 64:6337–43. 10.1158/0008-5472.CAN-04-0757 [DOI] [PubMed] [Google Scholar]
- 24.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell (2012) 21:822–35. 10.1016/j.ccr.2012.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrop R, John J, Carroll MW. Recombinant viral vectors: cancer vaccines. Adv Drug Deliv Rev (2006) 58:931–47. 10.1016/j.addr.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 26.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med (2000) 6:821–5. 10.1038/77558 [DOI] [PubMed] [Google Scholar]
- 27.Bridle BW, Boudreau JE, Lichty BD, Brunellière J, Stephenson K, Koshy S, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol Ther (2009) 17:1814–21. 10.1038/mt.2009.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulido J, Kottke T, Thompson J, Galivo F, Wongthida P, Diaz RM, et al. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol (2012) 30:337–43. 10.1038/nbt.2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brun J, McManus D, Lefebvre C, Hu K, Falls T, Atkins H, et al. Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther (2010) 18:1440–9. 10.1038/mt.2010.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pol JG, Zhang L, Bridle BW, Stephenson KB, Rességuier J, Hanson S, et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther (2014) 22:420–9. 10.1038/mt.2013.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol (2011) 33:369–83. 10.1007/s00281-011-0245-0 [DOI] [PubMed] [Google Scholar]
- 32.Kottke T, Thompson J, Diaz RM, Pulido J, Willmon C, Coffey M, et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated Treg modulation and interleukin-2. Clin Cancer Res (2009) 15:561–9. 10.1158/1078-0432.CCR-08-1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res (2008) 14:259–69. 10.1158/1078-0432.CCR-07-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res (2009) 15:2777–88. 10.1158/1078-0432.CCR-08-2342 [DOI] [PubMed] [Google Scholar]
- 35.Ungerechts G, Frenzke ME, Yaiw K-C, Miest T, Johnston PB, Cattaneo R. Mantle cell lymphoma salvage regimen: synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Ther (2010) 17:1506–16. 10.1038/gt.2010.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther (2011) 19:1737–46. 10.1038/mt.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng K-W, Myers R, Greenslade A, Mader E, Greiner S, Federspiel MJ, et al. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther (2013) 20:255–61. 10.1038/gt.2012.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A (2006) 103:12873–8. 10.1073/pnas.0605496103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolb EA, Sampson V, Stabley D, Walter A, Sol-Church K, Cripe T, et al. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group phase I consortium report. Pediatr Blood Cancer (2015) 62:751–8. 10.1002/pbc.25464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roulstone V, Khan K, Pandha HS, Rudman S, Coffey M, Gill GM, et al. Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors. Clin Cancer Res (2015) 21:1305–12. 10.1158/1078-0432.CCR-14-1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudin CM, Poirier JT, Senzer NN, Stephenson J, Loesch D, Burroughs KD, et al. Phase I clinical study of Seneca Valley Virus (SVV-001), a replication-competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res (2011) 17:888–95. 10.1158/1078-0432.CCR-10-1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med (2002) 8:793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 44.Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res (2015) 75:2139–45. 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 46.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med (2015) 373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387:1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med (2013) 369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med (2014) 6:226ra32. 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther (2016) 24:166–74. 10.1038/mt.2015.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res (2003) 9:327–37. [PubMed] [Google Scholar]
- 54.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res (2009) 15:2148–57. 10.1158/1078-0432.CCR-08-1332 [DOI] [PubMed] [Google Scholar]
- 55.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res (2008) 14:6674–82. 10.1158/1078-0432.CCR-07-5212 [DOI] [PubMed] [Google Scholar]
- 56.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res (2009) 69:2514–22. 10.1158/0008-5472.CAN-08-4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jha BK, Polyakova I, Kessler P, Dong B, Dickerman B, Sen GC, et al. Inhibition of RNase L and RNA-dependent protein kinase (PKR) by sunitinib impairs antiviral innate immunity. J Biol Chem (2011) 286:26319–26. 10.1074/jbc.M111.253443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer (2012) 130:1948–59. 10.1002/ijc.26219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawson KA, Mostafa AA, Shi ZQ, Spurrell J, Chen W, Kawakami J, et al. Repurposing sunitinib with oncolytic reovirus as a novel immunotherapeutic strategy for renal cell carcinoma. Clin Cancer Res (2016) 22:5839–50. 10.1158/1078-0432.CCR-16-0143 [DOI] [PubMed] [Google Scholar]
- 60.Jha BK, Dong B, Nguyen CT, Polyakova I, Silverman RH. Suppression of antiviral innate immunity by sunitinib enhances oncolytic virotherapy. Mol Ther (2013) 21:1749–57. 10.1038/mt.2013.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou W, Chen H, Rojas J, Sampath P, Thorne SH. Oncolytic vaccinia virus demonstrates antiangiogenic effects mediated by targeting of VEGF. Int J Cancer (2014) 135:1238–46. 10.1002/ijc.28747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shim KG, Zaidi S, Thompson J, Kottke T, Evgin L, Rajani KR, et al. Inhibitory receptors induced by VSV viroimmunotherapy are not necessarily targets for improving treatment efficacy. Mol Ther (2017) 25:962–75. 10.1016/j.ymthe.2017.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rommelfanger DM, Wongthida P, Diaz RM, Kaluza KM, Thompson JM, Kottke TJ, et al. Systemic combination virotherapy for melanoma with tumor antigen-expressing vesicular stomatitis virus and adoptive T-cell transfer. Cancer Res (2012) 72:4753–64. 10.1158/0008-5472.CAN-12-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchini A, Scott EM, Rommelaere J. Overcoming barriers in oncolytic virotherapy with HDAC inhibitors and immune checkpoint blockade. Viruses (2016) 8:9. 10.3390/v8010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov (2014) 13:673–91. 10.1038/nrd4360 [DOI] [PubMed] [Google Scholar]
- 66.Christiansen AJ, West A, Banks K-M, Haynes NM, Teng MW, Smyth MJ, et al. Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proc Natl Acad Sci U S A (2011) 108:4141–6. 10.1073/pnas.1011037108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bridle BW, Chen L, Lemay CG, Diallo J-S, Pol J, Nguyen A, et al. HDAC inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol Ther (2013) 21:887–94. 10.1038/mt.2012.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Champion BR, Rasiah N, Illingworth S, Besneux M, Lear R, Plumb D, et al. Developing tumor-localized, combination immunotherapies. Cancer Res (2016) 76 10.1158/1538-7445.AM2016-4875 [DOI] [Google Scholar]