Abstract
Bovine familial convulsion and ataxia, a heritable disorder, is present in crossbred Aberdeen Angus cattle in western Canada. Though the prevalence appears to be low, this genetic defect might become widespread if not controlled. Characteristic histological lesions were seen in the lingula, uvula, or both, of all affected calves regardless of their age.
Abstract
Résumé — Syndrome de convulsions et ataxie familial bovin en Saskatchewan et en Alberta. Le syndrome des convulsions et l’ataxie familial bovin est une maladie héréditaire présente chez les bovins de race Aberdeen Angus croisée de l’Ouest du Canada. Bien que la prévalence semble faible, cette anomalie génétique pourrait devenir répandue si des mesures n’étaient pas prises. Des lesions histologiques caractéristiques ont été observées dans la lingula et/ou l’uvula chez tous les veaux atteints, indépendamment de leur âge.
(Traduit par Docteur André Blouin)
Bovine familial convulsion and ataxia (BFCA) is an autosomal dominant heritable disorder with incomplete penetrance of purebred and crossbred Aberdeen Angus cattle, first reported in Scotland in 1968 (1). The disease has been controlled by elimination of cattle that transmit the disorder; currently, BFCA in Scotland seems to be of historical importance (2). In 1996, a single case of BFCA in a purebred Angus calf was reported for the first time in the United States (3). A similar condition was also reported in a crossbred Poll Hereford in Australia (4) and in Charolais cattle in the USA (5).
Newborn and young calves are affected by recurrent episodes of spastic seizures and residual ataxia. If affected calves survive, the frequency of seizures decreases and seizures eventually cease after 12 mo of age. Remission of seizures may be followed by remission of ataxia and by clinical normality by 2 y of age (6,7). Definitive diagnosis requires a clinical history of the disorder and demonstration of the characteristic histological lesions of proximal axonal swelling of Purkinje cells in the granular layer of the cerebellar cortex. These lesions have been reported to be rare in convulsing calves younger than 6 wk (6,7); therefore, confirmation of clinical diagnosis can be difficult in young calves.
This report documents the presence of BFCA on 4 different western Canadian farms. Contrary to previous reports, characteristic histological lesions were seen in the lingula, uvula, or both, of all affected calves regardless of their age, and progressive severity of the lesions appeared to be associated with increased age.
Case description
Between March and June 2002, 6 crossbred Aberdeen Angus calves (4 d to 3 mo old) from a herd of 22 second-calf Aberdeen Angus heifers in Saskatchewan were affected by central nervous system (CNS) clinical signs and submitted for necropsy to Prairie Diagnostic Services in Saskatoon and Regina. The heifers had been derived from 3 different sources and all had been bred by the same 2-year-old bull (50% Limousin [♀Canada] and 50% Aberdeen Angus [♂United State]). All the heifers and the bull were healthy and without any clinical nervous signs. The heifers received vitamins A, D, and E, as well as blackleg vaccine. Subsequently, the bull was removed from the breeding stock and slaughtered during the summer of 2002. During 2003, no calves showed clinical signs of BFCA on this farm.
All 6 calves submitted to necropsy were in good body condition and had similar clinical nervous signs. At birth, these calves had marked stiffness of the limbs, were unable to stand, and had recurrent spastic seizures a few hours later. They were alert with a good suckling reflex but were unable to stand on their own during the first few days. Subsequently, they were able to stand if the front limbs, hind limbs, or all 4 limbs were spread widely (Figure 1A) and to walk with mild to severe hypermetria and ataxia of all 4 limbs. Mild intention tremor of the head was present.
Figure 1.
A) Wide-based stance of an affected calf. B) Seizure with opisthotonus and rigid extension of all 4 limbs of an affected calf.
The recurrent seizures were characterized by rigid extension of extremities and neck, with occasional opisthotonus (Figure 1B) and intention tremor. When excited or stressed, the calves appeared to be more susceptible to relapse into seizures. Duration of seizures varied from 15 min to 10 h and frequency varied from a few times a day to a few times a week. During seizures, the eyes were open with decreased blinking frequency, resulting in exposure to mechanical friction with bedding.
Hematologic and biochemical analyses did not show any significant changes that could explain the clinical signs. An elevated creatinine kinase (399 to 1180 U/L; reference range 64 to 344 U/L) in the plasma of 3 calves tested was considered to be secondary to prolonged recumbency during seizures.
None of the affected calves had gross lesions in the brain. Two out of 6 calves had corneal edema and ulceration, consistent with exposure keratitis. Histologically, most obvious changes were restricted to the outer aspect of the cerebellar granular layer. There were multiple segmental fusiform axonal swellings (torpedoes and spheroids) that were homogeneously eosinophilic and argyrophilic in tissue sections stained by hematoxylin and eosin and Holmes’ (8) techniques, respectively (Figure 2B). Occasional swollen axonal segments were clearly in the proximal part of the axons of Purkinje cells, which appeared to be either shrunken with basophilic cytoplasm or mildly swollen with pale or mildly vacuolated cytoplasm (Figure 2B).
Figure 2.
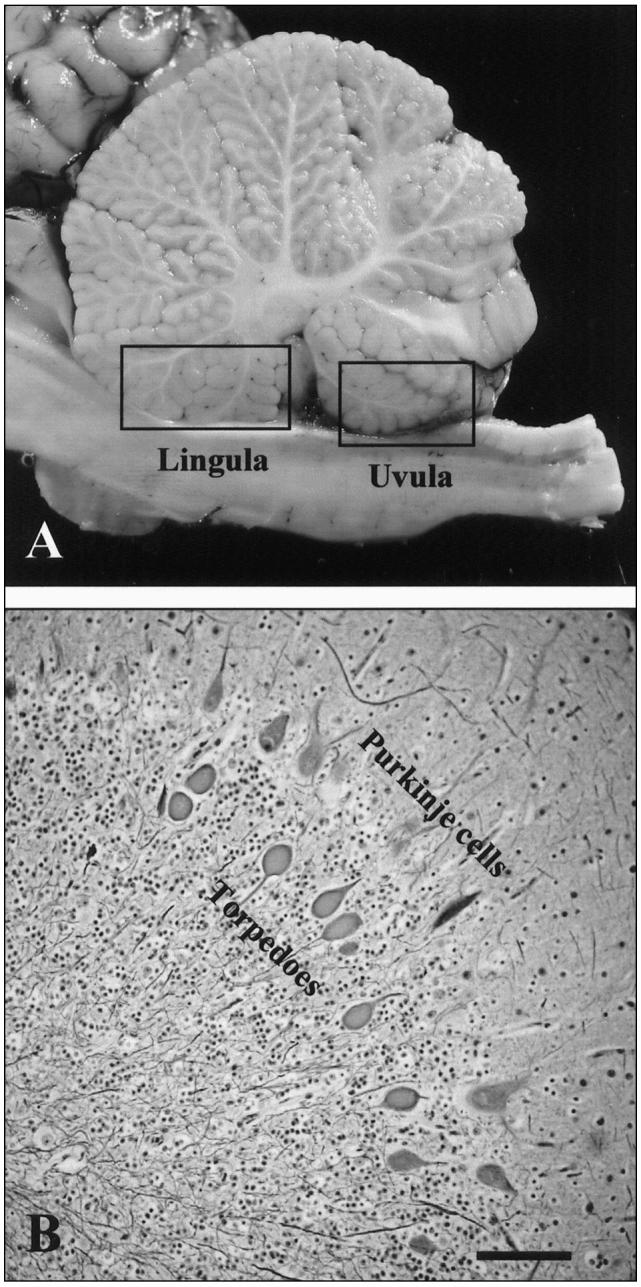
A) Regions of the lingula and uvula indicated on the medial cerebellar section. B) Swollen axons (torpedoes) in the outer aspect of the granular layer of the cerebellar cortex (Holmes’ stain). Bar = 100 μm.
Severity and distribution of the microscopic lesions appeared to be age related. Axonal swellings, a characteristic lesion of BFCA, were present in all calves, regardless of their age; however, in calves younger than 6 wk, the lesions were often restricted to the granular layer of the cerebellar lingula and uvula (Figure 2A). In older calves, the lesions were also present in the other regions of the vermis, as well as in the lateral cerebellar hemispheres.
Discussion
Since 1996, a total of 9 crossbred Aberdeen Angus calves (including the 6 described in this report) have been diagnosed with BFCA by the diagnostic laboratory at the Western College of Veterinary Medicine, University of Saskatchewan. These animals originated from 4 different farms (3 in Saskatchewan and 1 in Alberta). Based on clinical history, on 3 of the 4 farms, several other calves were affected by clinical signs similar to those of the 9 calves submitted for necropsy and diagnosed with BFCA.
The genetic relationship between the cattle on these various farms and the trading activities among farms were not investigated; therefore, it is not known if all or any of the affected animals shared a common ancestor.
Four of the 9 affected calves were younger than 6 wk and all 4 had characteristic axonal lesions of BFCA characterized by proximal axonal swelling of Purkinje cells in the granular layer of the cerebellar cortex of the lingula, uvula, or both (Figures 2A and 2B). These 4 calves were submitted from 3 different farms (2 in Saskatchewan and 1 in Alberta). In the remaining 5 calves (4 calves were 6- to 8-wk-old and 1 was 9-mo-old), similar axonal swellings were present, not only in the lingula and uvula but also in the other areas of the cerebellum; however, such axonal changes were always restricted to the outer aspect of the cerebellar granular layer, regardless of anatomical location in the cerebellum.
Based on clinical signs and history, cerebellar hypoplasia associated with bovine viral diarrhea virus (BVDV) infection was the condition most commonly suspected. Diagnostic ancillary testing failed to reveal antibodies to BVDV in sera from 3 affected calves that were tested to detect BVDV antigens by fluorescence antibody test or to isolate BVDV from the ileum, spleen, kidney, thymus, and liver, of the same 3 calves. Gross and microscopic changes in the CNS consistent with in utero BVDV infection were not observed. Storage disease of the CNS (alpha-mannosidosis) was also suspected, based on the clinical signs, but no microscopic lesions suggestive of this condition were present.
No follow-up for BFCA was performed on 3 out of 4 farms; however, lack of further submissions of similar cases from these farms suggests that this problem did not continue. On one farm, no further cases of BFCA occurred, after the suspected bull was culled.
The prevalence of BFCA appears to be low; however, the disease may be underdiagnosed and the prevalence may be higher than apparent from this study. Characteristic histologic lesions are often not very obvious in tissue sections stained with hematoxylin and eosin and, in calves younger than 6 wk, lesions are often restricted to small areas of the cerebellum, which might not be routinely sampled and examined.
Contrary to previous reports of rarity of histological lesions in convulsing calves younger than 6 wk (6,7), 4 out of 9 calves in this study were younger than 6 wk and all of them had characteristic histological lesions that were often restricted to the cerebellar lingula and uvula. The reason for this difference between the reports of previous studies and this study is not clear. It is possible that genetic mutation causing BFCA in Aberdeen Angus cross calves in western Canada is different from the one that caused the condition in Scotland, or it may be that Angus cross cattle population in western Canada is more prone to develop axonal morphological changes in young calves affected by BFCA than is the Angus population in Scotland, due to putative genotypic or phenotypic differences between these 2 populations. The pathogenesis of BFCA is unknown, but it may involve a metabolic disorder affecting Purkinje cells and their axons.
Bovine familial convulsions and ataxia has been classified as a cerebellar cortical abiotrophy (2). It has, however, some unique features compared with other cerebellar abiotrophies of domestic animals, such as the very early clinical onset (within a few hours after birth) and the presence of convulsions (1,6,7). In most cases, seizures are the dominant clinical sign during the first 2 to 3 mo, after which cerebellar ataxia becomes prominent, with a reduction in the duration and severity of seizures. Ataxia, usually the only residual clinical sign after 15 mo, has been reported to resolve completely after 2 y (6,7). Neurological lesions confined to the cerebellum explain the ataxia but do not account for the convulsions, which typically reflect prosencephalic disorders. It has been suggested that convulsive episodes may represent exacerbations of cerebellar signs rather than true cerebral seizures because of the absence of abnormal electroencephalogram findings in convulsive calves (3). During convulsions, the eyes are usually open with decreased frequency of blinking, thus explaining the presence of severe and chronic exposure keratitis in some cases.
Based on epidemiological data, it has been suggested that BFCA is an inherited disease associated with an autosomal dominant gene with incomplete penetrance (20% to 30%) (1,6,7). A penetrance of 27% (6 affected calves out of 22) was present in 1 Saskatchewan herd, whereas penetrance in the other 3 herds was not determined. Since not all of the animals affected by mutation will show clinical signs of BFCA, it is important to diagnose this disorder and eliminate the source from the genetic pool of a herd as soon as possible to ensure that this genetic defect is eliminated from the breeding herd. Most of the reported outbreaks appear to be associated with a carrier bull (4,6,7); therefore, culling of the carrier bull and its progeny appears to be the most effective control strategy (7).
In conclusion, the BFCA genetic mutation is present in Aberdeen Angus cross cattle in Canada and most likely in the United States, since the bull associated with the recent Canadian outbreak was sired by a bull from the USA. Accordingly, it is important that clinicians recognize and pathologists diagnose this disorder accurately at early stages of an outbreak in a herd. Elimination of carriers of this genetic mutation from the breeding stocks as soon as possible will prevent spreading of the genetic defect in Aberdeen Angus cattle in North America.
Acknowledgments
The authors thank Dr. D. Hupka-Butz for submitting some of the affected calves (used in this study) to the Prairie Diagnostic Services and for her assistance in data collection, as well as Dr. Lyall Petrie and Dr. Jim Orr for contributing previous diagnostic cases. CVJ
Footnotes
This study was supported in part by the Canadian Angus Association.
References
- 1.Barlow RM, Linklater KA, Young GB. Familial convulsions and ataxia in angus calves. Vet Rec. 1968;83:60–65. [Google Scholar]
- 2.Summers BA, Cummings JF, de Lahanta A. Veterinary Neuropathology. St. Louis: Mosby, 1995:301–305.
- 3.Wallace MA, Scarratt WK, Crisman MV, Prater DA, Jortner BS. Familial convulsions and ataxia in an Aberdeen Angus calf. Prog Vet Neurol. 1996;7:145–148. [Google Scholar]
- 4.Whittington RJ, Morton AG, Kennedy DJ. Cerebellar abiotrophy in crossbred cattle. Aust Vet J. 1989;66:12–15. doi: 10.1111/j.1751-0813.1989.tb09705.x. [DOI] [PubMed] [Google Scholar]
- 5.Cho DY, Leipold HW. Cerebellar cortical atrophy in a Charolais calf. Vet Pathol. 1978;15:264–266. doi: 10.1177/030098587801500212. [DOI] [PubMed] [Google Scholar]
- 6.Barlow RM. Further observations on bovine familial convulsions and ataxia. Vet Rec. 1979;105:91–94. [Google Scholar]
- 7.Barlow RM. Morphogenesis of cerebellar lesions in bovine familial convulsions and ataxia. Vet Pathol. 1981;18:151–162. doi: 10.1177/030098588101800202. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan DC, Hrapchak BB. Theory and Practice of Hostotechnology, 2nd ed. St. Louis: Mosby, 1980:256–257.



