Abstract
A newly developed immunohistochemical test was used for the first time to demonstrate the presence of Taenia saginata (Cysticercus bovis) antigens in the lymph nodes of a heifer calf experimentally inoculated with Taenia saginata eggs. The new test should aid in the differential diagnosis of eosinophilic lymphadenitis in cattle.
Abstract
Résumé — Cysticercose bovine : observation préliminaire sur la détection immunohistochimique d’antigènes de Taenia saginata dans les ganglions lymphatiques d’un veau infecté expérimentalement. Un test immunohistochimique récemment mis au point a été utilisé pour la première fois afin de démontrer la présence d’antigènes de Taenia saginata (Cysticercus bovis) dans les ganglions lymphatiques d’une jeune génisse inoculée expérimentalement avec des œufs de Taenia saginata. Le nouveau test devrait aider à raffiner le diagnostic différentiel avec la lymphadénite eosinophilique chez les bovins.
(Traduit par Docteur André Blouin)
Bovine cysticercosis, Taenia saginata cysticercosis, refers to the infection of cattle with metacestodes of the human tapeworm, T. saginata (1). Mature tapeworm proglottids, typically containing thousands of eggs, are commonly passed in the feces of infected individuals and, under unsanitary conditions, can lead to pasture or water contamination and the infection of cattle. Ingested eggs develop into cysticerci, which can be detected during meat inspection at the routinely inspected localization sites of the parasite, including heart, skeletal muscle, diaphragm, and esophagus (2). Differences in geographical isolates of the parasite and in the breed and age of cattle have been suggested as possible factors affecting the distribution of Cysticercus bovis (1,2). Events that occur between the eggs hatching in the duodenum or other parts of the small intestine and the ensuing cysticerci localizing in the different tissues and organs are not well delineated. Heath (3) made the early observation that the large size of ruminant lacteals was suitable for entry by oncospheres (hexacanth embryos), but it was the seminal work by Koudela and Trefný (4) on the relationship between the location of T. saginata metacestodes (C. bovis) and the local histophysiological attributes of bovine muscles and heart that led to the hypothesis that T. saginata oncospheres are released from blood capillaries into tissue cavities from where they enter lymph capillaries (4). Subsequent workers have assumed that the dissemination of the parasite occurs via the venous circulatory system (1). Although T. saginata cysticerci have been shown to exist in the lymphatic vessels of the heart, skeletal muscle, and liver (5), the actual contribution of the lymphatic circulation, if any, to the parasite’s dissemination in cattle is not known. Supporting evidence for the involvement of the lymphatic system has also come from observations in other Taenia spp. For instance, T. pisiformis oncospheres were observed in the lymph node of a rabbit 30 d following oral inoculation with eggs (6).
During the course of a different study, which was approved by the institutional animal care committee of the University of Saskatchewan, Saskatoon, to inoculate a group of cattle with T. saginata eggs obtained from Asia, a 10-month-old heifer calf was necropsied 62 d following oral inoculation. On gross examination, all lymph nodes appeared normal on their external and cut surfaces, with the exception of the cut surfaces of a submandibular and a submaxillary lymph node. The 2 nodes each had a small focus of caseous necrosis in the cortical region, measuring 1 to 2 mm. The lesions were further investigated by microscopic examination and immunohistochemical staining. In addition, 2 viable T. saginata cysticerci from the triceps muscle of the forelimb and 2 degenerate cysticerci from the diaphragm and tail muscle were also obtained from the experimental calf and used as positive sample controls. For negative sample controls, 2 normal lymph nodes were obtained during the routine postmortem examination of a calf that was not inoculated with T. saginata eggs (courtesy of Dr. Gary Wobeser, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon). The lymph nodes (test sample and negative sample controls) and T. saginata cysticerci (positive sample control) were placed in separate tissue cassettes (Histosette II; Simport Plastics, Beloeil, Quebec), labeled, and fixed by immersion in 10% buffered formalin for 24 h. Thereafter, the tissue cassettes were removed, immersed in 70% ethanol, processed, and embedded in paraffin, according to standard procedures (7). Tissue sections, 5 μm thick, were placed on slides and stained with hematoxylin and eosin (H&E). For immunohistochemical staining, sections were placed on poly-L-lysine-coated slides (Electron Microscopy Sciences, Fort Washington, Pennsylvania, USA), deparaffinized, and rehydrated by sequential immersion in xylene, ethanol (absolute, 95%, and 70%), and tap water. Endogenous peroxidases in the tissue sections were inactivated by immersing the slides in 1.2% H202 in methanol for 12 min at room temperature. The presence of T. saginata antigens in the sections was detected by using a newly developed immunohistochemical test for T. saginata cysticercosis; preliminary validation showed that the test is able to identify all lesions attributable to T. saginata cysticercosis and to differentiate them from other causes of meat condemnation, such as lesions of sarcocystosis and actinobacillosis (8). Briefly, the tissue sections were digested with 0.05% protease solution (Sigma Chemical, St. Louis, Missouri, USA) and washed 3 times with a commercial automation buffer (ESBE Labs, Biomeda, Calgary, Alberta). Nonspecific reactive sites were blocked by incubating slides sequentially with 4% horse serum and 1% nonfat skim milk for 10 min each. Monoclonal antibody 158C11A10 to T. saginata (9), diluted 1:1000 in phosphate buffered saline solution (PBSS), pH 7.4, containing 4% horse serum (assay diluent), was applied to slides and allowed to react with antigens in the tissue section for 2 h at 37°C; subsequently, unbound antibodies were washed off with PBSS. Biotinylated rabbit anti-mouse immunoglobulin (Ig) G, diluted 1:800 in assay diluent, was applied and allowed to react with the tissue sections on the slides for 30 min at 37°C; following this, the slides were washed with PBSS. Thereafter, avidin-biotinylated horseradish peroxidase complex (Vector Laboratories, Burlingame, California, USA) which was diluted according to manufacturer’s instructions, was applied to the tissue sections, followed by incubation at 37°C for 45 min. Slides were then washed in automation buffer and exposed to H2O2-activated 3,3’-diaminobenzidine tetrahydrochloride substrate for visibility. Color development was allowed to proceed for 4 min at room temperature, then the substrate was washed off with PBSS. Tissue sections were counterstained with hematoxylin and dehydrated with alcohol. The alcohol was subsequently removed by xylene and coverslips applied. Sections were examined microscopically for the presence of chromogen staining, which is indicative of specific antigen-antibody reaction.
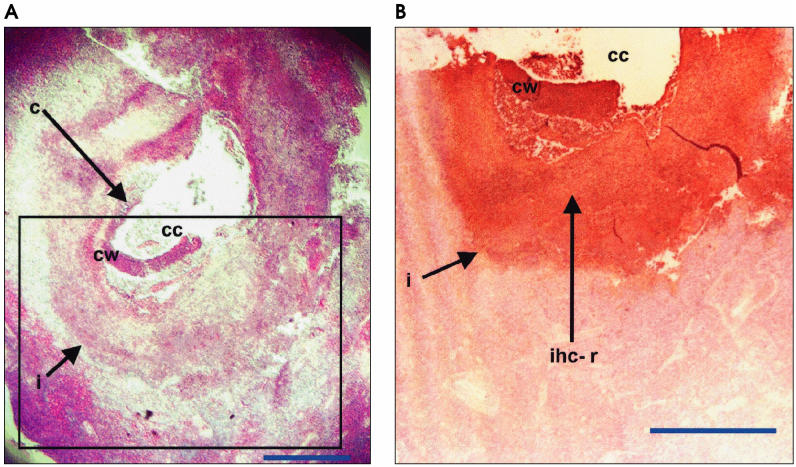
Microscopic examination of the H&E sections of the submaxillary and submandibular lymph nodes of the infected calf revealed a granulomatous reaction (Figures 1A and 2A), characterized by the presence of giant cells and infiltration of fibroblasts/fibrocytes, eosinophils, and lymphocytes (data not shown). A cystlike structure was observed in the submaxillary lymph node (Figure 1A). The wall contained an unorganized mixture of cells, including lymphocytes, macrophages, erythrocytes, reticular cells, eosinophils, giant cells, fibroblasts, and epithelioid cells. The interface between the wall and lymph node tissue consisted of a more organized mixture of similar inflammatory cells sandwiched between rows of epithelioid cells. The cavity contained some necrotic debris mixed with the inflammatory cells. The submaxillary lymph node (Figure 1A) was almost entirely depleted of lymphoid follicles, whereas numerous follicles were still present in the submandibular lymph node (Figure 2A). Immunohistochemical staining for T. saginata antigens showed diffuse but distinct color reactions around the lesions in the 2 lymph nodes obtained from the infected calf (Figures 1B and 2B). In the submaxillary lymph node, the color reaction circumscribed the cyst-like structure (Figure 1B) and provided evidence that the lesion identified by the monoclonal antibody was a Taenia cysticercus. No cysticercus or distinct parasite feature was evident in the submandibular lymph node and the color reaction (Figure 2B) was localized to a small area of the cortical region of the lymph node. No immunohistochemical staining was observed in control, normal lymph nodes, whereas the positive control viable and degenerate T. saginata cysticerci showed color reactions (data not shown) similar to the test samples.
Figure 1A and 1B.
Section of the submaxillary lymph node of a 10-month-old calf following experimental infection with Taenia saginata. The paraffin-embedded lymph node was sectioned and stained by hematoxylin and eosin (A) or, immunohistochemically, by using monoclonal antibody to Taenia saginata (B). Inset box in (A) represents area of the tissue section shown in (B). c — cysticercus; cc — cysticerus cavity; cw — cysticercus wall; i — interface between cysticercus and lymph node tissue. Bar = 1 mm.
Figure 2A and 2B.
Section of the submandibular lymph node of a 10-month-old calf following experimental infection with Taenia saginata. The paraffin-embedded lymph node was sectioned and stained by hematoxylin and eosin (A) or, immunohistochemically, by using monoclonal antibody Taenia saginata (B).<br>ihc-r — area of immunohistochemical reaction; l f — lymphoid follicle. Bar = 1 mm.
The presence of T. saginata antigens in the lymph nodes of an experimentally infected calf observed in this study supports an earlier report of the presence of the parasite in the parotid, submaxillary, and retropharyngeal lymph nodes in a 2-year-old native bull in Iran, although no information on the parasite or tissue section was presented in that report (11). In the present communication, the structure of the parasite is presented, and definitive identification made by immunohistochemistry.
The localization of parasite antigens in the lymph node might have occurred in 1 of 2 ways. Intact, early stages of the parasite might have migrated to the lymph node after emigration from the gastrointestinal tract. Alternatively, antigens or parasite fragments originating from a cysticercus located in another tissue might have drained into a local lymph node. The discrete immunohistochemical reaction and the presence of a cystic lesion in 1 of the lymph nodes suggest that an intact, juvenile metacestode can localize in the lymph node.
The presence of the parasite in 2 different lymph nodes in the same animal further supports the suggestion that lymphatic transportation of the parasite occurs. However, the relative role of lymphatic transfer compared with venous dissemination is unknown. Intense inflammatory reaction, which can limit the development of T. saginata cysticercus in the skeletal muscle, has been shown to occur in the lymph nodes of infected cattle (12). Accordingly, we hypothesize that venous dissemination may result in the establishment of the parasites at suitable sites, while lymphatic transfer may result in their elimination.
In spite of the previous report of C. bovis in the lymph node of a bull (11), the parasite is not usually considered to be a cause of lymphadenitis or eosinophilic lymphadenitis in cattle. The application of this immunohistochemical test for C. bovis could be a useful tool in investigation of the causes, and the differential diagnosis, of eosinophilic lymphadenitis in cattle (10). For example, lesions attributable to Sarcocystis do not always contain sarcocysts (10) and the application of this immunohistochemical test could help clarify the diagnosis of eosinophilic lymphadenitis by differentiating between sarcocystosis and cysticercosis (8) in the face of similar histopathological features.
References
- 1.Pawlowski ZS, Murrell KD. Taeniasis and cysticercosis. In: Hui YH, Sattar SA, Murrell KD, Nip W-K, Stanfield PS, eds. Foodborne Disease Handbook, 2nd ed. New York: Marcel Dekker, 2001:217–227.
- 2.Gracey JF, Collins DS. Meat Hygiene, 9th ed. London: Ballière Tindall, 1992:307–351.
- 3.Heath DD. The migration of oncospheres of Taenia pisiformis, T serialis and Echinoccocus granulosus within the intermediate host. Int J Parasitol. 1971;1:145–152. doi: 10.1016/0020-7519(71)90008-7. [DOI] [PubMed] [Google Scholar]
- 4.Koudela K, Trefný D. The causality of Cysticercus bovis location in the heart and in the skeletal muscles of cattle. Helminthologia. 1973;14:163–169. [Google Scholar]
- 5.Štěrba J, Dyková I, Machnicka B. Tissue reaction in the heart of cattle with a spontaneous and artificial Cysticercus bovis infection. Folia Parasitol (Praha) 1979;26:27–33. [PubMed] [Google Scholar]
- 6.Flatt RE, Moses RW. Lesions of experimental cysticercosis in domestic rabbits. Lab Anim Sci. 1975;25:162–167. [PubMed] [Google Scholar]
- 7.Anderson G, Gordon KC. Tissue processing, microtomy and paraffin sections. In: Bancroft JD, Stevens A, eds. Theory and Practice of Histological Techniques. 4th ed. New York: Churchill Livingstone, 1996:47–67.
- 8.Ogunremi O, MacDonald G, Geerts S, Brandt J. Diagnosis of Taenia saginata cysticercosis by immunohistochemical test on formalin-fixed and paraffin-embedded lesions. J Vet Diagn Invest 2004 (In press). [DOI] [PubMed]
- 9.Brandt JRA, Geerts S, De Deken R, et al. Monoclonal antibodybased ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–477. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 10.Bundza A, Feltmate TE. Eosinophilic myositis/lymphadenitis in slaughter cattle. Can Vet J. 1989;30:514–516. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoseinzadeh S, Shekarforoush SH, Khodakaram-Tafti A. An unusual case of cysticercosis in cattle: cysts in salivary glands. Trop Anim Health Prod. 1998;30:91. doi: 10.1023/a:1005039632157. [DOI] [PubMed] [Google Scholar]
- 12.Zain El , Din ESA. Immunopharmacological changes in experimental bovine cysticercosis. Sudan J Vet Res. 1981;3:93–96. [Google Scholar]