Abstract
It is important for public data repositories to promote the reuse of archived data. In the growing field of omics science, however, the increasing number of submissions of high-throughput sequencing (HTSeq) data to public repositories prevents users from choosing a suitable data set from among the large number of search results. Repository users need to be able to set a threshold to reduce the number of results to obtain a suitable subset of high-quality data for reanalysis. We calculated the quality of sequencing data archived in a public data repository, the Sequence Read Archive (SRA), by using the quality control software FastQC. We obtained quality values for 1 171 313 experiments, which can be used to evaluate the suitability of data for reuse. We also visualized the data distribution in SRA by integrating the quality information and metadata of experiments and samples. We provide quality information of all of the archived sequencing data, which enable users to obtain sufficient quality sequencing data for reanalyses. The calculated quality data are available to the public in various formats. Our data also provide an example of enhancing the reuse of public data by adding metadata to published research data by a third party.
Keywords: high-throughput sequencing; sequencing quality; public data; database
Background
The publication of primary data used as evidence is essential for ensuring transparency and reproducibility in scientific research, but it's also important for promoting the reuse of data in future research activities [1,2]. In the last decade, the rapid advance of high-throughput DNA sequencing (HTSeq) technologies has enabled omics research projects to produce massive amounts of data, which have huge potential for reuse from different perspectives [3]. An increasing number of sets of omics data are being produced by not only international consortiums, but also individual research projects [4]. However, only a portion of all archived data derived from large projects is frequently being reused, in contrast to data from individual studies. This is probably because users prefer to collect data from a single project that had a sufficient number of samples that were produced by experiments under reliable conditions, thus ensuring the quality of the data. To promote the reuse of combined sets of data from multiple projects, public repositories have to provide a filtering feature in data searches so that users can control the number of experiments and quality of data in their searches. Currently, data searches provided by repositories based on metadata described by the data submitter cannot be used for filtering by data quality. To enable such filtering, repositories have to provide information on the quality of sequence data.
As the number of submissions of data to repositories increases, the number of search results produced by inputting the same query also increases. To select an appropriate amount of data, sequencing quality is usually used to ensure that the data are sufficient for an analysis; however, only natural language metadata described bythe data submitter and a few quality informationdetails such as total sequence bases are available for public sequencing data to filter the number of data sets. Categorical values described in metadata can be used to filter the data, but they are not enough to retrieve data sets in a smaller pool of results. For example, when a user searches with the query “transcriptome data of mouse brain” in the Sequence Read Archive (SRA), a public HTSeq data repository, over 120 000 experiments are shown in the search results. To reduce the number of results and thus obtain the most suitable data set for analysis, the user needs to download all of the data and calculate the sequence quality, for instance, the read length or number of reads. Given the rapid increase in the amount of archived data, this is becoming increasingly unfeasible.
Providing information on data quality can also provide an insight into the data repository itself. Basic quality values, for example, mean and median levels of sequencing throughput, read length, or base call accuracy of a specific sequencing method, are important to obtain an overview of the archive. These values can be used to illustrate the overall distribution of data in the repository. The distribution can show the standard of data quality; thus, a user can use these values to filter out inappropriate data sets from among the thousands of search results.
Here, we provide the calculated sequencing quality data of all archived HTSeq experiments to allow repository users to control the amount and the quality of data in their searches. We also performed analyses to visualize the distribution of archived data by quality values to show the standard of data quality in the repository.
Data Description
Downloading of sequencing data
To calculate quality values of sequencing data, we downloaded the data from the SRA, which is the largest public repository for HTSeq data [5]. Sequencing data containing personal identification information that should be shared in a controlled-access manner are not archived in SRA. In this study, we downloaded open-access SRA data stored in FASTQ format from the FTP server of the DNA Data Bank of Japan [6].
We analyzed all of the publicly available HTSeq data submitted to SRA up until December 2015. The total number of sequenced samples was 1 171 313, and the number of sequenced bases was more than 2.7 trillion. The varieties of sequencing methods, sequencing instruments, and sequenced sample organisms are shown in Fig. 1; these were extracted from the metadata described by the data submitter. The most common sequencing method is the whole-genome shotgun (WGS) approach, which was employed for 426 841 samples, or 36.4% of the total. The number of different sequenced organisms is 33 961, based on the Taxonomy ID. The most commonly sequenced organism in SRA is human, with 216 896 samples, or 18.5% of the total, while the total number of samples whose scientific name contains “metagenome” is 244 457, or 20.9% of the total. The number of experiments counted by the sequencing instrument model used shows that Illumina HiSeq 2000 is the most commonly used instrument in SRA, with 542 332 experiments, or 46.3% of the total.
Figure 1:
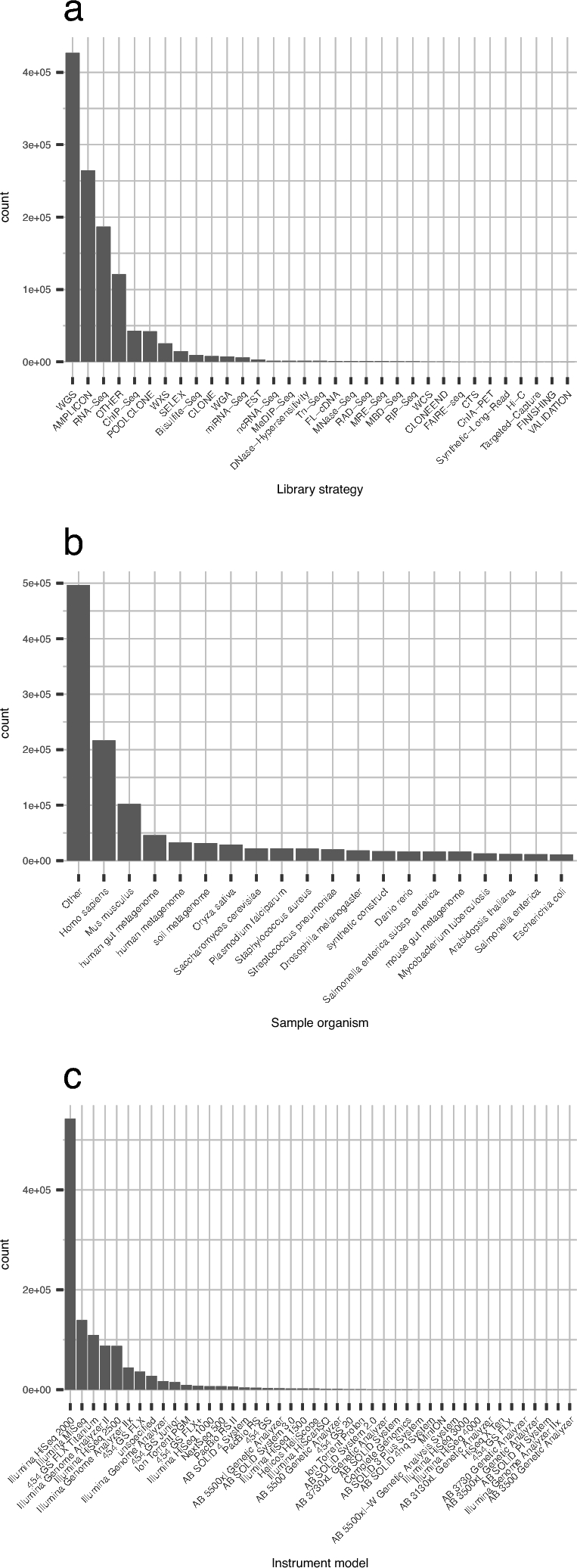
Performed sequencing experiments and sequenced samples of public data for quality calculation. (a) Bar plot of the top 20 library strategies. Values are categorical, retrieved from metadata described by the data submitter. (b) Bar plot of the top 20 sequenced sample organisms. Taxonomy information is retrieved from the NCBI taxonomy database and declared by the data submitter. (c) Bar plot of sequencing instrument models.
Calculation of sequence read quality
To enable filtering of the search results in the repository by quality information, we extracted sequence read quality values from raw sequencing data using FastQC. FastQC is one of the most popular software programs for performing quality control of high-throughput sequencing data [7]. By using the results from FastQC, we calculated comparable values of sequence data, such as the total number of reads, mean and median sequence read length, %GC, read duplicate percentage, mean and median base call accuracy, and percentage of failed base calling (N content) (Table 1). The read quality values were calculated for each downloaded set of sequencing run data in FASTQ format, and then assembled using the SRA Experiment ID.
Table 1:
Calculated sequence quality values and used modules of FastQC
| Calculated Quality Value | Numbers of Multiple Runs in an Experiment | Used FastQC Modules |
|---|---|---|
| Total number of reads | Added | Basic Statistics module |
| Mean/median read length | Average | Sequence Length Distribution module |
| %GC | Average | Basic Statistics module |
| Total duplicate percentage | Average | Duplicate Sequences module |
| Mean/median base call accuracy | Average | Per Base Sequence Quality module |
| N content | Average | Per Base N Content module |
We integrated the categorical values described inthe metadata of the sample and experiment with calculated read quality data. Experimental metadata were extracted from an SRA metadata XML file downloaded from the FTP server of the National Center for Biotechnology Information (NCBI). Sample information was extracted from the XML file downloaded from BioSample, a database maintained by the International Nucleotide Sequence Database Collaboration (INSDC) to archive information on biological materials [8].
Analyses
The state of the HTSeq repository visualized by the distribution of data quality
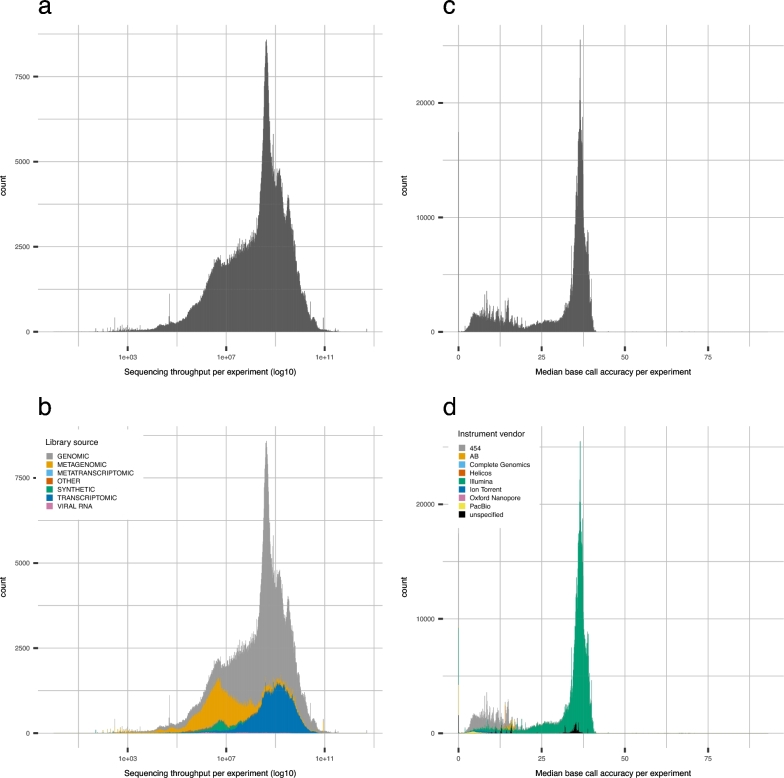
Providing sequence data quality enables users to control the number of search results from a data repository. The integration of information on data quality with metadata of samples and experiments can be used to develop a better search function. However, to offer a method of obtaining a suitable data set from thousands of search results, it is necessary to know the standard of data quality and the data distribution in the repository. To illustrate the state of publicly available HTSeq data using quality values, histograms were created for sequencing throughput, base call accuracy, and N content (Fig. 2, Supplementary Figs 1–3). As Fig. 1 shows, there is a huge bias in numbers of sequencing methods, sequenced organisms, and sequencing instrumentsused. Thus, we focused on the factor that defines the range of the quality values, not the count of data, which is probably affected by the bias of the number of sequencing instruments. To understand the data attribute that is decisive to its distribution, histograms were color-coded (Fig. 2b and d) or separated (Supplementary Figs 1 and 2) in terms of the metadata of sequencing experiments and sequenced sample organisms. In the histograms of sequencing throughput, library source, particularly genomic, transcriptomic, or metagenomic source of sequencing, clearly explains the distribution of sequenced bases (Fig. 2a and b, Supplementary Fig. 1). Overall, the mean value of throughput was 2.371e+09, and the median value was 3.349e+08. In the histogram of base call accuracy, as expected, the values are strongly affected by the choice of sequencing chemistry (Fig. 2c and d, Supplementary Fig. 2). The mean value of base call accuracy was 29.45, while the median value was 35.52. The histogram drawn by N content showed that 1 103 515 items, 94.2% of the data, had N at less than 1% of the total sequences (Supplementary Fig. 3). For the data with a higher proportion of N content, there may have been an error in the sample DNA preparation or sequencing operation.
Figure 2:
Data distribution in a public data repository by sequencing quality. (a, b) Histogram of sequencing throughput (a) and one color-coded by library source (b). (c, d) Histogram of base call accuracy (c) and one color-coded by instrument manufacturer (d).
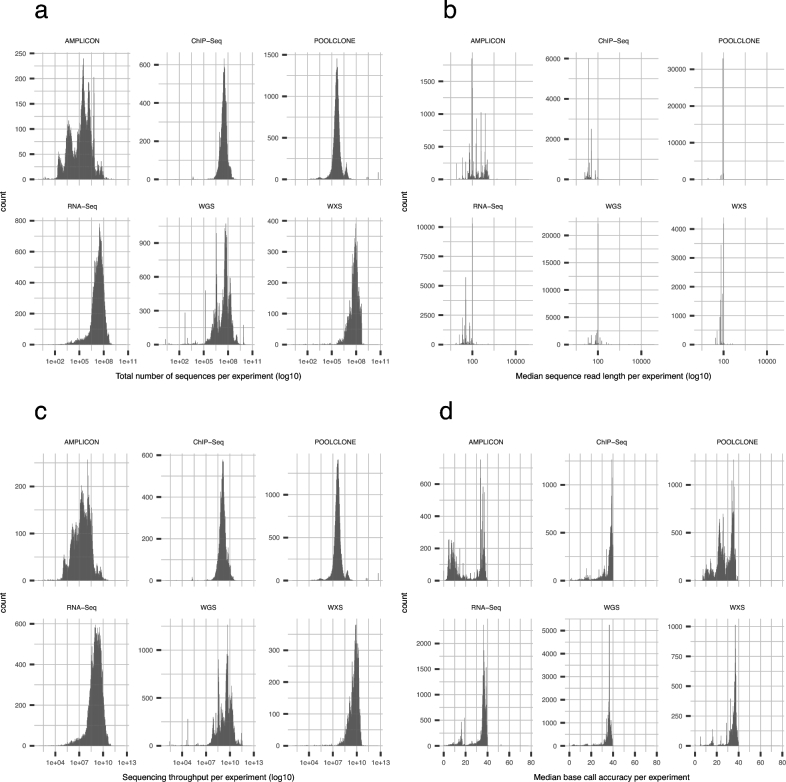
Data distribution by read quality for each sequencing method
SRA accepts the submission of various kinds of sequencing data, such as those obtained by WGS, RNA-Seq, ChIP-Seq, and metagenomic approaches, as well as many other DNA library construction strategies. To accomplish higher measurement accuracy and greater dynamic range, each sequencing method has ideal conditions regarding sequencing quality. We analyzed the distribution of data in each data set using a library strategy to investigate how many performed experiments achieved such ideal conditions. We employed 988 678 sets of data for this analysis, which were obtained through the sequencing of human samples via WGS, amplicon sequencing, RNA-Seq, ChIP-Seq, pooled clone sequencing, or whole-exome sequencing (WXS). We visualized the data distribution by creating a histogram for each library strategy (Fig. 3). The histograms were also separated by the sequencing instrument manufacturer to show which type of sequencing chemistry had been selected (Supplementary Fig. 4). In one of the six library strategies, namely amplicon sequencing, multiple types of sequencing chemistry were used, while the others were performed mostly by Illumina sequencing chemistry. The histograms indicate that the five library strategies require a larger number of sequence reads and higher base call quality. In contrast, experiments by other library strategies were performed with a short read length of around 100 bases, while some amplicon sequencing experiments were performed with longer sequence reads of hundreds of bases. A total of 66.3% of amplicon sequencing experiments were performed by non-Illumina sequencers, for which the average read length was 388.4. This is consistent with the standards of each sequencing strategy [9].
Figure 3:
Human data distribution for each library strategy. (a–d) Histograms separated by the top six library strategies. Data distribution is by the total number of sequences (a), median read length (b), sequencing throughput (c), and median base call accuracy (d) per experiment.
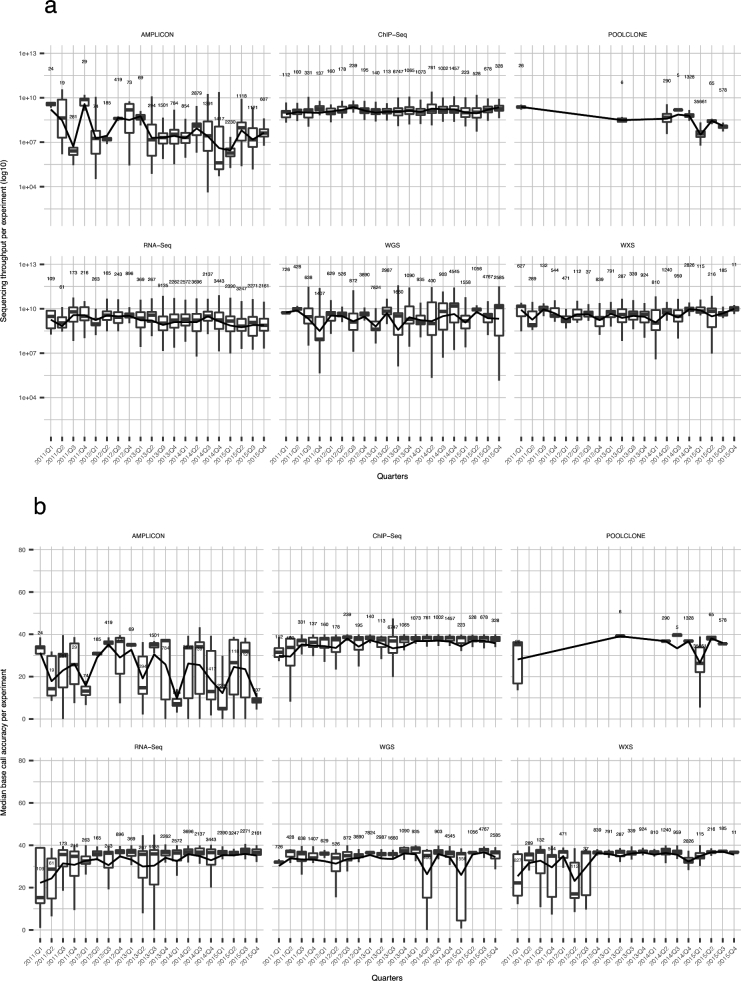
Changes of sequencing quality during SRA's history
Since 2007, when the first next-generation sequencing data were submitted to the SRA, there have been rapid advances in the sequencing technology regarding both the instruments and the chemistry, which have significantly improved the quality of sequencing data. The improved specs of sequencers have enabled various new sequencing methods to be developed, but have also helped improve the data quality output using existing methods. We visualized the changes of quality values for each sequencing method over time. A change in four sequencing qualities, total number of reads, read length, sequencing throughput, and base call quality of six library strategies, WGS, amplicon, RNA-Seq, ChIP-Seq, pooled clone, and WXS are visualized by box plots in quarterly time series (Fig. 4, Supplementary Fig. 5). While the plots of pooled clone sequencing could not be evaluated due to a lack of continuous data submission, the plots of the other strategies show their trends over time. The plots of amplicon sequencing show no specific tendency, probably indicating that such sequencing quality values are determined by the characteristics of each sequencing project, the surveying of which requires more detailed metadata. In ChIP-Seq and WXS, sequencing throughput increased slightly over time. In plots of base call accuracy, ChIP-Seq, RNA-Seq, WGS, and WXS showed increases of the value, possibly reflecting the improvement of sequencing technologies.
Figure 4:
Change of data distribution by sequencing quality over time. (a, b) Box plots separated by the top six library strategies, showing quarterly change. Data distribution is by the sequencing throughput (a) and median base call accuracy (b) per experiment. The numbers in the plots indicate the numbers of samples in a row. The lines connecting the boxes indicate changes of mean value.
Discussion
The increasing number of submissions of data to public high-throughput sequencing data repositories has made it difficult to reuse published data efficiently. By calculating quantitative variables of sequencing data and integrating them with information on experiments and sample organisms, we enabled an appropriately sized subset to be obtained from multiple projects archived in the repository. Without any quantitative information, users cannot choose a reliable data set from among thousands of search hits. When users search data with a query of sample-related information, such as a treatment of biological materials, the number of search results tends to be very small or too large for users to be able to browse through due to the lack of detailed metadata. It is also claimed that the metadata described by data submitters lack some important information or may contain errors [10]. In contrast, our results can provide information in a way that enables users to look into a large data set and control the amount of data output in their search by setting a threshold regarding the quality value.
Our approach also enables visualization of the data distribution to find the relative position of data in a data set of similar features. Moreover, it is now possible to show the distribution of read information and its change over time for each sequencing method. These features are useful when deciding on the conditions to set in a sequencing experiment. For example, from the results of our investigation on the distribution of sequencing throughput and base call accuracy, users can check whether the total number of sequenced bases is within the appropriate range for one's library source, and they can also evaluate whether the base call accuracy is sufficient to follow the standard quality of the instruments used. Though it is possible that an incorrect metadata description such as wrong usage of sequencing method categories can affect the interpretation of the result, the quality summary is useful to evaluate the users’ data by comparing to a similar data set.
The data of FastQC that we used to calculate the read information are also published on our web server (http://sra.dbcls.jp/fastqc). These data enable SRA users to examine read quality information before downloading sequencing data from the FTP server. They can also help users to avoid downloading data that do not match their objectives, which can decrease the cost of downloading. The calculated sequence statistics in this study are published as linked data, which can be accessed via the SPARQL endpoint, along with SPARQL query examples, to allow users to integrate these with other public biological linked data resources related to the SRA [11]. We will continue to calculate sequencing statistics for future data submission and update the summary.
Our study shows that efforts to extend metadata of existing public sequencing data by a third party can increase data accessibility and enhance the reuse of published data. Although it is important to publish primary data used in research, it is not possible to maintain a large repository of high-throughput sequencing data without sufficient economic and human resources [12]. To tackle the problem of the sustainability of data repositories, approaches to decrease the cost of hosting them have been proposed, including a new data compression strategy. As another method of increasing their efficiency, we also highlight the importance of biological data repositories, increasing their value by enhancing the reuse of data. We strongly believe that the use of open data is the best way of keeping them accessible.
Potential implications
The amount and the accuracy of sequencing data have been drastically changing in recent years. This means that database users have to care about the details of the experiment, for example, date of sequencing or sequencing equipment used for each database entry. The quality information of public sequencing data provided by our work can be used to evaluate the reliability of entries in biological databases, such as genome variations or gene expressions.
Methods
Data retrieval from the data repository
We downloaded data from the FTP server of the DNA Databank of Japan (ftp.ddbj.nig.ac.jp/ddbj_database/dra) by using the lftp command. Most of the data were downloaded as FASTQ format files. When data were only available in SRA format, we decompressed the data to FASTQ format by using the fastq-dump command of the SRA toolkit (v. 2.5.1). fastq-dump is performed with the –split-3 option to split paired-end files into individual FASTQ files. Downloaded data were analyzed by md5 checksum to confirm that they were not corrupt.
Extraction of sequencing quality information
First, we performed FastQC [7] via the command line with options –no-extraction and –threads 4. The versions of FastQC software used in this study were 0.10.0, 0.10.1, and 0.11.3, depending on the date when each sequencing run was performed. We confirmed that there were no differences in the results of the modules that we used among the versions. We parsed the result files of FastQC (fastqc_data.txt) by the bioruby [13] module bio-fastqc [14], which we developed based on biogem [15]. The results from paired-end reads were concatenated by calculating the average values for each quality value, excluding values of the total number of sequences that were summed. If an experiment involved multiple sequencing runs, quality values were also concatenated to create comparable values for each experiment. By using relation of SRA ExperimentID and BioSampleID, calculated quality values, experimental metadata, and sample organism metadata were assembled. The code is available online [16].
Publishing quality data as linked open data
We published the individual results of FastQC for each sequencing run on our web server [17]. Each set of sequencing quality data was converted into RDF format and deposited in the NBDC RDF portal [11]. We developed an ontology to describe sequencing quality information, namely sequence statistics ontology, and also published it in the NBDC RDF portal.
Visualization of the data distribution in the repository
Visualization of the distribution of data was performed using R language (v. 3.2.3) [18] and library ggplot2 (v. 2.1.0) [19]. The code is available online [15].
Additional files
Supplementary Figure 1: Data distribution of sequencing throughput for each set of metadata. (a–e) Histograms of sequencing throughput (a), separated by library strategy (b), library source (c), top 20 taxonomic scientific names (d), and instrument manufacturer (e).
Supplementary Figure 2: Data distribution of base call accuracy for each set of metadata. (a–e) Histograms of base call accuracy (a), separated by library strategy (b), library source (c), top 20 taxonomic scientific names (d), and instrument manufacturer (e).
Supplementary Figure 3: Data distribution by N content. (a–f) Histograms of N content percentage per experiment. Histograms of base call failure of overall (a), separated by library strategy (b), library source (c), sample organism (d), instrument manufacturer (e), and year of data submission (f). The y-axis is log 10 scale.
Supplementary Figure 4: Human data distribution for each library strategy separated by instrument manufacturer. (a–d) Histograms separated by the top 6 library strategies and instrument. Data distribution is by the total number of sequences (a), median read length (b), sequencing throughput (c), and median base call accuracy (d) per experiment.
Supplementary Figure 5: Change of data distribution by sequencing quality over time. Box plot of sequence quality per experiment over time. (a) Data distribution by total number of sequence reads per experiment. (b) Data distribution by median sequence read length per experiment.
Abbreviations
HTSeq: high-throughput sequencing; INSDC: International Nucleotide Sequence Database Collaboration; JST: Japan Science and Technology Agency; NBDC: National Bioscience Database Center; NCBI: National Center for Biotechnology Information; SRA: Sequence Read Archive; WGS: whole-genome shotgun; WXS: whole-exome sequencing.
Supplementary Material
Supplementary Figure 1: Data distribution of sequencing throughput for each set of metadata. (a–e) Histograms of sequencing throughput (a), separated by library strategy (b), library source (c), top 20 taxonomic scientific names (d), and instrument manufacturer (e).
Supplementary Figure 2: Data distribution of base call accuracy for each set of metadata. (a–e) Histograms of base call accuracy (a), separated by library strategy (b), library source (c), top 20 taxonomic scientific names (d), and instrument manufacturer (e).
Supplementary Figure 3: Data distribution by N content. (a–f) Histograms of N content percentage per experiment. Histograms of base call failure of overall (a), separated by library strategy (b), library source (c), sample organism (d), instrument manufacturer (e), and year of data submission (f). The y-axis is log 10 scale.
Supplementary Figure 4: Human data distribution for each library strategy separated by instrument manufacturer. (a–d) Histograms separated by the top 6 library strategies and instrument. Data distribution is by the total number of sequences (a), median read length (b), sequencing throughput (c), and median base call accuracy (d) per experiment.
Supplementary Figure 5: Change of data distribution by sequencing quality over time. Box plot of sequence quality per experiment over time. (a) Data distribution by total number of sequence reads per experiment. (b) Data distribution by median sequence read length per experiment.
Acknowledgments
The authors are grateful to Prof. Toshihisa Takagi and Dr. Itoshi Nikaido for thoughtful comments and discussions. The authors also thank to Dr. Yuichi Kodama for the comments as a DDBJ curator. Computations were performed on the NIG supercomputer at ROIS National Institute of Genetics.
Funding
This work has been supported by the National Bioscience Database Center of the Japan Science and Technology Agency.
Availability of source code and requirements
Project name: SRA Quanto
Project home page: https://github.com/inutano/sra-quanto
Operating systems: platform independent
Programming language: Ruby, R
Other requirements: Univa Grid Engine 8.4.0 or higher
License: MIT
Any restrictions to use by non-academics: N/A
Availability of supporting data and materials
The data set supporting the conclusions of this article is available in figshare [20], and snapshots of the code are available in the GigaScience database (GigaDB) repository [21].
Conflicts of interest
The authors declare that they have no competing interests.
Authors’ contributions
Conceptualization: T.O., T.N., and H.B. Methodology, software, investigation, visualization, writing original draft: T.O. Supervision: T.N. and H.B.
References
- 1. Organisation for Economic Co-operation and Development OECD Principles and Guidelines for Access to Research Data from Public Funding. Paris: OECD; 2007. http://www.oecd.org/science/sci-tech/38500813.pdf. (11 November 2016, date last accessed). [Google Scholar]
- 2. Sansone SA, Rocca-Serra P, Field D et al. Toward interoperable bioscience data. NatGenet 2012;44(2):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ball CA, Sherlock G, Brazma A. Funding high-throughput data sharing. Nat Biotechnol 2004;22(9):1179–83. [DOI] [PubMed] [Google Scholar]
- 4. Nakazato T, Ohta T, Bono H. Experimental design-based functional mining and characterization of high-throughput sequencing data in the sequence read archive. PLoS One. 2013;8(10):e77910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kodama Y, Shumway M, Leinonen R. The Sequence Read Archive: explosive growth of sequencing data. Nucleic Acids Res 2012;40(D1):D54–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mashima J, Kodama Y, Kosuge T et al. DNA data bank of Japan (DDBJ) progress report. Nucleic Acids Res 2016;44(D1):D51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andrews S. A quality control tool for high throughput sequence data. 2010. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/. (11 November 2016, date last accessed).
- 8. Barrett T, Clark K, Gevorgyan R et al. BioProject and BioSample databases at NCBI: facilitating capture and organization of metadata. Nucleic Acids Res 2012;40(D1):D57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 2016;17(6):333–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alnasir J, Shanahan HP. Investigation into the annotation of protocol sequencing steps in the sequence read archive. Gigascience. 2015;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Bioscience Database Center NBDC RDF Portal. https://integbio.jp/rdf/. (11 November 2016, date last accessed). [Google Scholar]
- 12. Cochrane G, Cook CE, Birney E. The future of DNA sequence archiving. Gigascience. 2012;1(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goto N, Prins P, Nakao M et al. BioRuby: bioinformatics software for the Ruby programming language. Bioinformatics. 2010;26(20):2617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tazro Inutano Ohta, ruby parser for FastQC, a quality control software for high-throughput sequencing data. https://rubygems.org/gems/bio-fastqc (11 November 2016, date last accessed).
- 15. Bonnal RJ, Aerts J, Githinji G et al. Biogem: an effective tool-based approach for scaling up open source software development in bioinformatics. Bioinformatics. 2012;28(7):1035–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tazro Inutano Ohta Summary of quantitative sequence information of the Sequence Read Archive. https://github.com/inutano/sra-quanto. (11 November 2016, date last accessed). [Google Scholar]
- 17. Database Center for Life Science DBCLS SRA. http://sra.dbcls.jp/. (11 November 2016, date last accessed). [Google Scholar]
- 18. R Core Team R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing,; 2015. https://www.R-project.org/. (11 November 2016, date last accessed). [Google Scholar]
- 19. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 20. Ohta T. A summary of sequencing quality of data archived in Sequence Read Archive: quality information of sequencing data archived in Sequence Read Archive calculated by FastQC. 2017; http://dx.doi.org/10.6084/m9.figshare.4498907.v2.
- 21. Ohta T, Nakazato T, Bono H.. Supporting data for “Calculating quality of public high-throughput sequencing data to obtain suitable subset for reanalysis from the Sequence Read Archive.” GigaScience Database. 2017; http://dx.doi.org/10.5524/100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Data distribution of sequencing throughput for each set of metadata. (a–e) Histograms of sequencing throughput (a), separated by library strategy (b), library source (c), top 20 taxonomic scientific names (d), and instrument manufacturer (e).
Supplementary Figure 2: Data distribution of base call accuracy for each set of metadata. (a–e) Histograms of base call accuracy (a), separated by library strategy (b), library source (c), top 20 taxonomic scientific names (d), and instrument manufacturer (e).
Supplementary Figure 3: Data distribution by N content. (a–f) Histograms of N content percentage per experiment. Histograms of base call failure of overall (a), separated by library strategy (b), library source (c), sample organism (d), instrument manufacturer (e), and year of data submission (f). The y-axis is log 10 scale.
Supplementary Figure 4: Human data distribution for each library strategy separated by instrument manufacturer. (a–d) Histograms separated by the top 6 library strategies and instrument. Data distribution is by the total number of sequences (a), median read length (b), sequencing throughput (c), and median base call accuracy (d) per experiment.
Supplementary Figure 5: Change of data distribution by sequencing quality over time. Box plot of sequence quality per experiment over time. (a) Data distribution by total number of sequence reads per experiment. (b) Data distribution by median sequence read length per experiment.