Abstract
Sepsis is one of the biggest challenges in critical care nowadays. Defining sepsis is a difficult task on its own and its diagnosis and treatment requires well trained, devoted personnel with interdisciplinary collaboration in order to provide the patients the best chance for survival. Immediate resuscitation, early adequate antimicrobial therapy, source control and highly sophisticated organ support on the intensive care units are all inevitable necessities for successful recovery.
To help fast and accurate diagnosis biomarkers have been measured for decades. Procalcitonin (PCT) is one of the most studied, but the results are conflicting. Sepsis means a very loose cohort of a large heterogeneous patient population, hence defining certain cut off values for PCT to differentiate between different severities of the disease is almost impossible. Clinicians first have to understand the pathophysiological background of sepsis to be able to interpret correctly the PCT results.
Nevertheless, PCT has been shown to have the best sensitivity and specificity to indicate infection, antibiotic appropriateness and stopping therapy.
In this article we will focus on some important aspects of pathophysiology and advice on how to implement that in the everyday clinical practice. We believe that this multimodal evaluation of the clinical picture together with PCT results can be a useful tool to make the most out of the PCT results, and do the best for patients on the ICU.
Key words: sepsis, septic shock, procalcitonin, antibiotic, biomarkers
INTRODUCTION
One of the most challenging tasks in critical care medicine is the treatment of serious infection related multiple organ dysfunction, termed in general as sepsis, and septic shock. Early detection of infection and the immediate start of resuscitation parallel with adequate antimicrobial therapy undoubtedly give the best possible chance for survival and received strong recommendation by the Surviving Sepsis Campaign guidelines [1]. However, while recognizing organ failure via objective signs is relatively easy, diagnosing infection as the possible underlying cause remains a challenge. Due to the non-specific properties of conventional signs of infection, such as body temperature and white cell count (WCC), biomarkers have been utilized to aid diagnosis for decades. One of the most studied biomarkers is procalcitonin (PCT) [2]. Its role in assisting antibiotic (AB) therapy has been studied extensively, with contradicting results. There are positive studies [3, 4] showing that a PCT-guided patient management reduced antibiotic exposure and length of antibiotic therapy without affecting patient outcomes. There are also negative studies, which could not show this benefit [5-7]. However, to understand the values and limitations of inflammatory biomarkers it is inevitable to understand the immunological background of critical illness determined mainly by the host response. Moreover, putting the results of these studies in context, based on new insights of the pathomechanism of sepsis and systemic inflammation generated mainly by the individuals’ host response, may explain the differences between the reported results and help the clinician to interpret PCT data with more confidence at the bedside.
SEPSIS SYNDROME AS A DISEASE
In most surgical and medical specialties we diagnose definitive diseases, which would indicate definitive treatment. However, defining, hence diagnosing sepsis is not that simple.
The term “sepsis syndrome” was invented during the designing of the protocol of one of the first prospective randomized trials in sepsis, performed by a group of scientists led by the late Roger Bone in Las Vegas in 1980 [8]. Several years later a statement paper was published by the same authors titled “Sepsis syndrome: a valid clinical entity” [9], after which the medical society started to deal with sepsis as with a definitive disease, which created false expectations:1) physicians wanted one single test with high sensitivity and specificity to diagnose sepsis, and 2) there was an urge to find an “antisepsis magic bullet”. Neither of these wishes have and will never ever come true.
Regarding the definition and diagnosis of sepsis, the classical signs of the “sepsis syndrome” such as fever/hypothermia, leukocytosis/leukopenia, tachycardia and hypotension, meant a very large and non-specific cohort of patients. For this reason, a consensus conference was brought together which defined the so called “consensus criteria” of sepsis, which has been used for decades in research and clinical practice alike [10]. However, the uncertainty about sepsis definitions lingered on that resulted the recently published new definitions as “Sepsis 3” [11]. In this, sepsis is defined as a “life- threatening organ dysfunction caused by a dysregulated host response to infection”. As categories only sepsis, septic shock, and organ dysfunction remained.
These efforts clearly show that finding the appropriate definition of sepsis has been a continuous challenge for more than 30 years. The difficulty in defining sepsis originates from its complex pathophysiology, which is affected by numerous individual variations of the host response. Furthermore, in most specialties diagnostic laboratory or radiological tests have very high sensitivity and specificity often reaching almost 95-100% [12]. However, in the case of sepsis, it is different, which makes not just the diagnosis, but the interpretation of the results of clinical trials and also epidemiological data very difficult.
THE IMMUNE RESPONSE FOR AN INSULT
The immune system is a complex network and the immune response to pathogens relies on both innate and adaptive components, dynamically defined as the pro-, and anti-inflammatory forces. The innate immune system (including the complement system, sentinel phagocyte and natural killer cells), is responsible for the eradication of the invaders, while the adaptive immune system’s role is to control the process and keep it localized to the site of the insult [13]. Under normal circumstances these mechanisms remain in balance. The innate system acts by broad recognition of antigens, mainly by triggering “pathogen-associated molecular patterns” (PAMP) of lipopolysaccharide elements of the surfaces of invading pathogens. When there is an imbalance due to the dysregulation of the pro-, and anti-inflammatory forces, the local response escalates into a systemic host response also termed as “cytokine storm” [14]. It was a surprising finding, that after trauma, burns, ischemia-reperfusion, pancreatitis, major surgery, etc., same or similar molecules are released mainly from the mitochondria of the injured or stressed cells that are found during PAMPs, and can also cause a cytokine storm. This process accompanying tissue injury is called “damage-associated molecular patterns” (DAMP). In the case of bacterial infection this similarity is due to the fact that the bacteria and the mitochondria (which is more-or-less an encapsulated bacterium) share very similar genetic background. This explains why tissue injury induced DAMP and bacterial infection induced PAMP manifest in similar host responses and clinical manifestations [15]. This similar pathophysiological response is summarized in Figure 1. This indicates that in addition to PAMP, DAMP can also cause the induction of the production of similar cytokines, hormokines and also PCT. This on the one hand provides the potential benefit of PCT in diagnosing infection (PAMP) but also limits its accuracy as levels may increase in scenarios without infection (DAMP). This is the reason, why unexpected PCT values (high or low) are often interpreted as “false negative” or “false positive”. However, understanding the nature of PCT production helps a lot in the interpretation of PCT values at the bedside.
Figure 1.
The molecular responses for damage (DAMP) and pathogen (PAMP) type insults
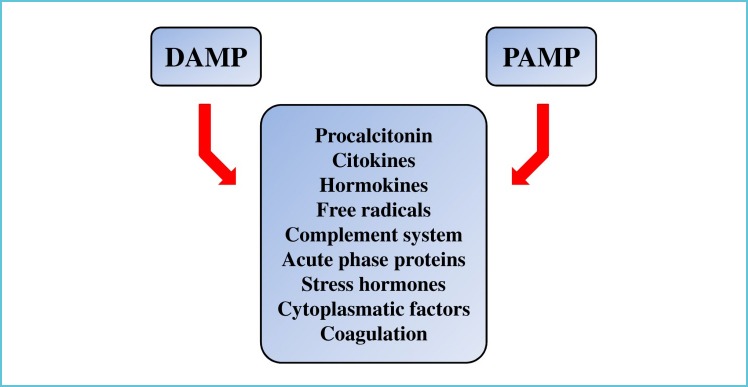
For further details, see text.
THE ROLE OF PCT IN DIAGNOSING INFECTION
The so called “sepsis biomarkers” do not recognize sepsis per se, but inflammation. The reasons have been explained in the previous paragraphs, namely that both damage and pathogen related insults can provoke a very similar inflammatory host response. Therefore, in this context, the right question is: whether the critically ill condition is due to infection or not? Because if it is, we should start anti-microbials or other source control. But if it isn’t, then anti-microbial therapy should not be commenced, due to its several undesired effects. Therefore, it is not “sepsis” what we treat, but organ dysfunction and infection.
Diagnosing infection on the ICU is not easy and requires a multimodal approach. Clinical signs are obviously the most important in recognizing critical illness and suspecting infection and even the source of infection, but they cannot prove it on their own. Conventional indicators such as fever/hypothermia, leukocytosis/leukopenia, tachypnea, tachycardia, hypotension, taken from the classical “sepsis-syndrome” criteria are non-specific, and in fact poor indicators of infection. To fill this gap inflammatory biomarker measurements have been developed [2]. Every biomarker has its own merit and limitations, but there is no “ideal” biomarker, and there may never be one. Biomarkers can support decision making but they will never be able to differentiate between inflammatory response for infection from host response for non-infectious insults with a 100% sensitivity and specificity due to the complex, overlapping pathomechanism of PAMP and DAMP. This is in sharp contrast with the diagnostic power of certain biomarkers used in the world of “definitive” diseases, where several laboratory parameters have this ability. Furthermore, learning how to use biomarkers is not easy either.
The two most commonly used markers in infection/sepsis diagnostics and for guiding therapeutic interventions are PCT and CRP [2]. One of the main limitations of CRP is that it moves “slowly”, and after a certain insult it reaches its maximum value usually 48 hours later. This is in general unacceptable on the ICU, as every hour delay in starting for example appropriate antibiotic treatment can affect mortality as indicated by the study of Kumar et al. [16]. Furthermore, levels are generally elevated in most ICU patients, making interpretation of CRP very difficult [17].
Procalcitonin is detectable in the serum within a few (4-6) hours after its induction, which is most often bacterial infection. During the “normal” course of an infection it reaches its peak within 24 hours and then starts its decline in the case of adequate treatment with levels reducing by roughly 50% daily according to its half-life [18]. Procalcitonin differentiates bacterial infections from systemic inflammatory response of other etiologies with higher sensitivity and specificity as compared to CRP [19], and also have a good prognostic value regarding survival [20]. However, interpreting PCT values on admission or after the onset of an acute insult, let it be infectious or not, is not simple. But this holds true for any biomarker, as they show a large scatter between patients with a seemingly similar clinical condition, hence single absolute values are difficult to interpret.
There are many studies reporting that PCT values correlate with severity and differ significantly in patients with SIRS, sepsis, severe sepsis and septic shock [21]. Clec’h et al., found that patients with septic shock had more than 10 times higher median PCT levels as compared to those admitted with shock of non-septic origin [22]. However, looking at the data carefully reveals that although there was a remarkable and statistically significant difference, but there is also a huge scatter and overlap of the PCT data between the groups (septic shock: 14 [0.3-767] vs. non-septic shock: 1 [0.15-36] ng/ml, respectively), which makes individual interpretation of a single measurement very difficult - a finding, which is generally true for every biomarker of inflammation. This has been reinforced by the same group in a subsequent study, in which they found that the median PCT value in medical vs. surgical patients differed both in SIRS: 0.3 (0.1-1.0) vs. 5.7 (2.7-8.3) ng/ml, and in septic shock: 8.4 (3.6-76.0) vs. 34.0 (7.1-76.0) ng/ml, respectively [23]. These differences and the large overlap can be explained by the PAMP and DAMP based host response. In certain cases there is a single PAMP or DAMP, but they can also occur in combination as PAMP+DAMP. The latter is bound to have a pronounced inflammatory response reflected in several times higher PCT values. Therefore, it has become clear that the same PCT value, in other words a given “normal” value, cannot be used in every condition. Medical patients with infection in general should have lower PCT values (single insult of PAMP) as compared to surgical patients with infection, where DAMP and PAMP are present at the same time. Moreover, it is also important to acknowledge, that any cellular injury, let it be direct tissue or ischemia-reperfusion injury without infection can result in elevation of PCT induced by a single DAMP type insult.
Although PCT absolute values have the above mentioned limitations, but there is overwhelming evidence that in most cases high PCT values indicate bacterial infection. The shortcomings of PCT absolute values might be compensated when the kinetics of PCT is taken into account to indicate infection.
PCT-ASSISTED ANTIBIOTIC THERAPY
There are three fundamental questions to be answered during our ward rounds when treating patients with suspected or proven infections on the ICU: 1) is there infection, in other words should we start empirical antibiotic therapy; 2) is the commenced antibiotic effective; and finally 3) when should we stop antibiotic treatment?
In this article we are giving some aspects to answer these questions referring to the result of previous studies which were performed at our department in the last few years in the field of procalcitonin and antibiotic therapy [24, 25, 26].
1. Is there infection?
It has been explained earlier that either PAMP or DAMD can induce PCT production. Serum levels of any biomarker show large scatter even in a seemingly homogenous patient population. That is why it is so difficult, almost impossible, to define an exact PCT value that indicates bacterial infection. Indeed, even the most accurate studies can only show 75-85% sensitivity and specificity. Unfortunately, most clinicians tend to interpret sepsis as a definitive disease, therefore they have false expectations from the role of the biomarkers in the diagnosis of infection, and they become “biomarker sceptic”, when they find high levels of PCT after a non-infectious insult, such as surgery, trauma, or after cardiopulmonary resuscitation. But those who know the patophysiology of inflammation (mechanism of DAMP, PAMP) are not surprised by this phenomenon, because they understand that this is due to the etiology and heterogeneity of patients, more precisely due to the individual immune response after a particular insult.
Our recently published results showed that PCT kinetics could give a much more reliable help to the clinicians’ decision making than absolute values. As it has a half-life of less than 24 hours, we hypothesized that kinetics may produce different pattern in those who receive adequate treatment as compared to those who don’t. In the EProK („Early Procalcitonin Kinetics”) study patients were enrolled who were thought to have infection [24]. From the enrolled 209 patients in 114 cases PCT was available from the previous day before the infection was suspected [25]. Throughout this 24 hours we found that PCT elevation was approximately twice higher in patients who turned out to have infection versus those who did not. So a more than 88% PCT elevation in 24 hours refers to infection with 75% (65-84) sensitivity and 79% (60-92) specificity (AUC 77%). It can be an absolute value independent indicator of infection.
It is important to note that if the patient is hemodynamically unstable and infection is likely, by definition he/she has septic shock or at least one cannot exclude it, hence antibiotic therapy shouldn’t be delayed but has to be commenced immediately, regardless of the PCT or any biomarker value [16]. However, if the patient is stable hemodynamically, and PCT is “low” or decreasing then we can wait, observe the patient and reassess later. What “low” means as an exact value is difficult to define, as it depends on the etiology and the patient. Therefore, we have to admit, that diagnosing infection with or without PCT remains a challenge.
2. Evaluating antibiotic appropriateness
After commencing empirical antibiotic therapy, it is indispensable to confirm appropriateness to correct treatment if needed as soon as possible because it is upmost vital. In septic shock every hour delay in starting adequate antibiotic therapy could have serious effect on survival [16]. But unnecessary overuse of antibiotics can also cause increased bacterial resistance, invasive fungal infections, side effects and increased costs [27]. Despite international guidelines are available to help in choosing the right medication with the best possible chance, unfortunately it seems that inappropriate empirical antibiotic therapy can be as high as 25-30% on the ICU [28, 29]. The gold standard for proving appropriateness of antibiotic therapy is the microbiological confirmation of the bacteria and its susceptibility. However, these results may come far too late, in reality days after the specimen had been sent, but treatment cannot be delayed. At present there is very little to help the clinicians at the early stage of patient care to confirm appropriate antibiotic treatment.
The above mentioned EProK study showed that there was a significant difference in the early kinetics of PCT between patients receiving appropriate as compared to those getting inappropriate antibiotic therapy (24). Serum PCT levels were measured right after ABs were commenced then 8 hourly in the first day. In those patients who received effective AB therapy PCT reached the highest level at 16 hours and started to decline at the end of the first day while those whose therapy turned out to be inadequate the PCT level continued to increase during the first day (Figure 2)These data suggest that early response of PCT within the first 24 hours of commencing empirical antibiotics in critically ill patients may help the clinician to evaluate the appropriateness of therapy.
Figure 2.
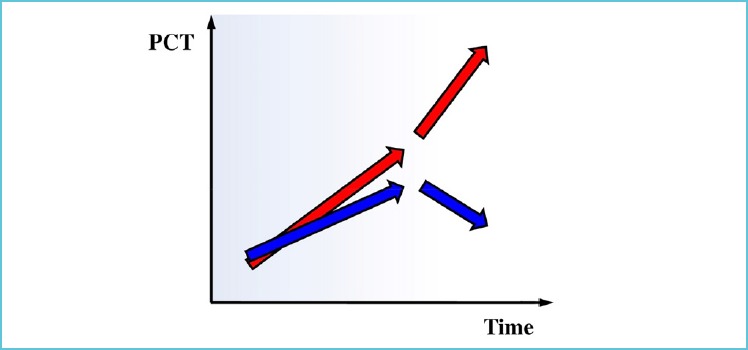
PCT kinetics during the first day of effective and ineffective empiric antibiotic therapy [24]
Measuring PCT when antibiotics were commenced, then 12-16 and 24 hours later can reveal a certain kinetics. In the case of continuous increase (red arrows), it is likely that the empirical ABs are inappropriate. However, a “roof-top” type PCT pattern, indicating, the levels peaked somewhere around 12-16 hours can indicate appropriate antibiotic therapy.
Once empirical antibiotics were commenced, daily re-evaluation of the situation is needed. In addition to the clinical picture and PCT levels, microbiology should also be taken into account. Microbiological data usually becomes available 2-3 days after specimen were sent, and then it’s time to re-evaluate the situation. The course of the clinical condition, combined with both the microbiological and PCT results, what we term as a multimodal approach, can assist us whether to continue, reconsider or change antibiotics and/or reassess organ support and most importantly, to stop antibiotics even on day 3 if they are considered unnecessary.
Despite no clinical improvement a decrease in PCT still may indicate that the infection is under control, but the patient needs more time to gain benefit from treatment. Therefore, ABs should be continued. On the contrary, if PCT is not decreasing or even increasing, these can be important signs that infection is not under control, hence source of infection and antibiotics (type, dose) should be reassessed. If antibiotics are appropriate, depending on PCT changes ABs should either be continued or other sources of infection should be looked for. In case of inappropriate ABs and no clinical improvement, regardless of the PCT, therapy should be changed.
If there is clinical improvement but no proof of infection (micro: negative), based on PCT changes (↓ or ↑) infection may be excluded and ABs stopped, or continued. Similar algorithms can be applied if ABs are appropriate. If ABs are inappropriate and PCT decreases, then one may consider the microbiology as false positive and stop ABs, because it is highly unlikely that there is clinical improvement and decreasing PCT if an infection is not under control due to inappropriate ABs. This scenario happens when there are pathogens (colonization for example), but no infection. Finally, in case of inappropriate ABs and unfavourable PCT changes, consultation with infectologists and microbiologists is recommended.
This multimodal evaluation could help to individualize suspected infection management in the early course of sepsis on the ICU.
3. Stopping antibiotic therapy
Procalcitonin, mainly due to its favourable kinetic profile can potentially be a useful biomarker for also the cessation of antibiotic treatment [30]. In the first trial on 600 ICU patients, the PRORATA study [4], PCT-guided antibiotic management was tested. Antibiotics were encouraged in case of elevated PCT levels, and discouraged when levels were low. The novelty of this trial was that investigators were encouraged to discontinue antibiotics when PCT concentration was less than 80% of the peak value or when absolute concentration of less than 0.5 ng/ml was reached. The same protocol was repeated in a large recent study on 1500 patients by de Jong et al., in a multicenter prospective trial [31]. The results were similar just like the previous one applying this approach shortened the duration of antibiotic treatment and the daily dose antibiotic consumption, in addition the mortality in this group was significantly lower in the PCT-group as compared to conventionally treated patients. In spite of the reinfection rate being higher in the PCT guided group the cumulative cost of antibiotics per patient was significantly lower. Despite the significantly shorter antibiotic therapy, they were unable to show any difference in outcome between the groups, in other words patients did not suffer harm from not receiving antibiotics for the length of time recommended by guidelines.
CONCLUSION
In this deadly battle of fighting the burden of serious infections on the ICU, we often keep missing the point. Although sepsis exists, just like critical illness, but precisely defining it is probably impossible due to its diversity in etiology, pathomechanism and clinical manifestation. Therefore, interpreting the results of sepsis studies is a daunting task. Procalcitonin is definitely one of the most reliable inflammatory markers in the critically ill to date, and there is also convincing evidence that its use to guide antibiotic therapy can rationalize starting, escalating and stopping antibiotic therapy. Furthermore, when the concept, highlighted in this paper is applied, PCT may also become cost effective, by not starting at all, or stopping antibiotic therapy early. However, starting or stopping antibiotic treatment is more complex than just treating one single figure or even the kinetics of PCT values. A multimodal, individualized concept, consisting of a) recognizing organ dysfunction, b) identifying the possible source, c) following the clinical picture and d) taking PCT and PCT-kinetics into account, is necessary to make the most out of your PCT and to do the best for your patients in your everyday practice. Indeed, it requires well-trained, devoted, thinking physicians who dial in all information such as the results of physical examination, laboratory data, and physiologicmeasurements and make the decisions. Therefore, PCT is not the answer, but it can certainly help, considering that we understand what’s going on in our patients.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Cooper-smith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Klein-pell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304-377 [DOI] [PubMed] [Google Scholar]
- 2.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, Müller B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600-607 [DOI] [PubMed] [Google Scholar]
- 4.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Régnier B, Brun-Buisson C, Chastre J, Wolff M; PRORATA trial group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463-474 [DOI] [PubMed] [Google Scholar]
- 5.Layios N, Lambermont B, Canivet JL, Morimont P, Preiser JC, Garweg C, Ledoux D, Frippiat F, Piret S, Giot JB, Wiesen P, Meuris C, Massion P, Leonard P, Nys M, Lancellotti P, Chapelle JP, Damas P. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med. 2012;40:2304-2309 [DOI] [PubMed] [Google Scholar]
- 6.Jensen JU, Lundgren B, Hein L, Mohr T, Petersen PL, Andersen LH, Lauritsen AO, Hougaard S, Mantoni T, Bømler B, Thornberg KJ, Thormar K, Løken J, Steensen M, Carl P, Petersen JA, Tousi H, Søe-Jensen P, Bestle M, Hestad S, Andersen MH, Fjeldborg P, Larsen KM, Rossau C, Thomsen CB, Ostergaard C, Kjaer J, Grarup J, Lundgren JD. The Procalcitonin and Survival Study (PASS) – a randomised multi-centre investigator initiated trial to investigate whether daily measurements biomarker procalcitonin and pro-active diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. Calculated sample size (target population): 1000 patients. BMC Infect Dis. 2008;8:91-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, Walker A, Bailey MJ, Johnson B, Millis D, Ding G, Peake S, Wong H, Thomas J, Smith K, Forbes L, Hardie M, Micallef S, Fraser JF; ProGUARD Study Investigators; ANZICS Clinical Trials Group. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J RespirCrit Care Med. 2014;190:1102-1110 [DOI] [PubMed] [Google Scholar]
- 8.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. New Engl J Med. 1987;317:653-658 [DOI] [PubMed] [Google Scholar]
- 9.Bone RC, Fisher CJ, Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989;17:389-393 [PubMed] [Google Scholar]
- 10.[No authors listed] American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-874 [PubMed] [Google Scholar]
- 11.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartori M, Cosmi B, Legnani CJ, Favaretto E, Valdré L, Guazzaloca G, Rodorigo G, Cini M, Palareti G. The Wells rule and D-dimer for the diagnosis of isolated distal deep vein thrombosis. J ThrombHaemost. 2012;10:2264-2269 [DOI] [PubMed] [Google Scholar]
- 13.Cavaillon JM, Adrie C, Fitting C, Adib-Conqui M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res. 2005;11:311-320 [DOI] [PubMed] [Google Scholar]
- 14.Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Raoof M, Chen Y. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 201; 464:104-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589-1596 [DOI] [PubMed] [Google Scholar]
- 17.Dandona P, Nix D, Wilson MF, Aljada A, Love J, Assicot M, Bohuon C. Procalcitonin increase after endotoxin injection in normal subjects. J ClinEndocrinolMetab. 1994;79:1605-1608 [DOI] [PubMed] [Google Scholar]
- 18.Meisner Michael. Procalcitonin – Biochemistry and Clinical Diagnosis, 1st edn UNI-MED Science, Germany: 2010 [Google Scholar]
- 19.Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, Ritz R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977-983 [DOI] [PubMed] [Google Scholar]
- 20.Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596-2602 [DOI] [PubMed] [Google Scholar]
- 21.Pupelis G, Drozdova N, Mukans M, Malbrain ML. Serum procalcitonin is a sensitive marker for septic shock and mortality in secondary peritonitis. Anaesthesiol Intensive Ther. 2014;46:262-273 [DOI] [PubMed] [Google Scholar]
- 22.Clec’h C, Ferriere F, Karoubi P, Fosse JP, Cupa M, Hoang P, Cohen Y. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32:1166-1169 [DOI] [PubMed] [Google Scholar]
- 23.Clec’h C, Fosse JP, Karoubi P, Vincent F, Chouahi I, Hamza L, Cupa M, Cohen Y. Differential diagnostic value of procalcitonin in surgical and medical patients with septic shock. Crit Care Med. 2006;34:102-107 [DOI] [PubMed] [Google Scholar]
- 24.Trásy D, Tánczos K, Németh M, Hankovszky P, Lovas A, Mikor A, László I, Hajdú E, Osztroluczki A, Fazakas J, Molnár Z. EProK study group. J Crit Care. 2016;34:50-55 [DOI] [PubMed] [Google Scholar]
- 25.Trásy D, Tánczos K, Németh M, Hankovszky P, Lovas A, Mikor A, Hajdú E, Osztroluczki A, Fazakas J, Molnár Z. Delta Procalcitonin Is a Better Indicator of Infection Than Absolute Procalcitonin Values in Critically III Patients: A Prospective Observational Study. J Immunol Res. 2016;2016:3530752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garnacho-Montero J, Huici-Moreno MJ, Gutierrez-Pizarraya A, López I, Márquez-Vácaro JA, Macher H, Guerrero JM, Puppo-Moreno A. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18: R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohl CA, Luther VP. Antimicrobial stewardship for inpatient facilities. J Hosp Med. 2011;1:S4-S15 [DOI] [PubMed] [Google Scholar]
- 28.Charles PE, Tinel C, Barbar S, Aho S, Prin S, Doise JM, Olsson NO, Blettery B, Quenot JP. Procalcitonin kinetics within the first days of sepsis: relationship with the appropriateness of antibiotic therapy and the outcome. Crit Care. 2009;13:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mettler J, Simcock M, Sendi P, Widmer AF, Bingisser R, Battegay M, Fluckiger U, Bassetti S. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: a prospective observational study. BMC Infect Dis. 2007;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, von Spiegel T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394:221-226 [DOI] [PubMed] [Google Scholar]
- 31.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, Loef BG, Dormans T, van Melsen GC, Kluiters YC, Kemperman H, van den Elsen MJ, Schouten JA, Streefkerk JO, Krabbe HG, Kieft H, Kluge GH, van Dam VC, van Pelt J, Bormans L, Otten MB, Reidinga AC, Endeman H, Twisk JW, van de Garde EM, de Smet AM, Kesecioglu J, Girbes AR, Nijsten MW, de Lange DW. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-827 [DOI] [PubMed] [Google Scholar]


