Abstract
Faithful chromosome segregation during mitosis requires that the kinetochores of all sister chromatids become stably connected to microtubules derived from opposite spindle poles. How stable chromosome bi-orientation is accomplished and coordinated with anaphase onset remains incompletely understood. Here we show that stable chromosome bi-orientation requires inner centromere localization of the non-enzymatic subunits of the chromosomal passenger complex (CPC) to maintain centromeric cohesion. Precise inner centromere localization of the CPC appears less relevant for Aurora B-dependent resolution of erroneous kinetochore–microtubule (KT–MT) attachments and for the stabilization of bi-oriented KT–MT attachments once sister chromatid cohesion is preserved via knock-down of WAPL. However, Aurora B inner centromere localization is essential for mitotic checkpoint silencing to allow spatial separation from its kinetochore substrate KNL1. Our data infer that the CPC is localized at the inner centromere to sustain centromere cohesion on bi-oriented chromosomes and to coordinate mitotic checkpoint silencing with chromosome bi-orientation.
Precise chromosome segregation during mitosis requires coordination of stable chromosome bi-orientation with anaphase onset, however the underlying mechanism is not clear. Here the authors show that inner centromere localization of the chromosomal passenger complex maintains centromeric cohesion on bi-oriented chromosomes and allows mitotic checkpoint silencing.
Accurate transmission of the genome during cell division requires that the duplicated chromosomes become faithfully segregated in mitosis. Failure to equally segregate the sister chromatids can result in gains and losses of whole chromosomes in the next generation of cells, a condition known as aneuploidy, which is frequently observed in cancer1. A prerequisite for error-free segregation is that all sister chromatids bi-orient on the mitotic spindle. This means that specialized multi-protein complexes that assemble at the centromeres of the sister chromatids (that is, kinetochores, KTs) become stably attached to microtubules (MTs) emanating from opposite poles of the mitotic spindle2. Chromosome bi-orientation is facilitated by several molecular mechanisms, including centromeric cohesion, the mitotic checkpoint and the chromosomal passenger complex3,4,5. Centromeric cohesin holds the sister-chromatids together until anaphase onset and promotes a back-to-back orientation of sister-kinetochores favoring bipolar microtubule capture3. The mitotic checkpoint prevents anaphase onset until all kinetochores have become attached to spindle microtubules and as such provides the necessary time for all chromosomes to bi-orient. And third, the chromosomal passenger complex (CPC), consisting of INCENP, survivin, borealin and Aurora B kinase, detects and resolves improper kinetochore-spindle connections that, when left uncorrected, would give rise to chromosome mis-segregations6. Detachment of incorrect kinetochore–microtubule (KT–MT) connections is a consequence of Aurora B-mediated phosphorylation of outer-kinetochore substrates, including components of the KMN (KNL1, MIS12 and NDC80 complex) network, which directly interact with microtubules7,8. Upon chromosome bi-orientation, the opposing pulling forces of the attached KT–MTs are resisted by centromeric cohesin, resulting in tension across sister kinetochores. Tension is thought to pull the outer-KT substrates out of the sphere of influence of Aurora B, resulting in the stabilization of bi-oriented attachments and anaphase onset9,10,11. Central to this ‘spatial separation' model is the inner centromere localization of Aurora B. However, there is ongoing debate whether this confined localization of Aurora B is indeed essential for chromosome bi-orientation6: apart from its localization at the inner centromere, a small pool of active Aurora B at or near the kinetochore has been described in mammalian cells and suggested to control KT–MT stability12,13. Moreover, tension was shown to directly stabilize in vitro reconstituted KT–MT attachments14, and Aurora B localization to the inner centromere appeared not to be required for faithful chromosome segregation in budding yeast or for viability of chicken DT40 cells15,16. This raised the question what function is executed by the inner centromere pool of Aurora B. Using various INCENP fusion proteins we subtly manipulated the chromosomal localization of Aurora B in human cells and found that, similar to the situation in budding yeast, precise inner centromere localization of the kinase was not required for either error correction or the stabilization of bi-orientated attachments. Yet, we found that, inner centromere localization of Aurora B supported silencing of the mitotic checkpoint once all chromosomes had bi-oriented. In addition, we found that inner centromere localization of the non-enzymatic subunits of the CPC appeared to be essential to maintain centromeric cohesion after chromosome bi-orientation and hence to prevent precocious sister chromatid separation before satisfaction of the mitotic checkpoint.
Results
Stable bi-orientation requires inner centromere localization of the CPC
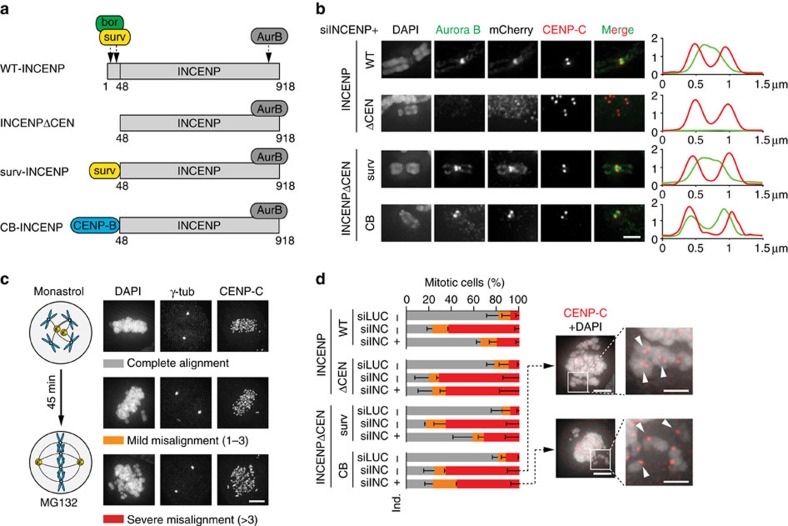
To study the function of inner centromere-localized Aurora B during mammalian mitosis, we made use of HeLa cell lines that ectopically expressed variants of the CPC scaffold protein INCENP from an inducible promoter, in conjunction with siRNA mediated knockdown of endogenous INCENP (Fig. 1a, Supplementary Fig. 1a). The N-terminal inner centromere-targeting domain (CEN-box, amino acids 1–47) of INCENP, which interacts with the CPC members survivin and borealin17, was either deleted (INCENPΔCEN) or replaced with different targeting moieties: Survivin (surv-INCENP), which re-localizes Aurora B to the inner centromere, or the centromere-targeting domain of CENP-B (CB-INCENP), which changes the position of Aurora B from predominantly inner centromeric to more prominent near the kinetochore. (Fig. 1a,b)11,18,19. Although some Aurora B remained associated with centromeric heterochromatin in CB-INCENP expressing cells, we will refer to this CB-INCENP-shifted pool of Aurora B as ‘kinetochore proximal'.
Figure 1. Inner centromere localization of the CPC is required for stable chromosome bi-orientation.
(a) Scheme of human CPC (bor, borealin; surv, survivin; AurB, Aurora B) and of INCENPΔCEN (deletion of aa 1–48), survivin-INCENPΔCEN (surv-INCENP) and CENP-B-INCENPΔCEN (CB-INCENP). In all experiments HeLa Flp-In T-REx cells expressing the indicated mCherry-tagged INCENP variants were used.+ ind., expression induced by doxycycline, − ind., no induction of expression. (b) IF of Aurora B, RFP (to detect mCherry), and CENP-C on chromosome spreads of nocodazole treated cells. 1D line graphs of Aurora B (green) and CENP-C (red) are shown on the right. Scale bar, 2 μm. Of note, although surv-INCENP and Aurora B accumulate at the inner centromere, in surv-INCENP-expressing prometaphase cells we also observed some localization of surv-INCENP and Aurora B over the chromosomal arms. Since survivin directly interacts with pH3T3 (refs 47, 48) and we frequently detected pH3T3 along the chromosomal arms, this most likely explains the additional arm localization. (c) Scheme of the bi-orientation assay (that is, release from a monastrol-induced mitotic arrest into medium containing MG132) and examples of the alignment categories. Scale bar, 5 μm. (d) Cells were transfected with siRNAs for Luciferase (siLUC) or INCENP (siINC) and subjected to the bi-orientation assay and chromosome alignment was assessed (n=2 exp., ≥150 cells per condition, error bars are s.e.m.). Representative images of two conditions (scale bar, 5 μm), and enlargements of selected image regions are shown on the right (scale bar, 2 μm). DNA is visualized using DAPI.
Analysis of INCENPΔCEN expressing cells, confirmed that removal of the CEN-box disrupted the inner centromere localization of Aurora B, similar to what has been reported for Sli15-delta N-terminus (Sli15-dNT), its analogue in S. cerevisiae (Fig. 1b, Supplementary Fig. 1b,c)16,19,20. Unlike Sli15-dNT, which supported chromosome bi-orientation in budding yeast, INCENPΔCEN did not rescue chromosome alignment in human cells lacking endogenous INCENP. In fact, we found a strong correlation between the inner centromere localization of Aurora B and stable chromosome bi-orientation in human cells (Fig. 1b–d).
Loss of inner centromere localized CPC weakens centromeric cohesion
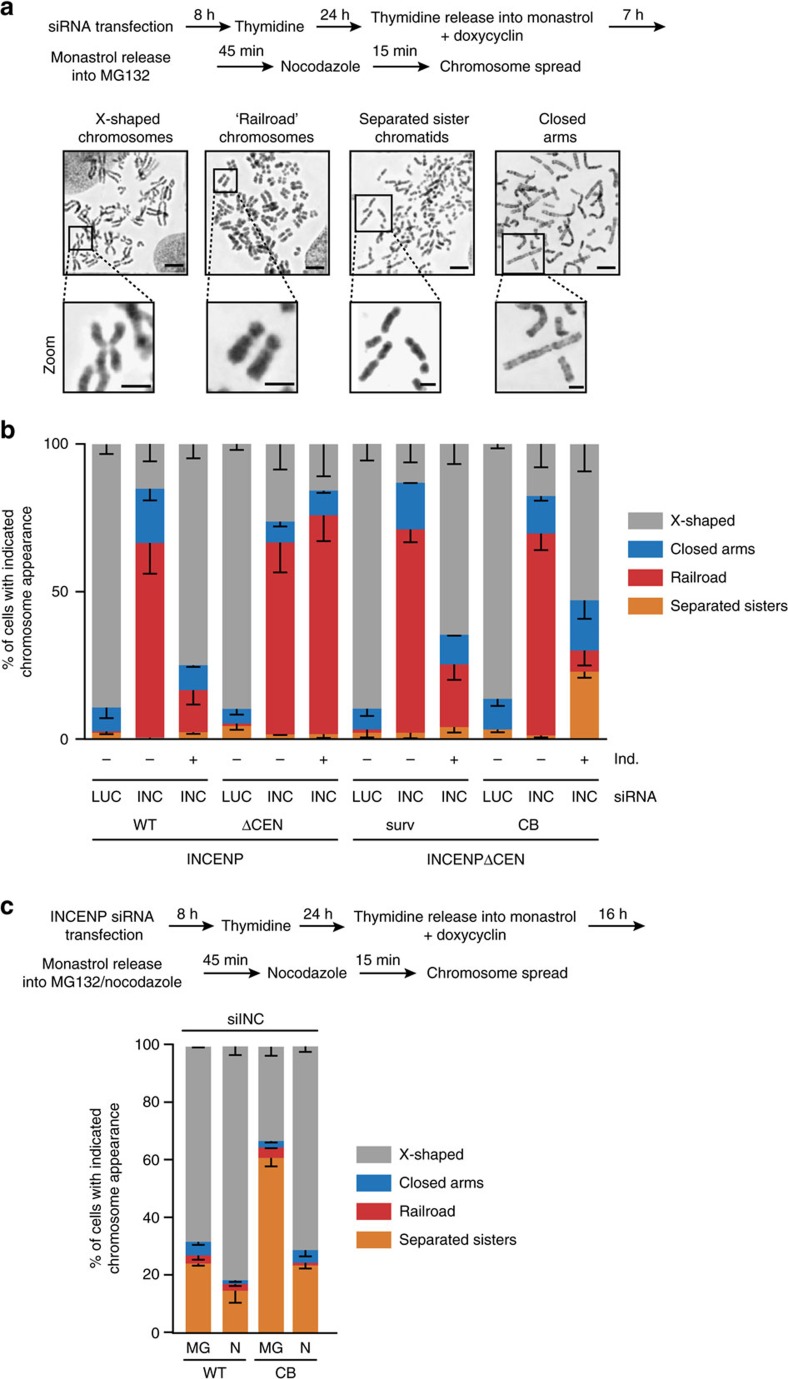
Close inspection of the misaligned chromosomes in cells with kinetochore-proximal Aurora B (that is, CB-INCENP-expressing cells) revealed the appearance of single sister chromatids. This was in marked contrast to cells lacking INCENP in which the misaligned chromosomes appeared as sister chromatid pairs (Fig. 1d, inset). This suggested that CB-INCENP expressing cells experienced problems in maintaining sister chromatid cohesion after bi-orientation, and we hypothesized that the inner centromere pool of Aurora B might be needed to stabilize centromeric cohesion21. Indeed, chromosome spreads of INCENP-depleted cells revealed that sister chromatids behaved as ‘railroads' (Fig. 2a,b), a phenotype seen in certain cohesinopathies, and explained by reduced centromeric cohesion, while most likely retaining at least some cohesion along the chromosomal arms, given the juxtaposition of the sister chromatids22,23 (Fig. 2a). The railroad chromosome phenotype was rescued after expression of WT-INCENP and surv-INCENP, but these chromosomes were still present in INCENP-ΔCEN expressing cells (Fig. 2b). This observation supports earlier evidence that Aurora B kinase activity is involved in cohesin removal from the chromosomal arms during prophase24,25,26,27, but is also needed for protection of centromeric cohesin21,26,28. The observation that INCENP-ΔCEN, which in human cells is mainly cytosolic and on certain fixation conditions slightly visible on chromatin (Supplementary Fig. 5), failed to rescue the cohesion defects induced by INCENP depletion (Fig. 2a,b), suggested that both the loss of arm cohesion, and as well as the cohesion protection at the centromere required robust chromosomal association (and maybe clustering) of INCENP and Aurora B. In cells expressing CB-INCENP we also no longer observed railroad chromosomes, but instead found an increase in the number of mitotic cells with fully separated sister chromatids (<5% for WT-INCENP versus >20% for CB-INCENP expressing cells, Fig. 2a,b). Remarkably, the fraction of cells with fully separated sister chromatids increased when the monastrol treatment, used to synchronize cells in mitosis, was prolonged from 7 to 16 h (∼24% for WT-INCENP versus ∼61% for CB-INCENP expressing cells, Figs 2c and 4g). This increase may be explained by a slow but gradual degradation of securin during a prolonged mitotic arrest, similar to what has been observed for cyclin B29,30. This most likely liberates a number of separase molecules from securin inhibition, which can cleave some of the cohesin rings, thereby ‘weakening' sister chromatid cohesion.
Figure 2. Loss of inner centromere localized CPC weakens centromeric cohesion.
(a,b) Cells were transfected with the indicated siRNAs, and synchronized according to the scheme (a), After the 45 min. accumulation in MG132 chromosome spreads were prepared (a) and the percentage of cells in which all chromosomes appeared as ‘X-shaped', ‘railroads', with ‘closed arms', or in which all sister chromatids were fully separated, was quantified (b, n=2 exp., ≥185 cells per condition, error bars are s.e.m.). Representative images of the different categories are shown in a. Scale bars, 5 μm, resp. 2 μm in zoom. (c) Cells were transfected with siINCENP and treated according to the depicted scheme. After a 16 h monastrol, treatment cells were either released into MG132 (MG) or into nocodazole (N). Chromosome spreads were scored for the indicated chromosome appearance (n=2 exp., ≥210 cells percondition, error bars are s.e.m.).
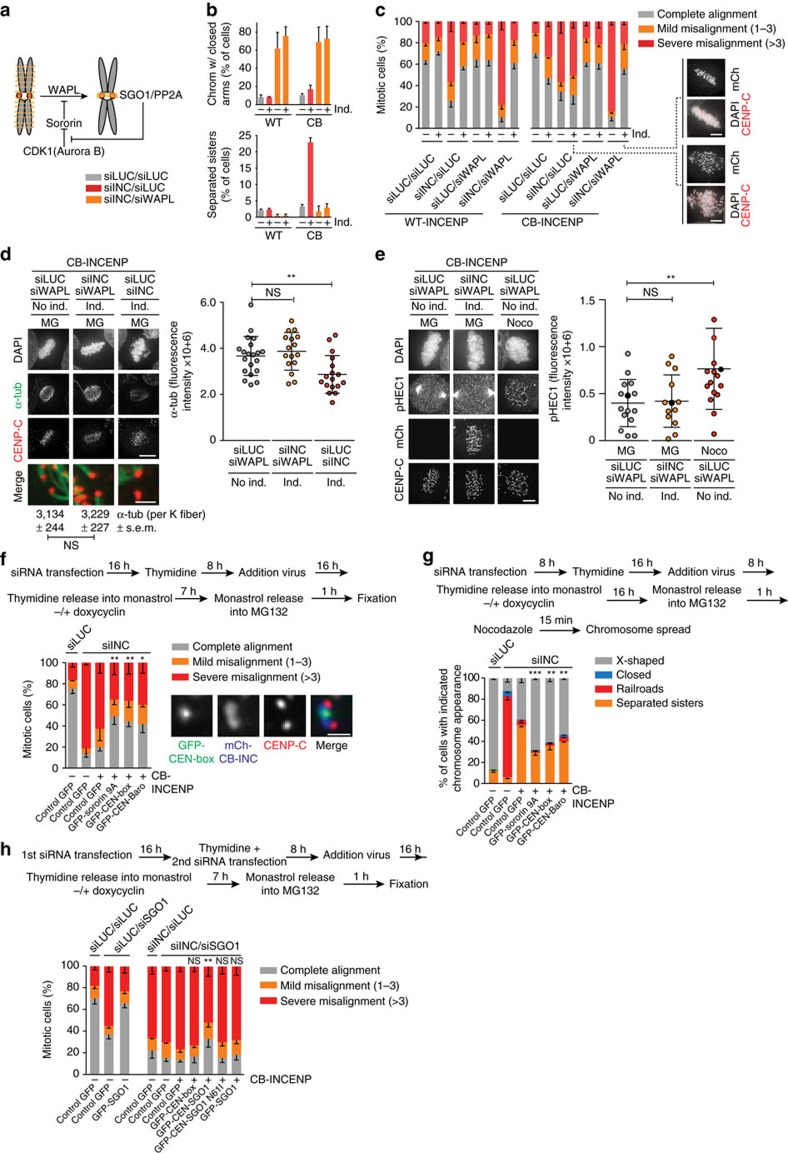
Figure 4. Cohesin maintenance rescues chromosome bi-orientation and promotes stable KT–MT attachments in CB-INCENP expressing cells.
(a) Scheme of the regulation of WAPL-dependent cohesin removal from chromosomal arms. (b) Quantification of the % of cells with chromosomes with fully closed arms (upper graph) and with fully separated sisters (lower graph) as a measure for efficiency of the siRNA-mediated WAPL knockdown (n=2 exp., ≥201 cells per condition, error bars are s.e.m.). Note that the conditions without siWAPL are also presented in Fig. 2b. (c) Cells were transfected with the indicated siRNAs and subjected to the bi-orientation assay (Fig. 1c). Chromosome alignment was assessed (n=3 experiments, ≥159 cells per condition, error bar is s.e.m., ns=not significant; ****P<0.0001; Chi-squared test for comparison of the indicated groups for % complete alignment). Representative images of two conditions are shown. (d) IF for α-tubulin and CENP-C in cells transfected with the indicated siRNAs and subjected to the bi-orientation assay followed by ice-cold treatment (Scale bar, 5 μm). FI quantifications of spindle α-tubulin (1 out of 2 exp., ≥16 cells per condition, bars: mean±s.d., ns=not significant; **P<0.01; unpaired t test). Numbers below merged images (scale bar: 1 μm) depict the mean FI of ≥17 individual K fibers. (e) IF for phospho-HEC1 (Ser44, pHEC1), mCherry and CENP-C (1 out of 2 exp., ≥13 cells per condition, bars: mean±s.d., ns, not significant; **P<0.01; unpaired t test). The corresponding data points of the images (scale bar: 5 μm) are coloured black in the graph. DNA is visualized using DAPI. Spindle poles were excluded from the quantification. (f,h) Cells±induction of CB-INCENP, were subjected to the depicted experimental set-up (siSGO1 was co-transfected directly after thymidine addition (h)), and chromosome alignment was assessed (n=3 exp, ≥240 cells per condition in (f) and ≥208 cells per condition in h, error bars are s.e.m., ns=not significant; ***P<0.001; Chi-squared test for differences between the indicated groups and the control GFP,+CB-INCENP group, for % complete alignment). Localization of GFP-CEN-box is shown in the IF images f. Scale bar, 1 μm. (g) Cells±induction of CB-INCENP, were subjected to the depicted experimental set-up and chromosome spreads were scored for the indicated categories (n=2 exp., ≥312 cells per condition, error bars are s.e.m., **P<0.01, ***P<0.001; Chi-squared test for differences between the indicated groups and the control GFP,+CB-INCENP group, for % separated sisters). Note that due to the fixation procedure used for the preparation of chromosome spreads, fluorescence was quenched and we could therefore not select for transduced cells. Hence we determined the frequency of cells with the indicated chromosome appearance in the total cell population. The observed rescue effect observed for sororin−9A, CEN-box and CEN-Baronase is most likely an underestimation.
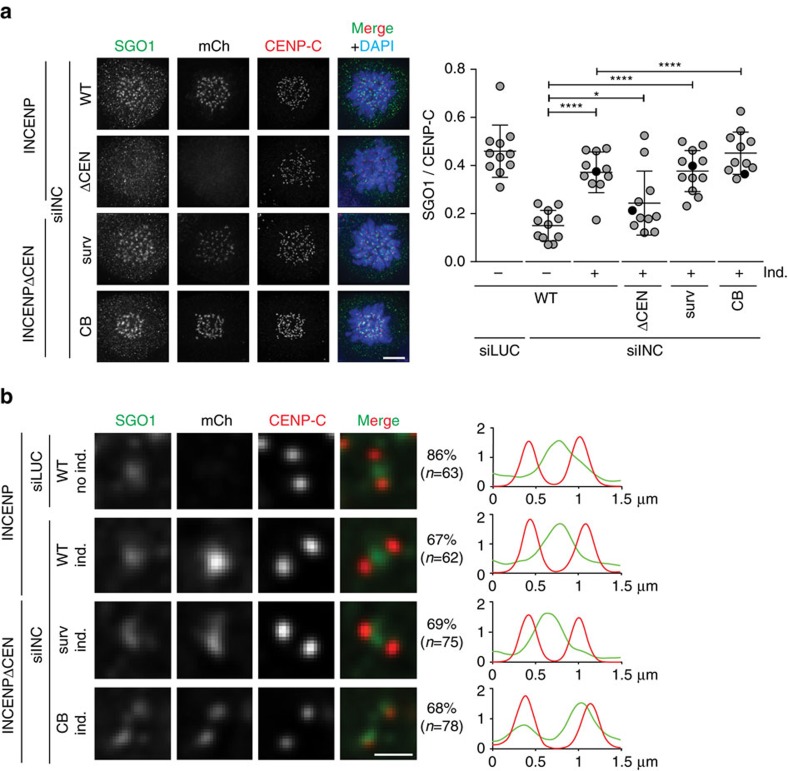
Collectively, our experiments suggest that CB-INCENP-localized Aurora B is proficient in resolving chromosomal arm cohesion, but insufficient in maintaining centromere cohesion. In line with these findings, we observed that loss of the CPC from the inner centromere correlated with a loss of the cohesin protector SGO1 from this site in prometaphase cells. In INCENP knockdown or INCENPΔCEN expressing cells, SGO1 was absent from both the inner centromere and kinetochores (Fig. 3a), in agreement with previous work26,31. Inner centromere localization of SGO1 was restored by expression of WT-INCENP and surv-INCENP, but not by expression of CB-INCENP (Fig. 3a). In the latter SGO1 was predominantly found at kinetochores (Fig. 3b), a SGO1 pool that does not support centromeric cohesion32,33,34. Thus our data show that inner centromere localized CPC correlates with inner centromere positioning of SGO1 and maintenance of centromeric cohesion after chromosome bi-orientation.
Figure 3. SGO1 is predominantly localized at kinetochores in CB-INCENP expressing cells.
(a) IF and quantification of fluorescence intensities (FI) of SGO1/CENP-C on centromeres and/or kinetochores in the depicted cell lines, transfected with the indicated siRNAs, and blocked in mitosis using STLC (1 exp. out of 2, 11 cells per condition, bars: mean±s.d., ns, not significant; *P<0.05; ****P<0.0001; unpaired t test). DNA is visualized using DAPI. Scale bar, 5 μm. (b) Representative sister kinetochores with SGO1, mCherry and CENP-C and 1D line graphs of SGO1 (green) and CENP-C (red) of cells transfected with the indicated siRNAs, blocked in mitosis using STLC and expressing INCENP variants that rescued SGO1 centromere/kinetochore localization (a). The percentage of kinetochore pairs showing that particular SGO1 staining pattern is shown on the right side of the images. Scale bar, 1 μm.
WAPL depletion rescues bi-orientation in cells with kinetochore-proximal Aurora B
We then asked whether reduced centromeric cohesion was causing the bi-orientation defect in CB-INCENP-expressing cells (Fig. 1d). To test this, we prevented cohesin removal by knockdown of the cohesin release factor WAPL or by overexpression of a sororin mutant (sororin-9A) that acts as a constitutive WAPL inhibitor (Fig. 4a,b)35,36,37,38. Indeed, retention of cohesin rescued chromosome alignment in CB-INCENP expressing cells (Fig. 4c,f). In our hands, WAPL depletion was slightly more effective in restoring chromosome bi-orientation than WAPL inhibition via overexpression of sororin-9A (Fig. 4c,f). On the other hand in an INCENP knock-down background, WAPL depletion appeared to exacerbate the bi-orientation defect (Fig. 4c), most likely because retention of arm cohesin delocalizes residual CPC from the inner centromere39. Remarkably though, even with Aurora B near kinetochores (Fig. 1b), bipolar, tension-generating, cold-stable end-on KT–MT attachments could be established when WAPL was depleted (Fig. 4d, Supplementary Fig. 2a,b). In agreement with this, the N-terminal tail of the Aurora B kinetochore substrate and microtubule binding protein HEC1/NDC80, was also no longer phosphorylated (Fig. 4e)12. Since Aurora B was capable of phosphorylating HEC1 Ser44 in CB-INCENP expressing cells in which microtubules were depolymerized by nocodazole, the absence of HEC1 phosphorylation in metaphase was unlikely to be due to a loss of function of the fusion protein (Supplementary Fig. 2c). Altogether, this argued that the bi-orientation defect observed after Aurora B displacement in CB-INCENP expressing cells, was a consequence of weakened centromeric cohesion that is unable to resist the opposing pulling forces originating from the attached KT–MT, a phenomenon known as cohesion fatigue40. In line with this, precocious sister chromatid separation in CB-INCENP expressing cells was most prominent, when cells were allowed to bi-orient on the mitotic spindle (release from monastrol into MG132) than when microtubules were absent (release from monastrol into nocodazole)(Fig. 2c).
Stable centromeric cohesion requires the CEN-box of INCENP
We next considered two possibilities why centromeric cohesion was less robust in CB-INCENP expressing cells: stable centromeric cohesion requires a pool of active Aurora B at the inner centromere, or it requires presence of the N-terminal CEN-box of INCENP, missing in CB-INCENP. The latter possibility predicts that expression of the CEN-box would be sufficient to rescue chromosome alignment in CB-INCENP expressing cells. In fact, this prediction appeared to be true; re-introduction of the CEN-box in CB-INCENP expressing cells rescued chromosome alignment to a similar extent as expression of a sororin-9A mutant, which has been reported to bypass the requirement for SGO1-PP2A in maintaining centromeric cohesion (Fig. 4f, Supplementary Fig. 3a–d)25,37. Fusing an active form of Aurora B (Baronase41) onto the CEN-box, guided Baronase localization to the inner centromere but did not further improve chromosome bi-orientation, indicating that the CEN-box itself is critical in stabilizing centromeric cohesion (Fig. 4f, Supplementary Fig. 3b). Indeed, analysis of chromosome spreads revealed that either transduction of sororin-9A or of the CEN-box in CB-INCENP expressing cells diminished the number of cells with fully separated sister chromatids and increased the fraction of cells with X-shaped chromosomes (Fig. 4g). Of note, the observation that sororin-9A expression predominantly rescued centromeric cohesion in CB-INCENP expressing cells instead of both arm and centromere cohesion as observed on WAPL depletion (Fig. 4b,g), is similar to what has been reported for the level of cohesion rescue by non-phosphorylatable sororin mutants in SGO1 depleted cells25,37. To test whether stabilization of centromeric cohesion involved inner centromere localized SGO1. We co-depleted endogenous INCENP and SGO1 in CB-INCENP expressing cells and re-introduced exogenous SGO1 or a fusion protein consisting of the CEN-box and SGO1 (CEN-SGO1)(Fig. 4h, Supplementary Fig. 3b). While exogenous SGO1 was capable of restoring chromosome bi-orientation in SGO1-depleted cells, it was incapable in INCENP and SGO1 double knockdown cells (Fig. 4h). Similarly, expression of the CEN-box did not support chromosome alignment in CB-INCENP expressing cells in which both INCENP and SGO1 were depleted (Fig. 4h). However, expression of a CEN-SGO1 fusion protein that localized to the inner centromere when CB-INCENP was expressed (Supplementary Fig. 3b), improved chromosome bi-orientation in INCENP/SGO1 double knockdown cells (Fig. 4h). Importantly, a CEN-SGO1 N61I mutant that does not bind PP2A did not improve chromosome bi-orientation, highlighting the importance of PP2A in SGO1-dependent cohesion protection (Fig. 4a,h)42,43,44. Taken together, our data suggest that the CEN-box in INCENP, either directly or through association with borealin and survivin, is needed to prevent precocious sister chromatid separation on chromosome bi-orientation. Our data further imply that Aurora B itself does not need to reside at the inner centromere to maintain centromeric cohesion or to allow the stabilization of bi-oriented KT–MT attachments (Fig. 4d,g, Supplementary Fig. 2a). We found that Aurora B localized near the kinetochore in CB-INCENP expressing cells, can correct erroneous KT–MT attachments (Fig. 4c), and provide the necessary positive feedback for Haspin-induced H3-T3 phosphorylation, and BUB1-mediated H2A-T120 phosphorylation, both of which are required for inner centromere recruitment of the CEN-box (Supplementary Fig. 3b–d)45,46,47,48. This raised the question why Aurora B kinase needs to be confined to the inner centromere.
Inner centromere localization of Aurora B supports mitotic checkpoint silencing
Microtubules that are stably attached to kinetochores are expected to silence the mitotic checkpoint49,50. However, we measured a significant metaphase delay in the CB-INCENP expressing cells in which we had knocked down WAPL (265 min, s.d.=128 min versus 52 min, s.d.=27 min and 50 min, s.d.=29 min, in respectively WAPL-depleted cells with endogenous INCENP or with ectopic WT-INCENP expression, Fig. 5a, Supplementary Fig. 4a). This suggested that despite stable bi-oriented attachments (Fig. 4d, Supplementary Fig. 2a), silencing of the mitotic checkpoint was impaired.
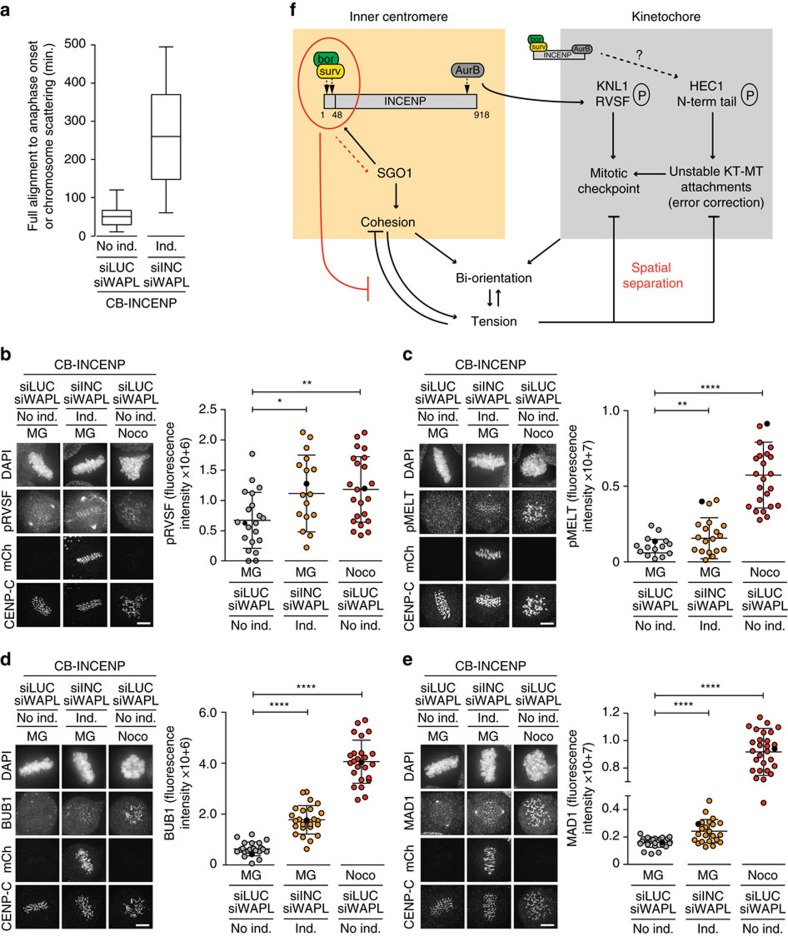
Figure 5. Spatial separation of Aurora B from KNL1 coordinates mitotic checkpoint silencing with bi-orientation.
(a) Time in metaphase for WAPL-and/or INCENP depleted cells±CB-INCENP (n=≥21 cells, see also Supplementary Fig. 4a–d). (b–e) IF of mCherry, CENP-C and KNL1-pRVSF-S60 (pRVSF) (b), KNL1-pMELT-T601 (pMELT) (c), BUB1 (d) and MAD1 (e) in cells±induction of CB-INCENP, transfected with indicated siRNAs and subjected to the bi-orientation assay. Quantifications of fluorescence intensities are shown on the right side of each panel (1 out of 2 exp., n=≥18 cells per condition, bars: mean±s.d., *P<0.05; **P<0.01; ****P<0.0001; unpaired t test, spindle poles were excluded from the quantifications). The corresponding data points of the images (scale bars, 5 μm) are coloured black in the graphs. (f) Model for how the CPC regulates the inner centromere and kinetochore to allow the build-up of tension upon bi-orientation, to counteract cohesion fatigue upon bi-orientation and tension, and to coordinate mitotic checkpoint silencing with chromosome bi-orientation.
The kinetochore protein KNL1 is an important signalling hub for the mitotic checkpoint. It recruits several checkpoint proteins including BUB1, BUB3, BUBR1 and MAD1, after phosphorylation of its MELT motifs by MPS1 (ref. 51). Phosphorylation of the KNL1 MELT motifs is antagonized by protein phosphatase 1 gamma (PP1γ), which is recruited to the N-terminal RVSF motif in KNL1 (refs 52, 53). Phosphorylation of this RVSF motif by Aurora B hampers PP1γ binding to KNL1 allowing optimal MELT phosphorylation and mitotic checkpoint activity52,53. Conversely, RVSF phosphorylation needs to go down in metaphase to allow kinetochore recruitment of PP1γ and silencing of the mitotic checkpoint53. We found that the phosphorylation status of the RVSF motif in KNL1 was enhanced on metaphase chromosomes of WAPL-depleted cells with Aurora B localized near kinetochores (Fig. 5b, Supplementary Fig. 2d). This correlated with enhanced MELT phosphorylation, increased kinetochore recruitment of BUB1, and low levels of MAD1 on all metaphase kinetochores (Fig. 5c–e, Supplementary Fig. 4e). Altogether, our data suggest that inner centromere localization of Aurora B is needed to spatially separate the kinase from its substrate KNL1, to promote mitotic checkpoint silencing on chromosome bi-orientation. As mentioned, in contrast to KNL1, the N-terminal tail of HEC1 was no longer phosphorylated and KT–MT attachments were stable in CB-INCENP expressing, WAPL-depleted cells (Fig. 4d,e, Supplementary Fig. 2a). This either implies that spatial separation of Aurora B from its KT substrates may be a more relevant mechanism for mitotic checkpoint silencing than to allow the dephosphorylation of the HEC1 N-terminus and the stabilization of bi-oriented attachments, or that due to increased tension (Supplementary Fig. 2b), even CB-INCENP positioned Aurora B can no longer reach HEC1. To discriminate between these two possibilities we expressed a fusion protein of INCENPΔCEN and the kinetochore protein MIS12 (MIS12-INCENP), to force localization of Aurora B at kinetochores11. However, in an INCENP knock-down background, the MIS12-INCENP expression levels did not restore phosphorylation of CENP-A and DSN1 to levels comparable to WT-INCENP expressing cells (Supplementary Fig. 5a–c), making the knock-down, add-back set-up unsuitable. We chose to perform experiments in the presence of endogenous INCENP and in the absence of WAPL, since this would allow us to analyse the mere consequence of maintaining a small pool of kinetochore-localized Aurora B while avoiding KT–MT attachment problems due to overall reduced levels of active Aurora B, or due to cohesion loss (Supplementary Figs 5c,6a). Cells were synchronized in G2 and subsequently released from a CDK1-inhibitor block to allow progression through mitosis (Supplementary Fig. 6b). Unlike WT-INCENP expressing cells, MIS12-INCENP expression resulted in an increased frequency of cells in metaphase. This metaphase accumulation was even more pronounced in MIS12-INCENP expressing cells than in CB-INCENP expressing cells, in which all endogenous Aurora B was redistributed in closer proximity of the KT (Supplementary Fig. 6a,c). This indicated that the small pool of KT-localized Aurora B in MIS12-INCENP expressing cells was highly effective in preventing mitotic checkpoint silencing and in delaying anaphase onset. Interestingly, when blocking cells in metaphase with MG132 for 90 min., we found that MIS12-INCENP expressing, WAPL depleted cells, remained fully aligned, similar to WT-INCENP and CB-INCENP expressing cells (Supplementary Fig. 6d). This implied that kinetochore-localized Aurora B did not detach bi-oriented attachments when centromere cohesion was preserved.
Discussion
Our work demonstrates that in human mitotic cells, inner centromere localization of the CPC is a prerequisite for stable chromosome bi-orientation because it ensures stable centromeric cohesion of the sister chromatids. Stable centromeric cohesion not only promotes tension across sister kinetochores on bi-orientation, but also prevents precocious sister chromatid separation due to cohesion fatigue when bipolar KT–MT attachments have been established (Fig. 5f). Prevention of cohesion fatigue is most likely critical for the first sister-chromatids pairs that have obtained tension-generating, bipolar attachments and that need to ‘wait' for the other sister chromatid pairs to become properly connected to the mitotic spindle. The centromere cohesion protective function of the CPC is dependent on the CEN-box of INCENP and requires Aurora B kinase activity to provide the necessary feedback for H3-T3 and H2A-T120 phosphorylation that promotes inner centromere docking of the CEN-box54,55,56. How the CEN-box confers centromeric cohesion stability remains to be resolved. Because direct fusion of survivin to INCENPΔCEN also rescued chromosome cohesion defects and chromosome bi-orientation, we consider it likely that the non-enzymatic CPC core complex17, consisting of the INCENP CEN-box, survivin and borealin, mediates this cohesion protective function. Moreover, the observation that the localization of the cohesin protector SGO1 was shifted from the inner centromere to kinetochores in CB-INCENP expressing cells, suggests a role for the CPC core complex in either driving SGO1 towards or retaining the cohesin protector at the inner centromere. However, since we were unable to assess the exact SGO1 location in CEN-box reconstituted cells, we cannot exclude the possibility that the CEN-box may also act independent of SGO1 in stabilizing centromeric cohesion, for instance by counteracting the decatenation of centromeric DNA. Interestingly, in budding yeast, the Sli15-dNT (the budding yeast analogue of INCENP-ΔCEN) mutant is synthetic lethal with mcm21Δ, ctf19Δ, and ctf18Δ (ref. 16). Although the mechanistic underpinning of this synthetic lethality is unclear, the observation that proteins encoded by these genes are involved in either recruitment or loading of (pericentromeric) cohesin57,58,59, suggested a potential functional connection of chromatin-localized CPC with cohesion16. Here we provide experimental evidence that the N-terminus of INCENP, most likely in conjunction with survivin and borealin, is critical for maintenance of centromeric cohesion after chromosome bi-orientation in human cells (Fig. 5f).
Remarkably, Aurora B does not need to be localized at the inner centromere to provide feedback for histone phosphorylation. Neither does Aurora B have to be at the inner centromere for its KT–MT error correction activity or to allow stabilization of correctly attached KT–MTs. This latter finding is in line with the situation in budding yeast in which truncation of the Sli15 N-terminus (which results in localization of the Aurora B homologue Ipl1 to kinetochores and maybe spindle microtubules instead of the inner centromere) only has a very minor effect on the fidelity of chromosome bi-orientation and segregation16. However, unlike Ipl1 in budding yeast, inner centromere confinement of Aurora B in human cells appears important to silence the mitotic checkpoint. Failure to silence the checkpoint is lethal in budding yeast60, however Sli15-dNT mutants are viable16, strongly suggesting that mitotic checkpoint silencing is not impaired in these mutants. We propose that the inner centromere localization of Aurora B allows it to become spatially separated from its kinetochore substrate KNL1 thereby supporting the recruitment of PP1 (Fig. 5f). We favour this model because both in prometaphase and metaphase Aurora B is mainly detected at the inner centromere. However, since the existence of a small but highly active KT pool of Aurora B has been suggested12,13, an alternative scenario could be that a KT pool of endogenous Aurora B is responsible for the phosphorylation of KNL1-RVSF. Since this putative KT pool of Aurora B is absent from bi-orientated chromosomes12, tension-dependent removal of this pool from kinetochores might be a way to allow the recruitment of PP1 by KNL1 and to support mitotic checkpoint silencing (Fig. 5f). Obviously, in CB-INCENP expressing cells the removal of this potential KT pool of Aurora B is bypassed. Although we were unable to visualize PP1 and could therefore not directly assess whether PP1 was absent from metaphase kinetochores in CB-INCENP expressing cells, the observed low levels of MELT phosphorylation, and BUB1 and MAD1 kinetochore levels, strongly suggest PP1 recruitment was hampered. While mitotic checkpoint silencing in budding yeast also requires PP1 kinetochore recruitment by KNL1 (known as Spc105 in budding yeast), there is currently no evidence supporting a role for Ipl1 in counteracting this Spc105-dependent PP1 recruitment60,61, and this most likely explains why Sli15-dNT mutants can tolerate kinetochore-localized Ipl1.
Finally, the observation that in CB-INCENP expressing cells, bi-oriented attachments were stabilized and HEC1-Ser44 was dephosphorylated, while the RVSF motif in KNL1 was still phosphorylated and thus most likely not recruiting PP1, implies that other phosphatases, or other PP1 regulatory subunits, are involved in the dephosphorylation of the HEC1 N-terminus and in the stabilization of attachments. Moreover, both in CB-INCENP and MIS12-INCENP expressing cells, in which Aurora B was redistributed respectively near or at the kinetochore, chromosome bi-orientation was maintained when cohesin removal was prevented. This re-raises the longstanding question how tension stabilizes KT–MT attachments, since it suggests that spatial separation of the HEC1 N-terminus from Aurora B kinase may not be the main mechanism. Formally, we cannot rule out that, despite being sufficient to prevent anaphase onset, the KT pool of Aurora B maintained in MIS12-INCENP expressing cells was too small to sustain phosphorylation of the more distant HEC1 N-terminus upon bi-orientation. Still, we argue that alternative models explaining how tension stabilizes attachments and how Aurora B discriminates between correct and incorrect KT–MT attachments need to be considered. Such models include direct mechanical stabilization of KT–MT attachments14, in combination with either a microtubule-associated HEC1 phosphatase or tension-dependent exposure of a HEC1 phosphatase-binding site on the kinetochore.
Methods
Cell lines and cell culture
HeLa cells (ATCC CCL-2) were cultured in Dulbecco's modified Eagle's medium (DMEM, Sigma Aldrich) supplemented with 6% Tetracycline Screened HyClone Fetal Bovine Serum (GE Healthcare), 1 mM ultraglutamine (Lonza) and streptomycin/penicillin (Sigma Aldrich). HeLa Flp-In T-REx cells (gift from S. Taylor, University of Manchester, Manchester, UK) were cultured in the same medium as HeLa cells that included 4 μg ml−1 Blasticidine S (Invitrogen). To generate stable cell lines, the pcDNA5/FRT/TO plasmids encoding VSV-INCENP-mCherry, VSV-INCENPΔCEN-mCherry, VSV-survivin-INCENPΔCEN-mCherry, VSV-CB-INCENPΔCEN-mCherry, VSV-MIS12-INCENPΔCEN-mCherry (verified using sequencing by Macrogen) were co-transfected with pOG44 (Invitrogen) using the standard FuGENE 6 (Promega) transfection protocol. After transfection, cells were selected in medium supplemented with 200 μg ml−1 Hygromycin B (Roche). To generate HeLa VSV-INCENP-mCherry and VSV-CB-INCENPΔCEN-mCherry cells stably expressing H2B-GFP, lentivirus was produced. HEK293T (ATCC, CRL-3216) cells were co-transfected with pWPT-H2B-GFP, pRSV, pMD2-G and pMDLG-I using the X-tremeGENE (Roche) transfection protocol. The transfected HEK293T cells were cultured as described above without Blasticidine S. After 48 h, viruses were harvested and the VSV-INCENP-mCherry and VSV-CB-INCENPΔCEN-mCherry expressing HeLa Flp-In T-REx cells were used as donor cells for viral transduction. HeLa cell lines expressing LAP-KNL1Δ87-1832 from a doxycycline-inducible promoter at a single integration site have been described and were used to characterize the pMELT-T601 antibody62. Spodoptera frugiperda (Sf) 9 insect cells (ATCC, CRL-1711) were cultured at 27 °C in Insect-XPRESS media (Lonza, Basel, Switzerland) supplemented with 5% FBS and penicillin/streptomycin. All cell lines were tested negative for mycoplasma.
siRNA transfections and cell synchronization
The following siRNAs were used: siLUC (Luciferase GL2 duplex; Dharmacon/D-001100- 01-20), siINCENP (Dharmacon/3′-UTR: GGCUUGGCCAGGUGUAUAU), siSGOL1 (Dharmacon/J-015475-12: GAUGACAGCUCCAGAAAUU), siWAPL (Dharmacon/J-026287-10: GAGAGAUGUUUACGAGUUU) and siKNL1 (Thermo Fisher Scientific/J-015673–05: GCAUGUAUCUCUUAAGGAA). siRNAs were reverse transfected using HiPerFect (Qiagen) at 20 nM for siLUC, siINCENP, siSGOL1, siKNL1 and 40 nM for siWAPL. Cells were seeded on 12 mm High Precision coverslips (Superior-Marienfeld GmbH & Co) in 24 wells plates. After 16 h of siRNA transfection, cells were synchronized in G1/S-phase by addition of 2.5 μM thymidine (Sigma Aldrich). When SGO1 was co-depleted with INCENP, siINCENP was first reverse transfected and cells were subsequently forward transfected with siSGO1 directly after thymidine addition. After 24 h, cells were released from the thymidine block into medium containing 20 μM S-trityl-L-cysteine (STLC, Tocris), or 100 μM monastrol (Sigma Aldrich). At the same time 1 μg ml−1 doxycycline (Sigma Aldrich) was added to induce protein expression. To assess the capacity to bi-orient chromosomes, monastrol was washed out 7 h later and medium containing either 10 μM MG132, or 0.83 μM nocodazole (Sigma Aldrich) (Calbiochem) was added for 45 min. Alternatively, cells were synchronized in G2 after the thymidine release via treatment with the CDK1 inhibitor RO3306 (5 μM, Calbiochem) for 6 h. Cells were subsequently released from the block by 3 × washing with PBS and fixed 2 h later. Reversine (250 nM, Sigma Aldrich) was used to inhibit MPS1.
Antibodies and immunofluorescence
For immunofluorescence (IF) of γ-tubulin, cells were fixed for 5 min with 4% PFA (Sigma Aldrich), washed once with PBS and permeabilized with ice-cold methanol. After blocking in PBS containing 0.05% Tween 20 and 3% BSA, coverslips were incubated at room temperature with the following primary antibodies: mouse anti-γ-tubulin (1:500, Sigma Aldrich—T5192), rat anti-RFP (1:500, ChromoTek—5F8, to detect mCherry) and guinea pig anti-CENP-C (1:500, MBL—PD-030, to visualize the kinetochores). For IF of GFP, Aurora B, SGO1, pDSN1, KNL1-pRVSF-KNL1, KNL1-pMELT, pCENPA, MAD1, BUB1, CREST, pH3T3 and pH2A-T120, a brief pre-extraction with 100 mM Pipes pH 6.8, 10 mM EGTA pH 8, 1 mM MgCl2, 0.2% Triton X-100 (PEM/T) was performed followed by addition of an equal volume of 4% PFA. After 5 min, the mixture was removed and 4% PFA was added for 5 min. After washing once with PBS, the coverslips were blocked as described above and subsequently incubated with rabbit anti-GFP (1:500, custom made), mouse anti-Aurora B (1:1,000, BD Transduction Laboratories–611083) or mouse anti-SGOL1 (1:1,000, Abnova–h00151648), rabbit anti-pCENPA-S7 (1:500, Millipore/Upstate–07-232), rabbit anti-pDSN1-S109 (1:2,000) and rabbit anti-KNL1-pRVSF-S60 (1:1,000, kind gifts of Iain Cheeseman63), rabbit anti-KNL1-pMELT-T601 (1:2,000, see below), mouse anti-MAD1 (1:1,000, Merck Millipore–MADE867), rabbit anti-BUB1 (1:1,000, Abcam–AB9000), human CREST (1:2,000, Cortex Biochem–cs-1,058), rabbit anti-pH3T3 (1:2,000, Upstate–07-424) and rabbit anti-pH2A-T120 (1:2,000, Active Motif–39,391). Anti-MAD1 was incubated overnight at 4 °C, whereas all other antibodies were incubated at room temperature for 2 h. Affinity-purified phospho-specific antibody recognizing KNL1-pMELT-T601 was generated by injection of rabbit with KLH-coupled MDLpTESHTSNLGSQC peptide and affinity purification of bleed-out serum (GenScript). For the cold-stable microtubule assay, cells were incubated with ice-cold medium for 5 min. Then the cells were pre-extracted with PEM/T as described above and mouse anti-α-tubulin (1:10,000, Sigma Aldrich–T5168) was used as primary antibody. Goat anti-mouse (A11029) or anti-rabbit (A11034) IgG-Alexa 488, goat anti-mouse (A11031), anti-rabbit (A11036) or anti-rat (A11077) IgG-Alexa 568 (Invitrogen), goat anti-guinea pig (A21450) IgG-Alexa 647 (Invitrogen) and goat anti-human (A21445) IgG-Alexa 647 (Invitrogen) were used as secondary antibodies (all 1:500). DNA was stained with 1 μg ml−1 4′,6-diamidino-2-phenylindole (DAPI, Sigma) for 2 min. The coverslips were washed once with PBS, dipped in 100% ethanol, dried and mounted onto glass slides using ProLong Antifade Gold (ThermoFisher) mounting media. Images were acquired on a deconvolution system (DeltaVision RT; Applied Precision) with a CoolSNAP HQ2 camera and a × 100/1.40 NA U Plan Apochromat objective (Olympus) using softWoRx software (Applied Precision). All images are maximum intensity projections of deconvolved stacks. For quantifications of immunostainings, all images of similarly stained experiments were acquired with identical illumination settings and analysed using ImageJ (National Institute of Health). An image J macro was used that automatically selected kinetochores, this selection were enlarged with 3 pixels (px), and this region of interest (ROI) was then used to measure fluorescence intensities in different channels. For background subtraction, a selected area surrounding the DAPI signal was selected, this area was enlarged with 4 px (ROI-A) and with 6 px (ROI-B). ROI-A was subtracted from ROI-B, and this selected region was used as background ROI. For quantification of α-tubulin fluorescence intensity per individual K-fibre, an ROI was selected surrounding part of the MT that extended 1 μm from the kinetochore. Statistical analyses were done in GraphPad Prism (methods and P values and are indicated in figure legends).
Chromosome spreads
Cells were synchronized as described for the bi-orientation assay. Forty five minutes after the release from monastrol into 10 μM MG132, nocodazole was added to a final concentration of 0.83 μM and incubated for 15 min. Cells were swollen by gradually increasing the concentration of Hank's Balanced Salt Solution (HBSS, Invitrogen) for 35 min. in 5% CO2 at 37 °C and cells were fixed by gradually increasing the concentration of MeOH/Acetic acid (3:1 ratio) at room temperature. Chromosomes were visualized using DAPI. When chromosome spreads were combined with IF for Aurora B, cells were released from a thymidine block into medium containing 0.83 μM nocodazole and they were allowed to swell in 55 mM KCl2 for 15 min in 5% CO2 at 37 °C. The cells were subsequently centrifuged onto coverslips in a 24 wells plate at 4,400 rcf for 1 min, permeabilized using PEM/T, and fixed with 4% PFA, as described above.
Live cell imaging
The HeLa cell lines stably co-expressing H2B-GFP and VSV-INCENPΔCEN-mCherry or VSV-CB-INCENPΔCEN-mCherry were transfected with siRNAs for INCENP and WAPL and seeded into 8-well chamber slides (Ibidi) and live cell imaging was started 5 h after thymidine release. Live cell imaging was performed using a DeltaVision microscope equipped with a CoolSNAP HQ2 camera and a × 60 objective. All image quantifications were performed using ImageJ.
SDS–PAGE and western blotting
Cell lines were seeded into 6 wells plates and synchronised and released from a thymidine block into medium supplemented with 20 μM STLC. Seven hours later cells were harvested and washed with PBS once followed by lysis in Laemmli buffer. SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and western blotting were performed using the standard Bio-Rad protocols. The nitrocellulose membranes were blocked in TBS/0.1% Tween 20 (TBST) containing 4% milk for 30 min and incubated with the following primary antibodies: mouse anti-INCENP (1:500, Invitrogen—39–2800), mouse anti-Aurora B (1:250, BD Transduction Labs—611,083), mouse anti-α-tubulin (1:10,000, Sigma—T5168), or rabbit anti-GFP (custom made). After washing in TBST, the membranes were incubated in HRP-conjugated secondary antibodies goat anti-mouse (1:2500, Dako—P0447) or goat anti-rabbit (1:2500, Dako—P0448) in TBST-4% milk ECL (Advansta) was used as substrate of HRP and chemiluminescence was measured using an Amersham Imager 600. Uncropped scans of western blots can be found in Supplementary Fig. 7).
BacMam virus production and transduction
pACEBac1-CMV encoding LacI-GFP, VSV-CEN-box-GFP (aa 1-63 of INCENP), VSV-CEN-Baronase-GFP (Baronase according to ref. 41), VSV-SGO1-GFP, VSV-CEN-Sgo1 WT/N61I-GFP and sororin WT/9A-GFP (sororin WT/9A manufactured by IDT), CENP-B-mCherry (aa 1-498 of CENP-B), VSV-INCENPΔCEN-mCherry, VSV-CB-INCENPΔCEN-mCherry and VSV-MIS12-INCENPΔCEN-mCherry, were transformed into EmBacY cells to generate recombinant bacmids. For bacmid transfections, 0.5 × 106 Sf9 cells per well were plated in a 6-well plate. Cells were transfected with X-tremeGENE 9 (Roche) using 3 μg recombinant bacmid (see below) and 8 μl transfection reagent in Insect-XPRESS media lacking FBS and penicillin/streptomycin. Cells were left for 5 days before harvesting the media (containing P1 virus). For production of a high titre P2 viral stock, 500 μl of P1 virus was used to inoculate a 60 ml suspension culture of log phase Sf9 cells at a density of 1.2 × 10̂6 cells per ml. Cultures were grown for 4 days followed by harvesting the P2 virus by spinning down the cells at 1000, g and collecting the supernatant. Viral stocks were stored at 4 °C in the dark. For optimal viral transduction and protein expression, the HeLa cell lines were cultured in RPMI (Sigma) medium containing supplements as described above. The cells were synchronized as described for the bi-orientation assay, and viral transduction was performed 24 h prior to fixation.
Statistical evaluation
Statistical analysis was performed with Prism Software (Graphpad software). Data are represented as means, together with s.e.m., of either two or three independent experiments, or as dot plots of individual cells of one representative experiment. Two-tailed Student's t-test was used to compare differences between groups of cells when immunofluorescence intensity/cell was measured. When categorical outcomes were scored a Chi-squared test was used. We performed the test on binary outcomes (complete alignment vs no complete alignment=mild+severe misalignment) for the indicated control group and experimental group, to assess whether the observed rescue of complete alignment was statistically significant.
Data availability
All relevant data supporting the findings of this study are available within the article and its Supplementary Information files, or from the corresponding author on request.
Additional information
How to cite this article: Hengeveld, R. C. C. et al. Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat. Commun. 8, 15542 doi: 10.1038/ncomms15542 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures and Supplementary References
Acknowledgments
We thank Dr G. Vader for valuable input, the Lens and Kops lab members for their feedback, Dr I. Cheeseman for generous gifts of reagents and Dr G. Kops for generation of the pMELT-T601 antibody and comments on the manuscript. This research was supported by The Netherlands Organization of Scientific Research (NWO-Vici 91812610 to S.M.A.L.; and NWO-Veni 91610036 to M.A.H.).
Footnotes
The authors declare no competing financial interests.
Author contributions R.C.C.H., M.A.H. and S.M.A.L. conceived and designed the study. S.M.A.L. wrote the manuscript with input from R.C.C.H., M.A.H. and M.J.M.V. R.C.C.H. and M.J.M.V. performed all experiments except the ones for Supplementary Fig. 4e. M.V. generated and characterized the KNL1-pMELT-T601 antibody in the lab of G. Kops. M.J.M.V. generated the BacMam viruses.
References
- Duijf P. H. & Benezra R. The cancer biology of whole-chromosome instability. Oncogene 32, 4727–4736 (2013). [DOI] [PubMed] [Google Scholar]
- Cheeseman I. M. The kinetochore. Cold Spring Harb. Perspect. Biol. 6, a015826 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y. Geometry and force behind kinetochore orientation: lessons from meiosis. Nat. Rev. Mol. Cell Biol. 13, 370–382 (2012). [DOI] [PubMed] [Google Scholar]
- Foley E. A. & Kapoor T. M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 14, 25–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R. & Khodjakov A. Thirty years of search and capture: The complex simplicity of mitotic spindle assembly. J. Cell Biol. 211, 1103–1111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn V. & Musacchio A. The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling. Front. Oncol. 5, 225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M. et al. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127, 983–997 (2006). [DOI] [PubMed] [Google Scholar]
- DeLuca J. G. et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969–982 (2006). [DOI] [PubMed] [Google Scholar]
- Tanaka T. U. et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108, 317–329 (2002). [DOI] [PubMed] [Google Scholar]
- Andrews P. D. et al. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253–268 (2004). [DOI] [PubMed] [Google Scholar]
- Liu D. et al. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 323, 1350–1353 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca K. F., Lens S. M. & DeLuca J. G. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J. Cell Sci. 124, 622–634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas G. V., DeLuca K. F. & DeLuca J. G. KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity. J. Cell Biol. 203, 957–969 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi B. et al. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468, 576–579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z. et al. Deconstructing Survivin: comprehensive genetic analysis of Survivin function by conditional knockout in a vertebrate cell line. J. Cell Biol. 183, 279–296 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. S. & Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature 497, 118–121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A. A. et al. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell 131, 271–285 (2007). [DOI] [PubMed] [Google Scholar]
- Eckley D. M. et al. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells. J. Cell Biol. 136, 1169–1183 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vader G. et al. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. 7, 85–92 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsztein A. M. et al. INCENP centromere and spindle targeting: identification of essential conserved motifs and involvement of heterochromatin protein HP1. J. Cell Biol. 143, 1763–1774 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P. & Stukenberg P. T. A centromere-signaling network underlies the coordination among mitotic events. Trends Biochem. Sci. 41, 160–174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelij P. et al. Warsaw breakage syndrome, a cohesinopathy associated with mutations in the XPD helicase family member DDX11/ChlR1. Am. J. Hum. Genet. 86, 262–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan G. et al. Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. EMBO J. 31, 71–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A., Hirano M. & Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16, 3004–3016 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T. et al. Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc. Natl Acad. Sci. USA 110, 13404–13409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Sullivan B. A. & Higgins J. M. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev. Cell 11, 741–750 (2006). [DOI] [PubMed] [Google Scholar]
- Gimenez-Abian J. F. et al. Regulation of sister chromatid cohesion between chromosome arms. Curr. Biol. 14, 1187–1193 (2004). [DOI] [PubMed] [Google Scholar]
- Tanno Y. et al. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 24, 2169–2179 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito D. A. & Rieder C. L. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 16, 1194–1200 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. et al. Substrate degradation by the anaphase promoting complex occurs during mitotic slippage. Cell Cycle 9, 1792–1801 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick T. D. et al. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev. Cell 11, 57–68 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. et al. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 10, 42–52 (2008). [DOI] [PubMed] [Google Scholar]
- Liu H., Jia L. & Yu H. Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr. Biol. 23, 1927–1933 (2013). [DOI] [PubMed] [Google Scholar]
- Tanno Y. et al. The inner centromere-shugoshin network prevents chromosomal instability. Science 349, 1237–1240 (2015). [DOI] [PubMed] [Google Scholar]
- Kueng S. et al. Wapl controls the dynamic association of cohesin with chromatin. Cell 127, 955–967 (2006). [DOI] [PubMed] [Google Scholar]
- Gandhi R., Gillespie P. J. & Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol. 16, 2406–2417 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Rankin S. & Yu H. Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol. 15, 40–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier M. R., Bekier M. E. 2nd & Taylor W. R. Regulation of sororin by Cdk1-mediated phosphorylation. J. Cell Sci. 124, 2976–2987 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarhuis J. H. et al. WAPL-mediated removal of cohesin protects against segregation errors and aneuploidy. Curr. Biol. 23, 2071–2077 (2013). [DOI] [PubMed] [Google Scholar]
- Daum J. R. et al. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr. Biol. 21, 1018–1024 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes Bastos R. et al. Aurora B suppresses microtubule dynamics and limits central spindle size by locally activating KIF4A. J. Cell Biol. 202, 605–621 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T. S. et al. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441, 46–52 (2006). [DOI] [PubMed] [Google Scholar]
- Riedel C. G. et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441, 53–61 (2006). [DOI] [PubMed] [Google Scholar]
- Tang Z. et al. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell 10, 575–585 (2006). [DOI] [PubMed] [Google Scholar]
- Tsukahara T., Tanno Y. & Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467, 719–723 (2010). [DOI] [PubMed] [Google Scholar]
- Yamagishi Y. et al. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330, 239–243 (2010). [DOI] [PubMed] [Google Scholar]
- Kelly A. E. et al. Survivin reads phosphorylated histone H3 Threonine 3 to activate the mitotic kinase aurora B. Science 330, 235–239 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. et al. Histone H3 Thr-3 phosphorylation by haspin positions Aurora B at centromeres in mitosis. Science 303, 231–235 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad B., Kuijt T. E. & Kops G. J. Kinetochore-microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint. Nat. Commun. 6, 8987 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchman E. C., Boehm F. J. & DeLuca J. G. Stable kinetochore-microtubule attachment is sufficient to silence the spindle assembly checkpoint in human cells. Nat. Commun. 6, 10036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr. Biol. 25, R1002–R1018 (2015). [DOI] [PubMed] [Google Scholar]
- Liu D. et al. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 188, 809–820 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis W. et al. Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat. Cell Biol. 16, 1257–1264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. et al. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr. Biol. 21, 1061–1069 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waal M. S. et al. Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep. 13, 380–387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S. et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281–294 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert C. A., Gravdahl D. J. & Megee P. C. The enhancement of pericentromeric cohesin association by conserved kinetochore components promotes high-fidelity chromosome segregation and is sensitive to microtubule-based tension. Genes Dev. 21, 278–291 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. M. et al. Pericentromeric sister chromatid cohesion promotes kinetochore biorientation. Mol. Biol. Cell 20, 3818–3827 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J. S. et al. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol. Cell Biol. 21, 3144–3158 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. S., Cross F. R. & Funabiki H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr. Biol. 21, 942–947 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N. et al. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 22, 900–906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugel M. et al. Arrayed BUB recruitment modules in the kinetochore scaffold KNL1 promote accurate chromosome segregation. J. Cell Biol. 203, 943–955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn J. P. et al. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell 38, 383–392 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Supplementary References
Data Availability Statement
All relevant data supporting the findings of this study are available within the article and its Supplementary Information files, or from the corresponding author on request.