Abstract
Anxious individuals tend to show a negative affective bias in attention that likely reflects reduced executive control, a cognitive function associated with the inferior frontal cortex (IFC), particularly its posterior segment, pars opercularis. Here, we investigated the relations among gray matter volume in the pars opercularis of IFC, trait anxiety, and negative biases in attention, in healthy participants. Sixty-two adults underwent structural magnetic resonance imaging scanning, completed a trait anxiety measure, and performed an Affective Go/No-Go (AGN) task. IFC volumes were extracted using Freesurfer, and negative bias scores were calculated from AGN performance. Trait anxiety correlated negatively with left IFC volume, and positively with the negative bias in reaction time. Furthermore, trait anxiety mediated the negative relation between the IFC volume and the negative bias measure. Overall, the present findings extend previous understanding of the IFC involvement in anxiety at the structural level, and may inform the development of intervention programs targeting anxiety.
Keywords: executive control, trait anxiety, negative bias, inferior frontal cortex, volumetric
Introduction
With nearly 60% of American college students reporting overwhelming anxiety within a 12 months interval (American College Health Association, 2015), we are witnessing what has started to be seen as an ‘epidemic of anguish’ (Wilson, 2015, August 31). Compounding the problem of high prevalence is the impact anxiety has on students’ academic and cognitive functioning (American College Health Association, 2015). Negative affective biases, often expressed as heightened sensitivity to negative or threatening information (Bar-Haim et al., 2007), are an important characteristic of anxiety (Cisler et al., 2009; Eysenck et al., 2007). These biases likely reflect compromised executive control of attention (Cisler et al., 2009), which has been linked to anomalies of the inferior frontal cortex (IFC), a region closely involved in control mechanisms (Aron et al., 2014). Here, we investigated the role of trait anxiety and IFC gray matter volume as personality and brain factors that influence negative affective biases in healthy young adults. Sampling from a nonclinical population where worsening mental health has become a pressing issue can provide insights into risk/resilience factors associated with anxiety symptoms in college students, and could in turn contribute to a better understanding of predispositions and the development of psychopathology (Montag et al., 2013).
Although the causal direction of the relation between anxiety and attention bias has not been completely resolved in the literature (Cisler et al., 2009), it has been recognized that anxiety can impair executive control by imposing demands on cognitive systems, including the ability to inhibit prepotent responses and to resist interference to task-irrelevant distracters (Eysenck et al., 2007). Impairment of these processes likely contributes to negative bias in attention, which can be captured by the Affective Go/No-Go (AGN) task. In this task, subjects are asked to respond to targets and withhold from responding to distracters, while targets and distracters differ in affective valence. Thus, when processing of negative information takes precedence, a negative affective bias can be observed as increased speed and/or more accuracy when attending to negatively (vs. positively) valenced information (Schulz et al., 2007). This pattern is typically shown by patients with affective disorders, whereas the opposite pattern is shown by healthy subjects (Erickson et al., 2005). Furthermore, in anxiety patients, the negative bias in reaction time on AGN was shown to predict worse response to a 12-week psychopharmacological treatment, whereas a positive bias predicted symptom improvement following treatment (Steiner et al., 2013). These results confirm that using AGN can reliably capture negative biases in attention, and identify potential links of such biases with impaired control and mechanisms underlying anxiety.
Previous evidence showing the role of the IFC in cognitive control and emotion regulation points to this region as a possible target for investigations of individual differences in affective biases and anxiety. IFC has been recognized as a region with great functional heterogeneity (e.g., Cai et al., 2014; Levy and Wagner, 2011), involved in a diverse range of processes such as basic processing of emotional and salient information (Kober et al., 2008; Seeley et al., 2007; Vytal and Hamann, 2010), response inhibition (e.g., Aron et al., 2014), and top-down emotion regulation (Denkova et al., 2010; Iordan et al., 2013; Ochsner et al., 2004). As much as it has been seen as an integrative ‘hub’ in emotion processing, recent research also points to possible functional dissociations within IFC, particularly along the anterior-posterior axis. Specifically, the anterior segment of IFC (approximately pars orbitalis and pars triangularis) has been typically identified as part of the ventral attention network (Corbetta et al., 2008) and implicated in bottom-up capture and reorientation of attention driven by salient information, whereas the more posterior segment, pars opercularis, has been typically identified as part of the cingulo-opercular network (Dosenbach et al., 2008) and implicated in active coping and top-down regulation of emotional responses. Related to performance on AGN, where a target is defined across a block of trials and a response is made on each trial in the face of distracting emotional information, cognitive demands are placed on the ability to sustain task sets and ensure performance in the context of concurrent processing of emotional information, more than on the spatial orientation of attention. Therefore, it seems that the posterior segment of IFC, i.e., pars opercularis, would be a region of specific interest to study individual differences on AGN.
Indeed, evidence from both structural and functional studies has linked the posterior IFC with anxiety disorders (Shang et al., 2014) and with performance in the cognitive and affective Go/No-Go tasks (Brown et al., 2015; Brown et al., 2012; Liddle et al., 2001; Swick et al., 2008, 2011), further supporting the role of this region in emotion-related control processes. A recent meta-analysis identified reductions in the left opercular volume in anxiety disorders, even after controlling for comorbid illnesses (Shang et al., 2014). Functional and lesion evidence points to bilateral involvement of posterior IFC in executive control (Swick et al., 2008, 2011), although traditionally a right lateralization was emphasized (Aron et al., 2014). Furthermore, there is evidence that trait anxiety modulates the left IFC responses to affective stimuli in control processing (Fales et al., 2010). Using a working memory task, Fales et al. (2010) found that higher anxiety was associated with stronger activation in a region in the left IFC in response to fearful (vs. neutral) targets, and with weaker activation in the same region to happy (vs. neutral) targets.
Based on these findings implicating IFC (particularly its posterior segment) in control mechanisms underlying negative affective bias in AGN tasks and in mechanisms sensitive to the modulation of anxiety, the current study targeted the IFC as a possible key region linked to anxiety and the negative bias measured with AGN. Given the suggestion that stable individual differences may be better reflected in structural changes (DeYoung et al., 2010), compared to task-dependent responses, a volumetric approach of studying brain-personality-behavior relations was employed (Dolcos et al., 2016). Using this multi-dimensional framework to study anxiety and its symptoms helps us identify risk/resilience factors and their relations across different levels of analysis, which can open up new venues for developing prevention and intervention programs.
Taken together, extant evidence shows that anxiety is associated with a negative affective bias in attention that can be measured with the AGN task, and points to the posterior IFC as the region associated with executive control and sensitive to anxiety levels in control processing. This suggests that a triadic relation may potentially exist among the posterior IFC, trait anxiety, and negative affective bias. However, such relations have not been examined at the level of individual differences. Thus, it remains unclear whether the involvement of the posterior IFC in negative affective bias can be detected at the structural level and, if so, whether it also reflects individual differences in anxiety levels. The present study examined the relations among the gray matter volume of pars opercularis, trait anxiety, and the negative affective bias measured by AGN, and tested the following hypotheses: (1) Trait anxiety is positively associated with measures of negative bias (Steiner et al., 2013), and negatively associated with measures of opercular volume (Shang et al., 2014); (2) Opercular volume is negatively associated with negative bias (Brown et al., 2012); (3) In line with the proposed relations between opercular volume and trait anxiety, and between trait anxiety and negative bias, we also explored the possibility that trait anxiety mediates the relation between volumetric measures of pars opercularis and measures of negative bias (Fales et al., 2010).
Materials and methods
Subjects
Analyses were performed on data from 62 (35 females) healthy young adults (average age = 23.129, SD = 3.877); there were no significant age differences between the female and male subjects [t(60) = 0.163, P > 0.5, two-tailed]. About 68.6% of the participants who provided race/ethnicity details were White, 25.5% Asian, 2.0% Black, 3.9% more than one race, and 11.9% of those who provided education-related details had a Master’s degree, 31.0% a Bachelor’s degree, 9.5% Postsecondary degrees, and 47.6% had High School diploma. Previous studies suggest that the relation between IFC gray matter volume and anxiety is likely to have a large effect size (Strawn et al., 2015), and the relation between anxiety and negative attention bias has been found to yield a medium to large effect size (Bar-Haim et al., 2007). None of the subjects had previously been diagnosed with neurological, psychiatric, or personality disorders. The research protocol employed in the present study was approved for ethical treatment of human participants by the Institutional Ethics Board.
Imaging protocol and MRI data processing
Structural scanning was conducted on a 1.5-T Siemens Sonata scanner. After the sagittal localizer, 3-D MPRAGE anatomical images were obtained using the following parameters: TR = 1600 ms; TE = 3.82 ms; FOV = 256 × 256 mm. This resulted in anatomical volumes with 112 axial slices and voxel size of 1 × 1 × 1 mm3. Cortical reconstruction was performed with the Freesurfer image analysis suite, Version 5.3.0 (Fischl, 2012), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). A semi-automatic workflow was adopted to ensure quality control at the following stages: Talairach registration, skull stripping, white matter surface reconstruction, and pial surface reconstruction. The outputs at each stage were manually inspected and corrected, if necessary, before implementing the next stage.
The Desikan atlas (Desikan et al., 2006) was used to extract the anatomical regions of interest (ROIs). To examine the specificity of the hypothesized effects to pars opercularis, we also extracted other ROIs both within and outside the IFC, and explored their relations with our variables of interests, trait anxiety and negative bias. The areas within the IFC included, in addition to pars opercularis, the pars triangularis and pars orbitalis. First, the whole IFC ROI was determined as the area delineated anteriorly by the rostral extent of the inferior frontal sulcus, posteriorly by the precentral gyrus, laterally by the lateral bank of the inferior frontal sulcus, and medially by the medial bank of the lateral orbital sulcus and/or the circular insular sulcus. Then, the subdivision pars opercularis was defined on this IFC ROI as the first gyrus from the precentral gyrus, pars triangularis the second, and pars orbitalis the third. The areas outside the IFC included the rostral anterior cingulate cortex (rACC), amygdala (AMY), rostral middle frontal cortex (RMF), and insula. Due to the involvement of the rACC in processing recruited by AGN (e.g., Liddle et al., 2001) and its noted volumetric reduction in affective disorders (Shang et al., 2014), the anatomical ROIs of this region were extracted to test the specificity of the identified relations. The rACC ROI was defined by the rostral extent of the cingulate sulcus, the superior frontal sulcus, and the genu of the corpus callosum, and the medial aspect of this cortex. To further probe the specificity of pars opercularis against other regions known to be involved in the processing of emotion-cognition interactions, we also extracted ROIs for AMY, RMF, and insula. The ROI for AMY was determined by Freesurfer’s probability atlas based on the characteristic T1 properties of this region aided by neuroanatomical constraints (Fischl et al., 2002). The ROI for RMF was defined between the superior frontal sulcus and the inferior frontal sulcus, rostrally limited by the rostral extent of the superior frontal sulcus, and caudally by the caudal extent of the middle frontal gyrus. The ROI for insula consisted of Freesurfer’s insula parcellation unit defined by the circular insula sulcus. For each subject, the gray matter volumes of these ROIs and the estimated intracranial volumes (ICVs) were extracted from Freesurfer results, and an index of the adjusted volume was obtained for each ROI by dividing the raw volumes by ICVs, and then multiplying them by 100. The resulting adjusted volumetric indices were used for group-level analyses.
Behavioral measures
Trait Anxiety. Trait anxiety was assessed using the State-Trait Anxiety Inventory-Trait (STAI) (Spielberger et al., 1983). Subjects rated how they generally felt about 20 statements, such as “I worry too much over something that really doesn’t matter”, using a 1–4 Likert scale (1 = not at all, 4 = very much so). Ratings of individual statements were summed to obtain a total score for each subject (ranging from 20 to 80). Higher scores were considered to reflect a vulnerability factor for anxiety disorders, and lower scores as potentially indexing lower vulnerability. The STAI has shown high internal consistency, test–retest reliability and good construct and concurrent validity (Spielberger et al., 1983). The Cronbach’s alpha for STAI in our sample was 0.861.
Depression Symptoms. To confirm that the effects investigated here were specific to anxiety and not to negative affect in general, measures of depression symptoms were also collected. Depression symptoms were assessed using the Beck Depression Inventory (BDI) (Beck et al., 1961). This scale consisted of 21 questions, and subjects answered these questions by choosing one of four possible choices, ranging in intensity from 0 to 3 (e.g., 0 = ‘ I do not feel sad’, 3 = ‘I am so sad or unhappy that I can’t stand it’). The corresponding numbers of the choices were summed to obtain a total score for each subject, ranging from 0 to 63, which would reflect the severity of depression symptoms. Considerable evidence has attested to the reliability and validity of BDI (Beck et al., 1988). The Cronbach’s alpha for BDI in our sample was 0.796.
Affective Go/No-Go (AGN)Task. AGN was administered as part of the Cambridge Neuropsychological Test Automated Battery (Cambridge-Cognition). The task comprised 20 blocks, with two practice blocks followed by 18 testing blocks. At the beginning of each block, a target word category and a distracter word category were designated. Then, a series of words were rapidly presented at the center of the screen, and subjects were instructed to make a button-press response to words from the target category, while withholding the response to words from the distracter category. Words from 3 categories were used (i.e., positive, negative, and neutral). For example, if the target category for a block is designated to be ‘Positive’, and distractor category ‘Negative’, then participants will press the button upon seeing the word ‘Happy’, and withhold the response upon seeing “Sad”. The 6 conditions of target-distracter categories assignment were balanced and repeated 3 times. There were 18 trials per block, and on each trial, each word was displayed for 300 ms, followed by a 900 ms response window before the next word was displayed. Subjects were instructed to respond as fast and accurate as possible. Percentages of hits (%Hits) and reaction times (RTs) for hit responses were recorded. Analysis focused on two conditions, one with positive targets and negative distracters (PosNeg), and the other with negative targets and positive distracters (NegPos). Measures of negative bias were created for percentages (NegBias_%Hits) and RTs (NegBias_RT) of hit responses, by subtracting %Hits and RTs for NegPos from those for PosNeg.
Given evidence suggesting that neutral information may also be perceived as negative by individuals with anxiety and thus influencing their performance (Morey et al., 2009), we also included a neutral condition. Measures of neutral bias were created between negative target/neutral distractor vs. neutral target/negative distractor conditions (i.e., NeuBias_%Hits, NeuBias_RT), to explore the sensitivity of this measure in relation to individual differences in trait anxiety and brain volumes. Thus, the lower the accuracy and the faster the response for negative targets relative to positive ones, the larger NegBias_%Hits and NegBias_RT. Similarly, the lower the accuracy and the faster the response to neutral relative to negative targets, the larger NeuBias_%Hits and NeuBias_RT.
Statistical analyses
Standardized scores were first calculated to detect outliers, using a criterion of three standard deviations. RTs were checked using a benchmark of 200 ms, to make sure all responses were valid. As a result, three subjects’ AGN measures and one subject’s right pars opercularis volumetric measure were identified as outliers (see Table 1 for the numbers of valid cases for all variables), and outliers were excluded analyses wise. One subject’s trait anxiety score was missing, and therefore that subject was not included in the analysis. Zero-order correlations were first used to assess the relations among the targeted variables (i.e., trait anxiety scores, performance measures on AGN, and opercular volumes) and among the comparison variables (i.e., depression scores, and pars orbitalis and pars triangularis, rACC, amygdala, rostral middle frontal cortex and insula). To test for the specificity of the effects to trait anxiety, depression scores were also included by testing the same effects using depression scores instead of trait anxiety scores. Similarly, the aforementioned comparison regions were included in the analysis to examine whether the identified effects were specific to the pars opercularis of IFC. To test our mediation hypothesis we conducted mediation analyses (Preacher and Hayes, 2008), with trait anxiety as the mediator, opercular volumes as the predictor, and AGN scores as the outcome variable. To probe the specificity of our proposed mediation model, we tested models including all 6 possible configurations of the variables of interest. Bias corrected confidence intervals (CIs) were used as an indicator of the significance of the mediation model. All statistical analyses were performed using SPSS for Windows, Version 20.0 (IBM, Released 2011).
Table 1.
Descriptive statistics of personality, cognitive performance and volumetric measures
| Variables | N | Mean | SD | Variables | N | Mean | SD |
|---|---|---|---|---|---|---|---|
| Anxiety | OPR volumes | ||||||
| STAI | 61 | 37.59 | 8.68 | L OPR | 62 | 0.42 | 0.08 |
| Depression | R OPR | 61 | 0.34 | 0.06 | |||
| BDI | 62 | 4.44 | 4.21 | Comparison brain regions volumes | |||
| AGN Performance | Within IFC | ||||||
| PosNeg_%Hit | 61 | 95.57 | 5.19 | L ORB | 61 | 0.15 | 0.03 |
| PosNeg_ RT | 62 | 475.79 | 63.84 | R ORB | 62 | 0.19 | 0.04 |
| NegPos_%Hit | 60 | 95.43 | 5.84 | L TRI | 61 | 0.29 | 0.06 |
| NegPos_ RT | 61 | 473.96 | 60.66 | R TRI | 61 | 0.35 | 0.06 |
| NegBias_%Hit | 59 | 0.31 | 7.00 | Outside IFC | |||
| NegBias_RT | 61 | – 0.83 | 31.37 | L AMY | 62 | 0.11 | 0.02 |
| NeuNeg_%Hit | 61 | 92.84 | 7.25 | R AMY | 62 | 0.10 | 0.03 |
| NeuNeg_RT | 61 | 532.96 | 69.68 | L rACC | 62 | 0.20 | 0.04 |
| NegNeu_%Hit | 61 | 94.35 | 5.60 | R rACC | 62 | 0.17 | 0.04 |
| NegNeu_RT | 60 | 496.42 | 59.02 | L RMF | 62 | 1.28 | 0.23 |
| NeuBias_%Hit | 61 | 1.52 | 6.74 | R RMF | 61 | 1.31 | 0.20 |
| NeuBias_RT | 60 | –33.14 | 49.57 | L Insula | 60 | 0.54 | 0.08 |
| R Insula | 61 | 0.55 | 0.08 | ||||
Notes: The differences in N for the listed variables are due to analysis-wise exclusion of outliers. STAI, State-Trait Anxiety Inventory-Trait (Spielberger et al., 1983); BDI, Beck Depression Inventory (Beck et al., 1961); AGN, Affective Go/No-Go task; PosNeg, the condition on AGN where positive stimuli are the targets and negative stimuli are the distracters; NegPos, the condition on AGN where negative stimuli are the targets and positive stimuli are the distracters; NegBias, negative bias; RT, reaction time; NeuNeg, the condition on AGN where neutral stimuli are the targets and negative stimuli are the distracters; NegNeu, the condition on AGN where negative stimuli are the targets and neutral stimuli are the distracters; NeuBias, neutral bias; OPR, pars opercularis; L, left; R, right; IFC, inferior frontal cortex; ORB, pars orbitalis; TRI, pars triangularis, rACC, rostral anterior cingulate cortex; RMF, rostral middle frontal cortex.
Results
Descriptive statistics of all variables are presented in Table 1. Paired-samples t tests indicated that, overall, %Hits [t(58) = 0.35, P = 0.73] and RTs [t(60) = 0.21, P = 0.84] did not differ between PosNeg and NegPos conditions, so we did not find an overall bias in favor of positive targets in our sample of healthy individuals, as previously reported by others (Erickson et al., 2005). Therefore, the rest of the analysis focused on the measures of relative bias, NegBias_%Hits and NegBias_RT. However, NegNeu and NeuNeg conditions did differ in RT [t(59) = –5.18, P = 0.00], although not so much in %Hits [t(61) = 1.76, P = 0.08]. This was consistent with the expectation that participants were faster responding to negative than neutral targets. Given our interest in the extent of the neutral bias in relation to trait anxiety and IFC volumes, we focused on NeuBias_%Hits and NeuBias_RT in the following analysis. Main correlation results are presented in Table 2, and correlation results concerning comparison variables are presented in Tables S1 and S2.
Table 2.
Correlations among measures of anxiety and depression, pars opercularis volumes, and AGN measures
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. STAI | 1 | 0.500 | –0.266* | –0.209 | 0.088 | 0.316* | 0.099 | –0.069 |
| N | 61 | 61 | 61 | 60 | 58 | 60 | 60 | 59 |
| 2. BDI | 1 | –0.093 | –0.080 | –0.024 | 0.262* | 0.175 | 0.191 | |
| N | 62 | 62 | 61 | 59 | 61 | 61 | 60 | |
| 3. L OPR | 1 | 0.556** | –0.103 | –0.108 | –0.051 | 0.174 | ||
| N | 62 | 61 | 59 | 61 | 61 | 60 | ||
| 4. R OPR | 1 | –0.016 | –0.086 | –0.018 | –0.001 | |||
| N | 61 | 58 | 60 | 60 | 59 | |||
| 5. NegBias_%Hit | 1 | –0.025 | –0.259* | –0.191 | ||||
| N | 59 | 59 | 59 | 59 | ||||
| 6. NegBias_RT | 1 | 0.104 | –0.170 | |||||
| N | 61 | 60 | 60 | |||||
| 7. NeuBias_%Hit | 1 | –0.080 | ||||||
| N | 61 | 59 | ||||||
| 8. NeuBias_RT | 1 | |||||||
| N | 60 |
Notes: AGN, Affective Go/No-Go task; STAI, State-Trait Anxiety Inventory-Trait (Spielberger et al., 1983); BDI, Beck Depression Inventory (Beck et al., 1961); OPR, pars opercularis; L, left; R, right; NegBias, negative bias; RT, reaction time; NeuBias, neutral bias;
P < 0.05;
P < 0.01.
Increased trait anxiety linked to decreased IFC volume and increased negative bias in RT
As predicted, trait anxiety was negatively correlated with the gray matter volume of the left pars opercularis (r = – 0.266, P = 0.039), and positively correlated with the negative bias in RT (r = 0.316, P = 0.014). The test between the correlations of trait anxiety with the left and right pars opercularis was not significant (t57 = 0.379). Trait anxiety was not correlated with the negative bias measure of %Hits (r = – 0.088, P = 0.513). None of the performance measures in AGN were directly associated with the opercular volumes. The same analysis was repeated for the identified correlations with all outliers included, which returned marginally significant correlations between trait anxiety and the left pars opercularis (r = – 0.215, P = 0.096), and between trait anxiety and the negative bias in RT (r = 0.226, P = 0.081).
To examine the specificity of trait anxiety, we explored the correlations concerning depression with pars opercularis and negative bias (Table 2). There were no significant correlations between depression and the bilateral pars opercularis (P > 0.4), despite a significant correlation between depression and negative bias (r = – 0.262, P = 0.041). To examine the specificity of the hypothesized effects to pars opercularis, we also explored the relations between a number of comparison brain regions, both within and outside the IFC, and our variables of interests, trait anxiety and negative bias (See Supplementary Materials Tables S1 and S2). Again, we did not find significant correlations for any of these regions with either trait anxiety or negative bias. Trait anxiety was positively correlated with the negative bias in RT, but not with the negative bias measure of %Hits, or any of the neutral bias measures (Table 2). These suggest selective relations among our target opercular IFC volumes, trait anxiety, and negative bias in RT. Mediation models involving trait anxiety, left opercular volume, and negative bias in RT were examined next.
Protective effect of IFC volume against negative bias through trait anxiety
Mediation analyses revealed that trait anxiety mediated the relation between the left opercular volume and negative bias in RT, such that a larger volume predicted a smaller negative bias via reduced trait anxiety (a = –26.80, P = 0.043; b = 1.14, P = 0.019; c = –32.00, P = 0.51; c’ = –1.31, P = 0.98; ab = –30.59, 95% CI = [–79.32, –4.78]; N = 60; Figure 1). This model remained significant after including age and gender as covariates (a = –27.35, P = 0.048; b = 1.12, P = 0.020; c = –41.98, P = 0.40; c’ = –11.35, P = 0.82; ab = –30.64, 95% CI = [–85.46, –4.81]; n = 60). Given the common correlations of opercular volume and negative bias with trait anxiety, we also tested the alternative model with negative bias in RT as the predictor and left opercular volume as the outcome variable. However, this model did not yield a significant mediation effect (ab = –0.0002, bootstrapped 95% CI = [–0.0006, 0.0000]; N = 60). This result points to the directionality of the model, that trait anxiety seems to be partially the mediating mechanism linking IFC volume (a brain level factor) to attention bias (a behavioral outcome), but not the other way around.
Fig. 1.
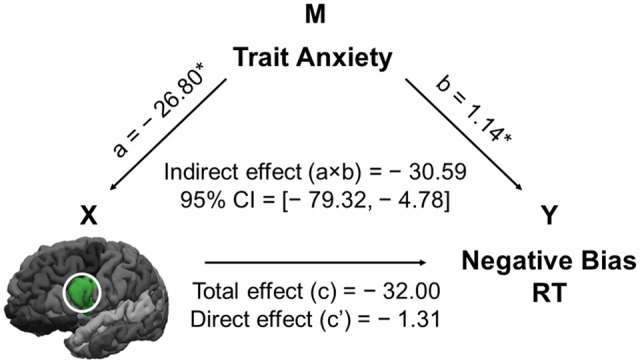
Protective Effect of IFC Volume against Negative Bias through Trait Anxiety. A schematic illustration of the mediation model showing that trait anxiety (M, the mediator variable) mediated the negative relation from the left opercular volume (X, the predictor variable) to negative bias on AGN (Y, the outcome variable). The left pars opercularis is shown in green and circumscribed by the white circle. Path a refers to the relation from X to M, and path b refers to the relation from M to Y, while controlling for X. Path c refers to the total effect from X to Y, and path c’ refers to the direct effect from X to Y controlling for M. The indirect effects were represented by the interaction term a × b, which was tested using bias-corrected bootstrapped 95% confidence intervals (CIs). Running the models with all outliers included did not yield significant results (ab = –18.02, 95% CI = [–69.92, 1.66]; N = 62). This might seem surprising, but is consistent with Cousineau and Chartier’s (2010) suggestion that an outlier may have a larger impact with a larger sample size compared to a smaller sample size. Unstandardized regression coefficients are displayed. *P < 0.05. STAI, State-Trait Anxiety Inventory-Trait (Spielberger et al., 1983); OPR, pars opercularis; AGN, Affective Go/No-Go task; RT, reaction time.
Given that the literature has emphasized the involvement of the right IFC in executive control, and also due to the lack of a significant difference between the correlations of left and right IFC with trait anxiety in our previous correlation analysis, we also tested a model using the right pars opercularis volume as the predictor, trait anxiety as the mediator, and negative bias as the outcome. This model yielded a significant indirect effect (ab = –39.97, 95% CI = [–107.91, –2.53]; N = 60). Overall, the findings of the current investigation do not seem to provide strong evidence for a laterality effect.
Discussion
The current investigation linked higher levels of trait anxiety in healthy young adults to smaller gray matter volumes in the left opercular segment of the IFC, and to larger negative biases in attention. It also identified that lower levels of trait anxiety played a mediating role in the protective effects of increased IFC volumes against negative affective biases. Importantly, such triadic relations were not found with the control variables (i.e., depression symptoms, comparison brain regions including the other two segments of IFC as well as rACC, amygdala, rostral middle frontal cortex, and insula). These findings provide initial evidence of an association between the IFC volume and a neuropsychological measure indexing a negative affective bias, and further show that this brain-behavior association occurs through mechanisms partially shared with trait anxiety.
Increased trait anxiety linked to decreased IFC volume and increased negative bias in RT
The present finding showing decreased opercular volumes in young healthy participants with higher anxiety levels advances previous evidence from the clinical literature documenting reduced IFC volumes in patients with anxiety disorders (Shang et al., 2014). Also, the finding of increased negative affective biases in attention in association with higher trait anxiety echoes previous findings of heightened negative biases in anxious populations (Bar-Haim et al., 2007). The opercular IFC has been previously involved in both response inhibition and in the regulation of emotional distractions (Aron et al., 2014; Denkova et al., 2010; Dolcos et al., 2006; Iordan et al., 2013), which seemingly is also implicated in the negative affective biases in attention (Eysenck et al., 2007). Thus, the common associations of the IFC volume and negative biases with trait anxiety also point to a link between impaired executive function and anxiety symptoms. Contrary to our expectations, the current study did not find a direct association between opercular volumes and negative bias measures at the structural level, although an indirect pathway was identified through trait anxiety. It is possible that the negative attention bias in response to valenced target words is tapping into cognitive processes too specific to manifest in the ROI-based volumes, although similar Freesurfer-based approaches have proved effective in identifying links with trait anxiety (Dolcos et al., 2016).
Importantly, the correspondence between the current findings obtained in a sample of healthy individuals and previous findings obtained in patients diagnosed with affective disorders suggests that the mechanisms linking anxiety with negative biases and IFC might be shared between subclinical and clinical populations. This reinforces the idea that studying risk/resilience factors of affective disturbances in healthy populations can inform our understanding of the etiology and development of affective disorders (Montag et al., 2013), and supports the possibility that intervention strategies may be translatable between subclinical and clinical target populations.
Protective effect of IFC volume on negative bias through trait anxiety
The finding of a partial mediation involving trait anxiety suggests that the opercular IFC volume has an indirect effect on behavioral measures of cognitive control through mechanisms related to trait anxiety. This finding links previous understanding of the involvement of IFC in anxiety (Shang et al., 2014) with its role in executive control (Aron et al., 2014) and emotion regulation (Denkova et al., 2010; Dolcos et al., 2006; Iordan et al., 2013), and extends previous functional neuroimaging evidence showing an additive influence of emotional and control processes in IFC on AGN (Brown et al., 2015; Brown et al., 2012). Our mediation findings emphasize the integrated influence of factors across different levels leading to behavioral symptoms, and further supports the idea that impaired executive control may be one of the central manifestations of anxiety symptoms (Eysenck et al., 2007) that can be effectively examined using a brain-personality-behavior framework.
The interpretation of the current mediation results may be informed by previous functional neuroimaging evidence showing that interaction between trait anxiety and affective valence also engages IFC. Using a two-back task, Fales et al. (2010) showed that higher trait anxiety was associated with stronger IFC activation in response to negative stimuli, and weaker activation in response to positive stimuli, suggesting that highly anxious people might recruit IFC to a greater extent in response to negative stimuli. Interestingly, although we did not find strong evidence that directly supports an effect of lateralization, our current findings show that a smaller left IFC gray matter volume was associated with higher trait anxiety, and that the left IFC is associated with a stronger protective effect on negative bias. Although previous theorizing has emphasized a right-lateralization of control (Aron et al., 2014), the current finding of a stronger effect in the left IFC may reflect a shift of emphasis in the control system due to the type of control required by the task demand, i.e., the presence of emotional interference with cognitive processing. Nonetheless, as has been pointed out in recent discussions regarding frontal lobe lateralization (Miller et al., 2013), functional specializations of frontal lobe regions may be more comprehensive than what could be sufficiently characterized by a lateralization account based on a simple theme.
Caveats
Our mediation results suggest a possible specificity in the directionality of effects, such that the mediation model was only significant when the IFC volume was placed as the predictor, but not as the outcome of negative bias in attention. However, mediation models only capture static relations among the variables in discussion, and thus further empirical studies are needed to verify the directions of these relations by manipulating and assessing changes at different levels in a longitudinal design. With this caveat in mind, based on the current results, it appears more likely that changes at the brain level, such as training targeting IFC-related cognitive control functions, may be able to trigger favorable effects of reducing anxiety symptoms and strengthening attentional control, ultimately correcting the cognitive/behavioral bias associated with anxiety. This lends promise to possible future avenues for prevention and intervention studies in healthy populations, which become particularly important in light of the high prevalence and relevance of anxiety issues in today’s society.
Conclusion
In summary, the current study showed that in healthy subjects, lower trait anxiety scores were associated with larger gray matter volumes in the left IFC and smaller negative affective bias in attention, and that trait anxiety mediated the relation between the IFC volume and negative bias. These results extend previous understanding of the role of IFC in emotion-cognition interaction in anxiety from functional neuroimaging studies to the structural level, and inform the development of future tools targeting the prevention and reduction of anxiety and negative affective biases in healthy populations.
Funding
This research was supported by the National Alliance for Research on Schizophrenia and Depression (currently, the Brain & Behavior Research Foundation), Canadian Psychiatric Research Foundation (currently, Healthy Minds Canada), University of Alberta, and University of Illinois at Urbana-Champaign. The authors declare no conflicts of interest with respect to the authorship or the publication of this article.
Conflict of interest. None declared.
Supplementary Material
References
- American College Health Association. (2015). American College Health Association-National College Health Assessment II: Undergraduate Student Reference Group Executive Summary, Spring 2015. Hanover, MD: American College Health Association. [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18,177–85. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van IJzendoorn M.H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin, 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Garbin M.G. (1988). Psychometric properties of the Beck Depression Inventory - 25 years of evaluation. Clinical Psychology Review, 8, 77–100. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry 4, 561–71. [DOI] [PubMed] [Google Scholar]
- Brown M.R.G., Benoit J.P.A., Juhas M., et al. (2015). fMRI investigation of response inhibition, emotion, impulsivity, and clinical high-risk behavior in adolescents. Frontiers in Systems Neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.R.G., Lebel R.M., Dolcos F., et al. (2012). Effects of emotional context on impulse control. NeuroImage, 63, 434–46. [DOI] [PubMed] [Google Scholar]
- Cai W., Ryali S., Chen T., Li C.S., Menon V. (2014). Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. The Journal of Neuroscience, 34,14652–67. doi: 10.1523/jneurosci.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge-Cognition. Cambridge neuropsychological test automated battery. Http://www.Camcog.Com/cantab-tests.Asp.
- Cisler J.M., Bacon A.K., Williams N.L. (2009). Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive Therapy and Research 33,221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron 58, 306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousineau D., Chartier S. (2010). Outliers detection and treatment: a review. International Journal of Psychological Research, 3, 58–67. [Google Scholar]
- Denkova E., Wong G., Dolcos S., et al. (2010). The impact of anxiety-inducing distraction on cognitive performance: A combined brain imaging and personality investigation. Plos One, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31,968–80. [DOI] [PubMed] [Google Scholar]
- DeYoung C.G., Hirsh J.B., Shane M.S., Papademetris X., Rajeevan N., Gray J.R. (2010). Testing predictions from personality neuroscience: Brain structure and the big five. Psychological Science, 21, 820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Kragel P., Wang L.H., McCarthy G. (2006). Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport, 17, 1591–4. [DOI] [PubMed] [Google Scholar]
- Dolcos S., Hu Y., Iordan A.D., Moore M., Dolcos F. (2016). Optimism and the brain: Trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Social Cognitive and Affective Neuroscience, 11, 263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K., Drevets W.C., Clark L., et al. (2005). Mood-congruent bias in affective Go/No-Go performance of unmedicated patients with major depressive disorder. American Journal of Psychiatry, 162, 2171–3. [DOI] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N., Santos R., Calvo M.G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7,336–53. [DOI] [PubMed] [Google Scholar]
- Fales C.L., Becerril K.E., Luking K.R., Barch D.M. (2010). Emotional-stimulus processing in trait anxiety is modulated by stimulus valence during neuroimaging of a working-memory task. Cognition & Emotion, 24, 200–22. [Google Scholar]
- Fischl B. (2012). FreeSurfer. NeuroImage, 62,774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33,341–55. [DOI] [PubMed] [Google Scholar]
- IBM. (Released 2011). IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. [Google Scholar]
- Iordan A.D., Dolcos S., Dolcos F. (2013). Neural signatures of the response to emotional distraction: A review of evidence from brain imaging investigations. Frontiers in Human Neuroscience, 7, 200.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. (2008). Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage, 42, 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224, 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle P.F., Kiehl K.A., Smith A.M. (2001). Event-related fMRI study of response inhibition. Human Brain Mapping, 12, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A., Crocker L.D., Spielberg J.M., Infantolino Z.P., Heller W. (2013). Issues in localization of brain function: The case of lateralized frontal cortex in cognition, emotion, and psychopathology. Frontiers in Integrative Neurosciences, 7, 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C., Reuter M., Jurkiewicz M., Markett S., Panksepp J. (2013). Imaging the structure of the human anxious brain: A review of findings from neuroscientific personality psychology. Reviews in the Neurosciences, 24,167–90. [DOI] [PubMed] [Google Scholar]
- Morey R.A., Dolcos F., Petty C.M., et al. (2009). The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. Journal of Psychiatric Research, 43,809–17. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–99. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–91. [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Fan J., Magidina O., Marks D.J., Hahn B., Halperin J.M. (2007). Does the emotional Go/No-Go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Archives of Clinical Neuropsychology 22, 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience 27(9),2349–56. doi: 10.1523/jneurosci.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Fu Y., Ren Z., et al. (2014). The common traits of the ACC and PFC in anxiety disorders in the DSM-5: Meta-analysis of voxel-based morphometry studies. PLoS One, 9, e93432.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Steiner A.R., Petkus A.J., Nguyen H., Wetherell J.L. (2013). Information processing bias and pharmacotherapy outcome in older adults with generalized anxiety disorder. Journal of Anxiety Disorders 27, 592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn J.R., Hamm L., Fitzgerald D.A., Fitzgerald K.D., Monk C.S., Phan K.L. (2015). Neurostructural abnormalities in pediatric anxiety disorders. Journal of Anxiety Disorders 32, 81–8. doi: 10.1016/j.janxdis.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage, 56,1655–65. [DOI] [PubMed] [Google Scholar]
- Vytal K., Hamann S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: A voxel-based meta-analysis. Journal of Cognitive Neuroscience 22(12),2864–85. doi: 10.1162/jocn.2009.21366. [DOI] [PubMed] [Google Scholar]
- Wilson R. (2015, August 31). An epidemic of anguish, The Chronicle of Higher Education.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


