Abstract
Recent research has implicated altered neural response to interpersonal feedback as an important factor in adolescent depression, with existing studies focusing on responses to feedback from virtual peers. We investigated whether depressed adolescents differed from healthy youth in neural response to social evaluative feedback from mothers. During neuroimaging, twenty adolescents in a current episode of major depressive disorder (MDD) and 28 healthy controls listened to previously recorded audio clips of their own mothers’ praise, criticism and neutral comments. Whole-brain voxelwise analyses revealed that MDD youth, unlike controls, exhibited increased neural response to critical relative to neutral clips in the parahippocampal gyrus, an area involved in episodic memory encoding and retrieval. Depressed adolescents also showed a blunted response to maternal praise clips relative to neutral clips in the parahippocampal gyrus, as well as areas involved in reward and self-referential processing (i.e. ventromedial prefrontal cortex, precuneus, and thalamus/caudate). Findings suggest that maternal criticism may be more strongly encoded or more strongly activated during memory retrieval related to previous autobiographical instances of negative feedback from mothers in depressed youth compared to healthy youth. Furthermore, depressed adolescents may fail to process the reward value and self-relevance of maternal praise.
Keywords: adolescence, depression, social threat, social reward, social cognition
As many as one in seven adolescents struggle with depression (Kessler, 1994), yet existing interventions are ineffective for 40–50% of depressed adolescents (Kennard et al., 2006). As depression typically onsets during adolescence (Costello et al., 2011), research is needed to identify developmentally-sensitive and neuroscience-informed targets for prevention and intervention. Recent research has implicated neural response to interpersonal feedback as a potentially important factor in understanding risk for and maintenance of adolescent depression (Davey et al., 2011; Masten et al., 2011; Silk et al., 2014). This work is consistent with theoretical models of depression, which highlight the critical role of interpersonal sensitivity (Coyne and Downey, 1991; Rudolph and Conley, 2005; Slavich and Irwin, 2014). Neural response to interpersonal feedback may be particularly relevant during adolescence, a developmental period in which interpersonal sensitivity is often exacerbated due to both neurobiological changes and reorganization within the social context (Nelson et al., 2005).
Behavioral research documents increased sensitivity to interpersonal threats, such as peer rejection and romantic breakups, in adolescent depression (Brown and Harris, 1989; Lewinsohn et al., 1999; Hammen and Brennan, 2001; Abela et al., 2005). Furthermore, social rejection from peers often precedes the onset of depressive symptoms in adolescence (Nolan et al., 2003; Rudolph and Conley, 2005). Yet, the neurobiological underpinnings of interpersonal sensitivity in adolescent depression are only beginning to be elucidated. Two recent studies examined how adolescent depression is associated with neural response to negative social feedback. Masten et al. found that elevated activity in the subgenual anterior cingulate cortex (sgACC) during exclusion on a virtual ball-tossing task was associated with increases in depressive symptoms over 1 year in adolescents (Masten et al., 2011). Silk et al. (2014) used a virtual peer evaluation task and found that a clinical sample of depressed adolescents had increased activation of the amygdala, anterior insula, striatum and sgACC in response to simulated peer rejection relative to healthy controls. These studies suggest that adolescents with and at risk for depression are more responsive to negative peer evaluation in regions of the brain involved in monitoring and evaluating emotional salience.
To better focus intervention efforts for adolescent depression, it would be important to know whether this increased neural sensitivity to interpersonal threat is limited to negative feedback from peers or extends to other salient interpersonal relationships. In the present study, we focus on feedback from mothers. Despite the increased value placed on peer relationships during adolescence, adolescents continue to care what their parents think about them (Steinberg and Silk, 2002). Criticism from parents often increases during adolescence, as the adolescent begins to seek independence and assert his or her own conflicting views on issues over which parental authority was previously accepted (Steinberg and Silk, 2002). A recent study examined the neural response to maternal criticism in typically developing adolescents who listened to audio clips of maternal feedback during neuroimaging (Lee et al., 2015). In response to maternal criticism compared to neutral feedback, adolescents showed increased brain activity in subcortical-limbic regions but decreased activity in regions of the brain that subserve cognitive control of emotion. Although no studies have investigated neural response to maternal criticism in depressed adolescents, we anticipated that the affective response to maternal criticism would be even stronger in depressed adolescents than in typically developing adolescents. This is consistent with research conducted by Hooley et al. in depressed adults, which demonstrates that adults with remitted depression exhibit greater amygdala activation in response to maternal criticism compared to healthy adults (Hooley et al., 2005; Hooley et al., 2009).
Although the neuroimaging literature has been relatively consistent in implicating altered social threat responding in adolescent depression, it is less clear whether there are alterations in sensitivity to social rewards in adolescent depression. Studies have clearly documented blunted striatal response to monetary reward in depressed youth and youth high in depressive symptoms (Forbes et al., 2006; Forbes et al., 2009; Forbes et al., 2010; Morgan et al., 2013). Yet, results from studies incorporating socially relevant rewards have been less consistent. Silk et al. (2014) found that depressed and healthy youth did not differ in striatal response to peer acceptance. Davey et al. (2011) similarly found no difference between depressed and healthy adolescents in striatal response to being rated as ‘liked’ by virtual peers; however, depressed participants demonstrated increased amygdala response to being liked compared to healthy participants.
Given these inconclusive findings, we sought to examine whether depressed adolescents differed from non-depressed adolescents in neural response to maternal praise. No studies have examined neural response to parental praise, which presumably might be less salient than positive feedback from peers, in adolescents with depression. In adults, Hooley et al. (2009) found that individuals with remitted depression demonstrated reduced DLPFC and ACC response to praise. In healthy adolescents, Whittle et al. (2012) found that reduced rostral anterior cingulate activation to clips of one’s own mother’s positive affect, relative to an unfamiliar mother’s positive affect, was associated with higher depressive symptoms. These studies suggest that adolescent depression could be associated with reduced neural response to maternal positivity.
Furthermore, despite problems in social functioning and feelings of diminished self-worth in adolescent depression (Orth et al., 2008; Rudolph et al., 2009), it is not known whether adolescents with depression show altered neural response in brain regions that support social-cognitive and self-referential processing in response to social-evaluative feedback. A social-cognitive brain network includes areas involved in perceiving and making attributions about another person’s thoughts and feelings (Blakemore, 2008). These social cognitive processes involve activation of a network of regions that includes the posterior superior temporal sulcus (pSTS), and the temporoparietal junction (TPJ), the temporal poles, the precuneus and the mPFC. Social evaluative feedback requires not only social perception and cognition but also self-inferential processing to interpret the personal relevance of the feedback and integrate it with other autobiographical material stored in memory. This involves the parahippocampal gyrus, which plays an important role in memory encoding and retrieval (Eichenbaum et al., 2007), as well as cortical midline structures such as the ventromedial prefrontal cortex (VMPFC), the medial parietal cortex (MPC) and the posterior cingulate cortex (PCC) (Northoff et al., 2006; Pfeifer et al., 2007).
No studies that we are aware of have investigated social cognitive or self-referential processing in response to social evaluative feedback in adolescents with depression. However, Pfeiffer et al. have shown activation of these regions in healthy adolescents when they are asked to reflect on what they believe other adolescents think of them (Pfeifer et al., 2009). Whittle et al. (2012) demonstrated increased activation of brain regions involved in self-referential and social cognitive processing, such as the precuneus, in response to video clips of mothers expressing positive emotions compared to an unfamiliar adult expressing these emotions. In adults, Morgan et al. (2015) found that depressed mothers with a greater number of depressive episodes showed less precuneus activation when viewing video clips of positive affect displayed by their child compared to an unfamiliar child and greater precuneus response when viewing negative clips of their children, relative to negative clips of an unfamiliar child. This suggests that depressed mothers may have greater self/social processing of personally relevant negative stimuli and reduced self/social processing of personally relevant positive stimuli.
In the present study, we extended this work to investigate self-referential and social cognitive processing in depressed adolescents in response to personally relevant evaluative feedback from mothers. We compared a group of adolescents in a current episode of major depressive disorder (MDD) to healthy control (CON) adolescents previously reported on in Lee et al. (2015). We focused on group differences in response to criticism and praise from mothers across regions of the brain involved in processing threat and reward, as well as self-referential and social information. We hypothesized that, unlike healthy youth, adolescents with MDD would exhibit increased neural sensitivity to criticism and decreased sensitivity to praise in neural networks involved in processing threats and rewards compared to neutral comments. We also hypothesized that adolescents with MDD, unlike CON, would show increased activation of self/social information processing networks in response to maternal criticism and decreased activation of self/social information processing networks in response to maternal praise compared to neutral comments.
Method
Participants
Participants were 48 adolescents (36 female, ages 9–17, M[s.d.]age = 14.58 [1.81]). Twenty adolescents had a current diagnosis of MDD based on DSM-IV criteria (American Psychological Association, 1994) and 28 were healthy controls with no psychiatric history. MDD and CON adolescents did not differ in age, pubertal status, gender, or race (all P’s > 0.05).
Youth were recruited from pediatricians’ offices, community advertisements and mental health clinics. MDD youth were included if they were on a stable dose of SSRI medication but still met criteria for MDD (N = 1). Participants were excluded if they were taking other psychoactive medications or had metal objects in their body. CON youth were excluded if they met current or lifetime DSM-IV diagnosis for any psychiatric disorder. MDD youth were excluded if they had a current diagnosis of obsessive-compulsive disorder, post-traumatic stress disorder, conduct disorder, substance abuse or dependence and ADHD combined type or predominantly hyperactive-impulsive type, or a lifetime diagnosis of bipolar disorder, psychotic depression, schizophrenia, schizoaffective disorder, or a pervasive developmental disorder. Nine MDD youth had a current or past diagnosis of one or more comorbid anxiety disorders. Other comorbid diagnoses in MDD youth included dysthymia (N = 1), oppositional defiant disorder (N = 2) and enuresis (N = 1). Past diagnoses in the MDD youth included anorexia (N = 1), ADHD (N = 1), alcohol and substance abuse (N = 1) and enuresis (N = 2).
Procedure
Informed consent/assent was obtained from participants and their parents at the initial assessment, and all research procedures were approved by the Institutional Review Board. Participants completed two laboratory visits. During the first visit, participants completed a structured diagnostic interview and mothers recorded audio clips to be used during the fMRI assessment on the second visit.
Clinical information
Participants and parent(s) were interviewed to determine the youth's psychiatric history using the Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime version (K-SADS-PL, Kaufman et al., 1997). Parents and youth were interviewed separately, with clinicians integrating data from both informants to arrive at a final diagnosis. Interviews were carried out by trained BA- and MA-level clinicians. Fifteen percent of interviews were double coded and there were no diagnostic disagreements (kappa = 1.0). Anxiety severity was measured by child-report on the Screen for Childhood Anxiety Disorders (SCARED; Birmaher et al., 1997).
Stimuli and experimental paradigms
During the fMRI scan, participants listened to their mother’s comments about them, delivered via MRI compatible headphones. There were 2 audio clips for critical, praising, and neutral comments, each lasting for 30 s. We followed similar procedures used in previous studies (Hooley et al., 2005; Hooley et al., 2009) for obtaining audio clips. Each mother was asked to produce two 30 s audio clips describing things that bother her about her child [critical statements beginning with ‘Name, one thing that bothers me about you is…’, i.e. not doing chores or attitudes towards family member(s)], two 30 s audio clips describing things that she especially likes about her child (praising statements beginning with ‘Name, one thing I really like about you is…’, i.e. sense of humor, being a nice person, willingness to help out, and academic and extracurricular achievements), and two 30 s neutral clips (neutral statements: something your child won’t find interesting, i.e. grocery shopping, parent work or chores, and weather). Mothers were instructed to formulate their critical remarks based on something they had shared with their child on more than one occasion, so that youth would not be exposed to new and potentially disturbing information in the scanner.
There was one block each for critical, praising, and neutral conditions. Each block (run) consisted of two 30.06 s comment presentations (30 s audio clip with 0.06 s additional duration to match with our TR 1.67 s) and three 30.06 s rest periods. Each began with a rest period, followed by one comment presentation, the second rest period, another comment presentation, and then the last rest period. To minimize possible emotional carry-over after listening to criticism or praise from parents, the neutral block was presented first and the order of two other blocks was counterbalanced across participants.
Subjective ratings and debriefing
Participants rated how happy, sad, angry and frustrated they felt on a 1–5 point scale following completion of each block in the scanner. Sad, angry and frustrated ratings were combined to assess negative affect and happy ratings were used to index positive affect. Participants were carefully debriefed following completion of the scan.
Objective ratings of clips
In order to examine whether there were objective differences in the emotional intensity of praise, criticism and neutral clips obtained from the mothers of MDD and CON youth, each audio clip was independently rated by a panel of 11 undergraduate and graduate students who were not aware of diagnostic group assignment. After listening to each clip, they were asked to rate, using a 1–10 scale, (1) how positive or negative the comment was (depending on whether it was a criticism or a praising comment) and (2) how happy or upset they would feel if that comment was made about them. These ratings were combined to reflect the positivity and negativity of each clip.
BOLD functional MRI acquisition, preprocessing and analysis
Imaging acquisition
Images were acquired on a 3T Trio scanner (Siemens, Erlangen, Germany). Thirty-two 3.2-mm slices were acquired parallel to the AC-PC line using a posterior-to-anterior echo planar (EPI) pulse sequence (T2*-weighted imaged depicting BOLD signal; TR = 1670 ms, TE = 29 ms, FOV = 205 mm, flip angle = 75). Each image was acquired in 1.67 s, allowing 18 scans per 30.06 s trial consisting of either a 30.06 s-second comment presentation or rest period. There were three blocks. Each block lasted for 150.3 s (2.505 min). Ninety images (150.3s/TR = 1.67 s) were collected in each block. High-resolution T1-weighted MPRAGE images (1 mm, axial) were also collected for use in cross-registration.
fMRI data preprocessing
fMRI analyses were conducted using locally developed NeuroImaging Software (NIS) (Fissell et al., 2003) and Analysis of Functional Neuroimaging (AFNI) software (Cox, 1996). Functional imaging data were corrected for motion using 3dVolReg implemented in AFNI using the first image as a reference. Quadratic trends within runs were removed and outliers over 1.5 interquartile range (IQR) from the 25th or 75th percentiles were Windsorized using niscorrect to remove non-physiological spikes. Data were temporally smoothed using a 4 point Gaussian filter and converted to %-change based on the median of the timeseries. Data were co-registered to the Colin-27 Montreal Neurological Institute (MNI) template using the Automated Image Registration package’s (AIR (Woods et al., 1993)) 32 parameter non-linear automated warping algorithm and spatially smoothed using a 6 mm full width at half maximum (FWHM) filter.
Statistical analyses
Whole brain analyses: neural responses to maternal criticism and praise compared to neutral comments
To examine group differences in temporal dynamics of neural responses to maternal criticism or praise vs neutral comments, two random-effects whole-brain voxelwise ANOVA’s were conducted with participant as a random factor and group (MDD vs CON), condition (criticism vs neutral, or praise vs neutral) and time (18 scans across 30.06 s) as fixed factors. This model-free analysis was employed to account for empirical variation in the shape of the hemodynamic response (e.g. sustained activity or early deactivation) rather than relying on hemodynamic responses to have a canonical shape. Group X condition X time interaction effect maps were thresholded at an uncorrected P < 0.001. The group-level statistical maps were thresholded at voxel-wise P < 0.001 and corrected for multiple comparisons by using an empirically determined minimum cluster size to achieve a brain-wise corrected P < 0.05, via AFNI’s 3dClustSim with smoothing estimated via AFNI’s 3dFWHMx, version 16.1.04 ‘acf’ procedure. The P < 0.001 threshold is in keeping with recent recommendations for valid cluster size estimation (Woo et al., 2014), and the recent version of ClusterSim responds to a recent methods-critique (Eklund et al., 2016) by accounting for non-Gaussian autocorrelation in estimating smoothness of the data and fixing a historical bug. Our cluster sizes were determined using 5000 Monte Carlo simulations, third-nearest neighbor (NN3) clustering, and one-sided thresholding. Both the uncorrected voxel-wise P value and contiguity threshold necessary to achieve a brain-wise corrected P < 0.05 are reported with each test described below.
Furthermore, we used Guthrie and Buchwald (1991)’s method to control for Type I error across the many evaluated temporal samples (0∼30.06 s) within functional ROIs. As a temporal analog of contiguity thresholding, this technique restricts statistical significance to regions in which there are more consecutive scans each statistically significant at P < 0.05 than would be expected by chance given the temporal autocorrelation of the data (r = 0.50–0.66 after removing 2 principal components, which accounted for ∼75% of the variance in the time-series). Using this technique, Monte Carlo simulations suggested that group X condition interactions significant for 2–3 consecutive scans would be considered to have a temporal region significant at P < 0.05.
Empirically detected regions are interpreted and presented in figures if they met all of the following criteria: (1) they showed significant 3 way interaction effects; (2) they showed significant condition differences within MDD; (3) they were > 65% gray matter; and (4) they have been implicated in emotional, memory, social, or self-related processing in existing literature (via Neurosynth.org; Yarkoni et al., 2011). For regions meeting these criteria, we further examined whether brain activation was associated with subjective ratings of maternal clips. We also examined whether results remained statistically significant controlling for youths’ symptoms of anxiety, since anxiety may also influence brain response to social evaluative feedback (Guyer et al., 2008).
Results
Subjective and objective ratings of clips
Paired sample t-tests were conducted to compare post-scan ratings (made on a 1–5 scale) regarding participants’ subjective experience of praise, criticism and neutral clips (Table 1). As expected, participants rated that their mothers’ critical remarks made them feel more negative than her neutral remarks and her praising remarks made them feel happier than her neutral remarks. Independent samples t-tests (Table 2) indicated that MDD and CON youth did not differ significantly from each other in their subjective ratings of how positive they felt following praise clips or how negative they felt following criticism clips. However, MDD youth rated themselves as more negative following neutral clips than CON youth. There were no significant differences between clips made by mothers of MDD and CON youth in objective ratings (made on a 1–10 scale) by an independent panel regarding the positivity of praise clips, the negativity of criticism clips, or the positivity or negativity of neutral clips (Table 2).
Table 1.
Participants’ subjective ratings of maternal expressed emotion audio clips made following each block during neuroimaging on a 1–5 scale
| Criticism clips [M (s.d.)] | Praise clips [M (s.d.)] | Neutral clips [M (s.d.)] | t-statistic | Sig. (P-value) | |
|---|---|---|---|---|---|
| Negative rating | 2.01 (0.87) | — | 1.42 (0.59) | 5.49 | <0.001 |
| Positive rating | — | 3.40 (1.01) | 2.65 (1.21) | 5.09 | <0.001 |
Table 2.
Group differences in maternal expressed emotion audio clip ratings
| MDD group [M (s.d.)] | CON group [M(s.d.)] | t-statistic | Sig. (P-value) | Cohen’s d | |
|---|---|---|---|---|---|
| Subjective negative ratings | |||||
| Criticism clips | 2.17 (0.75) | 1.89 (0.95) | 1.07 | 0.289 | 0.32 |
| Neutral clips | 1.70 (0.64) | 1.23 (0.46) | 2.98 | 0.005** | 0.83 |
| Subjective positive ratings | |||||
| Praise clips | 3.15 (1.04) | 3.57 (0.96) | –1.45 | 0.154 | 0.41 |
| Neutral clips | 2.25 (1.12) | 2.93 (1.21) | 1.97 | 0.055+ | 0.57 |
| Objective negative ratings | |||||
| Criticism clips | 5.35 (0.70) | 5.28 (0.75) | 0.34 | 0.739 | 0.09 |
| Neutral clips | 1.62 (0.38) | 1.49 (0.36) | 1.20 | 0.238 | 0.35 |
| Objective positive ratings | |||||
| Praise clips | 6.91 (1.00) | 6.71 (0.72) | 0.79 | 0.434 | 0.23 |
| Neutral clips | 1.75 (0.47) | 1.85 (0.47) | −0.731 | 0.468 | 0.21 |
Notes. Subjective ratings were made on a 1–5 scale. Objective ratings were made on a 1–10 scale. **P < 0.01, +P <0.10.
fMRI results
Group differences in neural response to maternal criticism compared to neutral comments
A group (MDD vs CON) X condition (criticism vs neutral) X time (0–30.06 s) interaction was observed in several brain regions as shown in Figure S1 (P < 0.001, 21 voxels contiguity). After controlling for Type I error across temporal samples, brain regions that showed significant group X condition interactions for 2–3 consecutive scans within functional ROIs are shown in Table 3 and Table S1). Of particular interest, there was a significant group X condition interaction in the left parahippocampal gyrus (average activity from 8.35 to 30.06 s; Table S1 and Figure S3). Adolescents with MDD showed greater activity in the left parahippocampal gyrus in response to maternal criticism compared to neutral comments. CON youth showed greater activity in response to neutral comments than criticism in the same region (Figure 1). This result remained significant when controlling for symptoms of anxiety (Table S3). Activity in the left parahippocampal gyrus in response to criticism was not associated with subjective ratings of criticism (r = –0.10, P = 0.497).
Table 3.
Brain regions showing a group X condition (criticism vs neutral) X time interaction (P < 0.001, 21 voxels contiguity)
| Size | Talairach coordinates of centroid |
Temporal regions: significant group X condition | Pairwise comparisons |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain region | BA | (mm3) | X | Y | Z | F value | MDD | CON | |
| L Superior Parietal Lobe | 7 | 7518 | –12 | –62 | 56 | 3.1 | 15.03 ∼ 30.06 s | Crit > Neut | n.s. |
| R Postcentral Gyrus | 5 | 788 | 41 | –45 | 61 | 3.41 | 10.02 ∼ 15.03 s | n.s. | Crit < Neut |
| 23.38 ∼ 30.06 s | Crit > Neut | n.s. | |||||||
| L Postcentral Gyrus | 6 | 6730 | –32 | –19 | 47 | 2.5 | 13.36 ∼ 26.72 s | Crit > Neut | n.s. |
| R Postcentral Gyrus | 2/40 | 1182 | 57 | –30 | 49 | 2.47 | 10.02 ∼ 18.37 s | n.s. | Crit < Neut |
| 21.71 ∼ 30.06 s | Crit > Neut | n.s. | |||||||
| R Precuneus | 7 | 2955 | 14 | –71 | 49 | 2.85 | 20.04 ∼ 30.06 s | Crit > Neut | n.s. |
| R Postcentral Gyrus | 3 | 788 | 47 | –20 | 42 | 2.54 | 10.02 ∼ 20.04 s | Crit > Neut | n.s. |
| 21.71 ∼ 26.72 s | Crit > Neut | n.s. | |||||||
| R Cingulate Gyrus | 31 | 1083 | 30 | –34 | 34 | 2.61 | 11.69 ∼ 20.04 s | n.s. | Crit < Neut |
| 21.71 ∼ 28.39 s | n.s. | n.s. | |||||||
| L Angular Gyrus extending to IPL | 39 | 1215 | –35 | –68 | 36 | 2.63 | 10.02 ∼ 30.06 s | n.s. | Crit < Neut |
| R Medial Frontal Gyrus | 10/11 | 722 | 3 | 60 | –12 | 4.53 | 25.05 ∼ 28.39 s | n.s. | n.s. |
| a L Parahippocampal Gyrus | 35/36 | 854 | –30 | –28 | –17 | 2.66 | 8.35 ∼ 30.06 s | Crit > Neut | Crit < Neut |
| L Culmen | 985 | –10 | –29 | –22 | 2.77 | 8.35 ∼ 16.7 s | Crit > Neut | Crit < Neut | |
| R Culmen | 821 | 29 | –32 | –26 | 3.59 | 6.68 ∼ 28.39 s | Crit > Neut | Crit < Neut | |
Note. BA, Brodmann area; F value, F value of centroid; R, Right; L, Left; n.s., no significant difference between conditions; IPL, Inferior Parietal Lobe.
Average brain activity across the temporal regions that displayed significant group X condition interactions and time-series in these regions are presented in Figure 1 and Figure S3, respectively. Other regions not consistent with Neurosynth.org brain maps implicated in emotional, memory, social, or self-related processing in the literature.
Fig. 1.
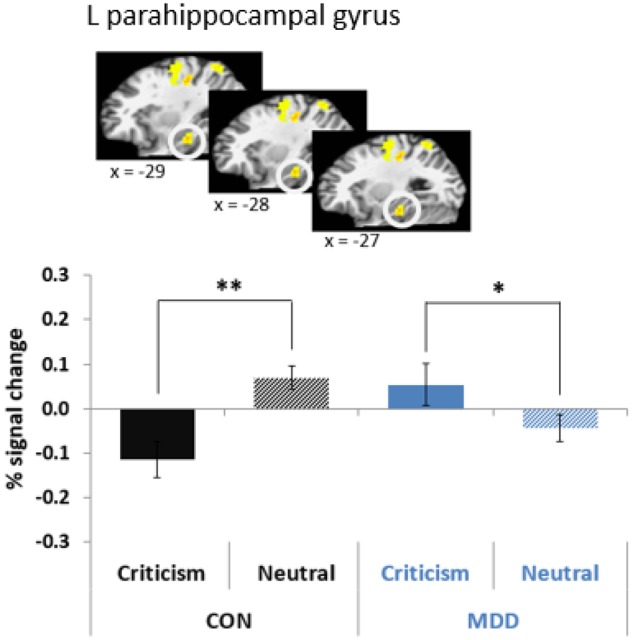
Significant group (MDD vs CON) X condition (criticism vs neutral) in the left parahippocampal gyrus (average activity from 8.35 to 30.06 s) (*P < 0.05, **P < 0.01).
Group differences in neural response to maternal praise compared to neutral comments
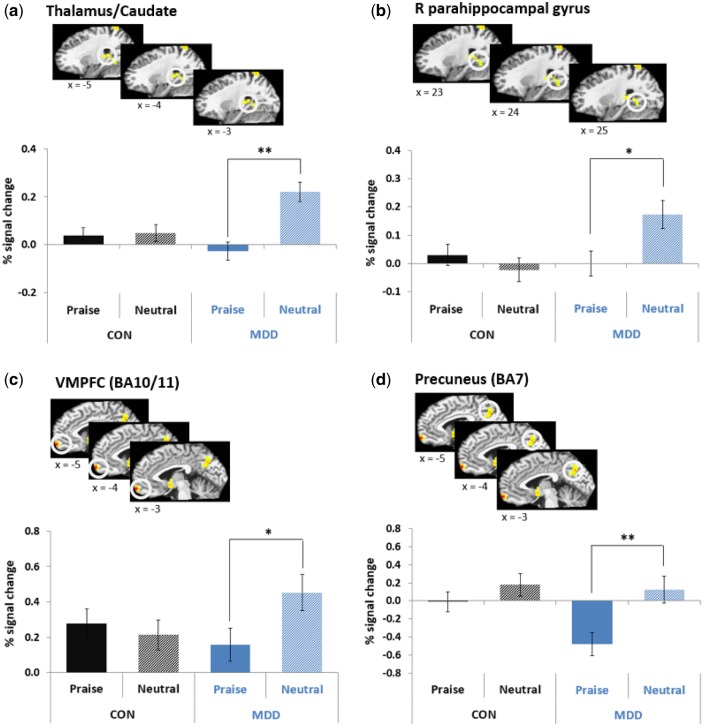
A group X condition X time interaction was again observed in several brain regions (P < 0.001, 18 voxels contiguity; Figure S2). After controlling for Type I error across temporal samples, brain regions that showed significant group X condition interactions for 2–3 consecutive scans within functional ROIs are shown in Table 4 and Table S2. These included the right thalamus extending to the caudate (3.34–10.02 s, 13.36–20.04 s), right parahippocampal gyrus (3.34–8.35 s), VMPFC (13.36–30.06 s) and precuneus (15.03–18.37 s; see Table S2 and Figure S4). As presented in Figure 2, pairwise comparisons revealed that MDD adolescents showed less activity in response to maternal praise compared to neutral comments in the thalamus/caudate, right parahippocampal gyrus, VMPFC, and precuneus; while CON youth did not show significant differences in neural responses to praise vs neutral comments in these regions. These results remained significant when controlling for symptoms of anxiety, with the exception of the right parahippocampal gyrus (Table S4). Brain activation in response to praise was not associated with subjective ratings of praise in any of these regions (r’s range from –0.11 to 0.17, all P’s > 0.19).
Table 4.
Brain regions showing a group X condition (praise vs neutral) X time interaction (P < 0.001, 18 voxels contiguity)
| Size | Talairach coordinates of centroid |
Temporal regions: significant group X condition | Pairwise comparisons |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain region | BA | (mm3) | x | Y | z | F value | MDD | CON | |
| L Postcentral Gyrus | 5 | 755 | –16 | –43 | 63 | 3.44 | 21.71 ∼ 30.06 s | Praise > Neut | n.s. |
| R Superior Parietal Lobule | 7/40 | 3907 | 36 | –54 | 58 | 2.51 | 13.36 ∼ 20.04 s | Praise < Neut | n.s. |
| L Precentral Gyrus | 6 | 624 | –41 | –5 | 57 | 3.07 | 21.71 ∼ 30.06 s | Praise > Neut | n.s. |
| a Precuneus | 7 | 2758 | 2 | –65 | 38 | 3.41 | 15.03 ∼ 18.37 s | Praise < Neut | n.s. |
| R Inferior Frontal Gyrus | 44/45 | 1051 | 56 | 12 | 13 | 2.79 | 25.05 ∼ 30.06 s | Praise > Neut | Praise < Neut |
| R Middle Occipital Gyrus | 19 | 1871 | 34 | –73 | 9 | 2.56 | 21.71 ∼ 28.39 s | Praise > Neut | n.s. |
| R Cuneus | 18/17 | 2200 | 8 | –86 | 11 | 2.95 | 11.69 ∼ 16.7 s | Praise < Neut | n.s. |
| a R Thalamus extending to Caudate | –– | 821 | 23 | –34 | 10 | 2.64 | 3.34 ∼ 10.02 s | Praise < Neut | n.s. |
| b 13.36 ∼ 20.04 s | Praise < Neut | n.s. | |||||||
| L Lingual Gyrus | 17/18 | 3217 | –23 | –81 | 3 | 4.13 | 23.38 ∼ 26.72 s | Praise > Neut | n.s. |
| Subgenual ACC extending to Caudate | 25 | 624 | –5 | 0 | –4 | 2.92 | 18.37 ∼ 25.05 s | n.s. | n.s. |
| a R Parahippocampal Gyrus | 37 | 886 | 24 | –48 | –2 | 2.45 | 3.34 ∼ 8.35 s | Praise < Neut | n.s. |
| a Medial Prefrontal Cortex | 11/10 | 624 | –1 | 60 | –12 | 3.8 | 13.36 ∼ 30.06 s | Praise < Neut | n.s. |
| R Anterior Middle Temporal Gyrus | 21 | 1379 | 54 | 3 | –14 | 3.17 | 20.04 ∼ 30.06 s | n.s. | Praise < Neut |
Note. BA, Brodmann area; F value, F value of centroid; R, Right; L, Left; n.s., no significant difference between conditions.
Average brain activity across the temporal regions that displayed significant group X condition interactions and time-series in these regions are presented in Figure 2 and Figure S4, respectively. Other regions not consistent with Neurosynth.org brain maps implicated in emotional, memory, social, or self-related processing in the literature.
Two temporal regions in the thalamus extending to caudate showed similar patterns of activity in group X condition interactions, so only the first segment is shown in Figure 2.
Fig. 2.
Significant group (MDD vs CON) X condition (praise vs neutral) in a) the right thalamus/caudate (average activity from 3.34 to 10.02 s), b) right parahippocampal gyrus (average activity from 3.34 to 8.35 s), c) VMPFC (BA10/11) (average activity from 13.36 to 25.05 s) and d) precuneus (BA7) (average activity from 15.03 to 18.37 s) (*P < 0.05, **P < 0.01).
Discussion
The results of the present study suggest that depressed and healthy adolescents differ from each other in the way that their brains process social evaluative feedback from their mothers, including both praise and criticism. First, we found that, unlike low-risk controls, adolescents in a current episode of MDD exhibited increased neural response to critical clips, relative to neutral clips, in the parahippocampal gyrus, a brain region involved in episodic memory encoding and retrieval. Conversely, depressed adolescents showed a blunted response to maternal praise clips relative to neutral clips in the parahippocampal gyrus. Depressed adolescents also showed a blunted response to praise relative to neutral clips in areas involved in reward and self-referential processing, such as the thalamus/caudate, ventromedial prefrontal cortex and precuneus. These findings are largely consistent with current theoretical models of depression, which highlight the critical roles of interpersonal sensitivity, altered self-perception and attenuated reward processing (Coyne and Downey, 1991; Joiner and Metalsky, 1995; Allen and Badcock, 2003; Rudolph and Conley, 2005; Orth et al., 2008; Pizzagalli, 2014; Slavich and Irwin, 2014), and add to an emerging literature delineating the neural substrates of these alterations. Findings suggest that altered neural responses to interpersonal feedback in adolescent depression are not limited to feedback from peers, but extend to other important interpersonal relationships during this period, such as the mother-adolescent relationship.
Consistent with a larger literature showing depressed adolescents’ heightened neural response to social threat cues, we find that this pattern extends to criticism from mothers. However, while most previous studies identified heightened amygdala response to threat (Beesdo et al., 2009; Yang et al., 2010; Tao et al., 2012), in the present study the pattern of heightened response to critical comments was seen across a different portion of the medial temporal lobe, primarily encompassing the parahippocampal gyrus. The parahippocampal gyrus has been implicated in episodic memory encoding and retrieval (Eichenbaum et al., 2007) and memory encoding of emotionally salient stimuli compared to neutral stimuli (Murty et al., 2010). It is possible that that maternal criticism may be more strongly encoded or more strongly activated during memory retrieval related to previous autobiographical instances of negative feedback from mothers in depressed youth compared to healthy youth, although future research using memory encoding and retrieval paradigms would be needed to support this possibility. Alternatively, depressed youth may actually have more memories of perceived maternal criticism, consistent with behavioral studies reporting higher levels of conflict in the families of depressed youth (Sheeber et al., 2001), or they may attach greater salience to their negative emotional memories. Conversely, depressed adolescents showed a blunted response to maternal praise clips relative to neutral clips in the parahippocampal gyrus, possibly suggesting reduced salience or disrupted memory of autobiographical memories of mothers providing praise. Again, future research using explicit memory paradigms could be helpful in examining whether depressed adolescents might demonstrate altered memory encoding or retrieval of maternal praise.
Findings also revealed a pattern of attenuated reward processing in the caudate and thalamus among depressed youth when listening to maternal praise clips compared to neutral clips. The caudate is a dorsal portion of the striatum involved in processing the rewarding outcomes of actions (Balleine et al., 2007). Findings may suggest that depressed youth find listening to mothers’ praise them less rewarding than listening to their mothers talk about random neutral topics (i.e. weather, work). This possibility is consistent with research suggesting that individuals with depression are uncomfortable with receiving positive feedback, perhaps because it is not consistent with their own self-perception (Swann et al., 1992). Alternatively, depressed youth may actually experience the neutral comments as rewarding because they signal safety by indicating that the adolescent will not be exposed to evaluative feedback during the remainder of the clip. This is consistent with the timecourse shown in Figure S1, which reveals that MDD youth’s caudate/thalamus response to neutral clips peaks in the first 6 s of the clip. Interestingly, depressed youths’ blunted response to praise extended to the thalamus, which plays key a role in filtering information. A recent study in healthy adolescents suggests that the thalamus sends an alerting signal to the striatum when reward is anticipated (Cho et al., 2013). Reduced thalamus activation to maternal praise may be related to reduced reward expectancy in adolescents with depression.
Blunted caudate activation to praise in adolescents with depression is consistent with previous research demonstrating reduced striatal, and specifically caudate response, to monetary reward in depressed youth and youth high in depressive symptoms (Forbes et al., 2006; Forbes et al., 2009; Forbes et al., 2010; Morgan et al., 2013). However, this is the first study of which we are aware to report reduced neural reward response to a social reward in adolescent depression. Two recent studies examining neural response to social reward from virtual adolescent peers showed no differences in striatal response in depressed and healthy adolescents (Davey et al., 2011; Silk et al., 2014). It may be that peer social rewards are more motivationally salient to adolescents compared to both monetary rewards and social reward from parents, thus activating depressed adolescents in a way that monetary and parental reward does not. An important direction for future research will be to use comparable tasks to examine differences in neural response to social reward from different social companions (i.e. peers, parents, romantic partners) in both depressed and healthy adolescents, as it appears that the type of social relationship may be an important modulator of reward responding in adolescent depression.
Findings also revealed attenuated response to praise relative to neutral clips among depressed adolescents in midline cortical areas involved in social cognition and self-referential processing, such as the VMPFC and the precuneus. Listening to random neutral comments that were not emotional or explicitly relevant to the child generated greater activation in these regions among depressed youth than direct positive comments about the child. Interestingly, both the VMPFC and precuneus have been shown to be activated by real as opposed to imagined autobiographical events, supporting the possibility that depressed adolescents’ reduced activity in these regions in response to maternal praise may be related to limited memory of real-world maternal praise in these youth (Summerfield et al., 2009). It is also possible that depressed youth may fail to experience maternal praise as self-relevant, or as congruent with their own self-perception. Interestingly, this finding parallels Morgan et al. (2015)’s finding that depressed mothers with a greater number of depressive episodes showed less activation in self-referential brain regions, such as the precuneus, when viewing video clips of positive affect displayed by their child compared to an unfamiliar child. Together, these findings suggest that depression in both adolescence and adulthood may be associated with a failure to experience positive aspects of interpersonal relationships as personally relevant or salient to one’s self-identity.
The present study has several limitations. First, the cross-sectional design makes it impossible to determine whether altered neural response to maternal feedback is a risk factor or correlate of adolescent depression. Relatedly, it is unclear the extent to which previous interactions between the mother and adolescent earlier in development, as well as the quality of the mother-adolescent relationship, might moderate neural response to maternal praise and criticism during adolescence. Existing research suggests that parent-offspring relationship quality may influence neural response to social evaluative feedback (Hooley et al., 2012; Tan et al., 2014). Additionally, the present investigation focused only on mothers, despite evidence that the father-adolescent relationship also plays a role in adolescent depression (Sanford et al., 1995). There is also strong evidence of gender and age differences in rates of depression (Kessler, et al., 2001), as well as interpersonal sensitivity (Rudolph, 2002); however, given our relatively small sample size, we were not able to investigate gender and age differences. It is also interesting to note that adolescents with depression showed altered neural reactivity to praise and criticism in the absence of significant group differences in subjective appraisals. The lack of statistically significant differences in subjective ratings may be partly a function of low power to detect small to moderate effects. Nevertheless, it does suggest that the BOLD response may be more sensitive to differences in how depressed and healthy adolescents process social value feedback compared to subjective self-report.
Finally, it is important to acknowledge that although the ecological validity of the task used in this study is a strength of the investigation, the use of personally-relevant stimuli requires a tradeoff with standardization of experimental stimuli. We used real audio recordings of mothers expressing genuine feelings toward their adolescents in order to maximally engage neural networks involved in the processing of real-world social interactions. This approach precluded us from standardizing clips between subjects; however, we were able to demonstrate that clips did not differ between the mothers of depressed and healthy subjects in terms of emotional intensity as perceived by objective raters. Another challenge was to develop neutral stimuli that would be non-emotional but comparable to criticism and praise clips in other perceptual characteristics. Clips of mothers talking about non-emotional information, such as weather and groceries, could be differentially engaging to different youth. Although objective raters did not detect any differences in the emotional quality of neutral clips, depressed youth did rate neutral clips as more negative compared to healthy youth. For this reason, we examined response over time to both neutral and emotional clips within groups, rather than calculating a difference score.
Despite these limitations, this study presents an additional ecologically-valid approach to investigating how adolescents process interpersonally-relevant stimuli, helping to move beyond a historical focus on static emotional faces and extending recent research on peer feedback to another key interpersonal domain. The use of a well-characterized clinical sample of adolescents currently experiencing a depressive episode was also an important strength. Findings suggest potential developmentally-sensitive and neuroscience-informed targets for prevention and intervention of adolescent depression. Specifically, heightened activity in the parahippocampal gyrus in response to maternal criticism suggests that one valuable direction for experimental therapeutics in adolescent depression may be to focus on therapeutic approaches to altering the strength and salience of negative interpersonal emotional memories. A parallel approach informed by findings on response to maternal praise may be to develop mechanisms to increase activation of memory, self-referential and reward processing brain networks during the receipt of positive social feedback. This is an important implication, as existing interventions for depression focus primarily on the normalization of responses to negative information. These therapeutic targets might be altered via psychosocial interventions as well as neurocognitive training and neurofeedback. Such approaches may help depressed adolescents develop more adaptive neural responses to interpersonal praise and rejection.
Supplementary Material
Acknowledgments
The authors are grateful to Marcie Walker, Katie Burkhouse, Terri Nicely and Karen Garelik for their assistance in data acquisition. The authors also thank the participants and their families. This research was supported by a National Institute of Drug Abuse grant R21DA024144 to J.S.S. and R.E.D., Principal Investigators.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Abela J.R., Hankin B.L., Haigh E.A., Adams P., Vinokuroff T., Trayhern L. (2005). Interpersonal vulnerability to depression in high-risk children: the Role of insecure attachment and reassurance seeking. Journal of Clinical Child and Adolescent Psychology, 34(1),182–92. [DOI] [PubMed] [Google Scholar]
- America Psychological Association. (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn, Washington, D.C: Author: American Psychiatric Associatio. [Google Scholar]
- Allen, N. B., Badcock, P. B. (2003). The social risk hypothesis of depressed mood: evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin, 129(6), 887–913. [DOI] [PubMed]
- Balleine B.W., Delgado M.R., Hikosaka O. (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience 27(31), 8161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. (2008). The social brain in adolescence. Nature Reviews Neuroscience 9(4), 267–77. [DOI] [PubMed] [Google Scholar]
- Birmaher, B., Khetarpal, S., Brent, D., et al. (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36(4), 545–53. [DOI] [PubMed]
- Brown G.W., Harris T.O. (1989). Stressful Life Events and Illness. New York: Guilford Press. [Google Scholar]
- Cho, Y. T., Fromm, S., Guyer, A. E., et al. (2013). Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage, 66, 508–21. [DOI] [PMC free article] [PubMed]
- Costello E.J., Copeland W., Angold A. (2011). Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? Journal of Child Psychological Psychiatry 52(10), 1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research 29, 162–73. [DOI] [PubMed] [Google Scholar]
- Coyne J.C., Downey G. (1991). Social factors and psychopathology: stress, social support, and coping processes In: Rosenzweig M. R., editor. Annual Review of Psychology, Vol. 42, pp. 401–425. Palo Alto, CA: Annual Reviews, Inc. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Allen N.B., Harrison B.J., Yucel M. (2011). Increased amygdala response to positive social feedback in young people with major depressive disorder. Biological Psychiatry 69(8), 734–41. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113(28), 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience 30, 123–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fissell K., Tseytlin E., Cunningham D., et al. (2003). Fiswidgets: a graphical computing environment for neuroimaging analysis. Neuroinformatics 1(1), 111–25. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., May C.J., Siegle G.J., et al. (2006). Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychological Psychiatry 47(10), 1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., et al. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., et al. (2010). Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of American Academy Child Adolescence Psychiatry 49(2), 162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie D., Buchwald J.S. (1991). Significance testing of difference potentials. Psychophysiology 28(2), 240–4. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Lau J.Y., McClure-Tone E.B., et al. (2008). Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry 65(11), 1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C., Brennan P.A. (2001). Depressed adolescents of depressed and nondepressed mothers: tests of an Interpersonal Impairment Hypothesis. Journal of Consulting and Clinical Psychology 69(2), 284–94. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Gruber S.A., Parker H.A., Guillaumot J., Rogowska J., Yurgelun-Todd D.A. (2009). Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Research 172(1), 83–91. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Gruber S.A., Scott L.A., Hiller J.B., Yurgelun-Todd D.A. (2005). Activation in dorsolateral prefrontal cortex in response to maternal criticism and praise in recovered depressed and healthy control participants. Biological Psychiatry 57(7), 809–12. [DOI] [PubMed] [Google Scholar]
- Hooley J.M., Siegle G., Gruber S.A. (2012). Affective and neural reactivity to criticism in individuals high and low on perceived criticism. PLoS ONE 7(9), e44412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner, T. E., Metalsky, G. I. (1995). A prospective test of an integrative interpersonal theory of depression: A naturalistic study of college roommates. Journal of Personality and Social Psychology, 69(4), 778–88. [DOI] [PubMed]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of American Academy Child Adolescence Psychiatry 36(7), 980–8. [DOI] [PubMed] [Google Scholar]
- Kennard B., Silva S., Vitiello B., et al. (2006). Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS). Journal of American Academy Child Adolescence Psychiatry 45(12), 1404–11. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. (1994). Lifetime and 12-month prevalence of DSM-III–R psychiatric disorders in the United States: results from the National Comorbidity Study. Archives of General Psychiatry 51(1), 8–19. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C., Avenevoli, S., & Merikangas, K. R. (2001). Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry, 49(12), 1002–14. [DOI] [PubMed]
- Lee, K. H., Siegle, G. J., Dahl, R. E., Hooley, J. M., Silk, J. S. (2015). Neural responses to maternal criticism in healthy youth. Social Cognitive and Affective Neuroscience, 10(7), 902–12. [DOI] [PMC free article] [PubMed]
- Lewinsohn P.M., Allen N.B., Seeley J.R., Gotlib I.H. (1999). First onset versus recurrence of depression: differential processes of psychosocial risk. Journal of Abnormal Psychology 108(3), 483–9. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Eisenberger N.I., Borofsky L.A., McNealy K., Pfeifer J.H., Dapretto M. (2011). Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Development and Psychopathology 23(1), 283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, J. K., Ambrosia, M., Forbes, E. E., Cyranowski, J. M., Amole, M. C., Silk, J. S., . . . Swartz, H. A. (2015). Maternal response to child affect: Role of maternal depression and relationship quality. Journal of Affective Disorders, 106–113. [DOI] [PMC free article] [PubMed]
- Morgan J.K., Olino T.M., McMakin D.L., Ryan N.D., Forbes E.E. (2013). Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiological Disease 52, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E., Pine D.S. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine 35(2), 163–74. [DOI] [PubMed] [Google Scholar]
- Nolan S.A., Flynn C., Garber J. (2003). Prospective relations between rejection and depression in young adolescents. Journal of Personality and Social Psychology 85(4), 745–55. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage 31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- Orth U., Robins R.W., Roberts B.W. (2008). Low self-esteem prospectively predicts depression in adolescence and young adulthood. Journal of Personality and Social Psychology 95(3), 695–708. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Lieberman M.D., Dapretto M. (2007). “I know you are but what am I?!”: neutral bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience 19(8), 1323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. (2009). Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Development 80(4), 1016–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D. A. (2014). Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annual Review of Clinical Psychology, 10, 393–423. [DOI] [PMC free article] [PubMed]
- Rudolph, K. D. (2002). Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health, 30(4), 3–13. [DOI] [PubMed]
- Rudolph K.D., Conley C.S. (2005). The socioemotional costs and benefits of social-evaluative concerns: do girls care too much? Journal of Personality 73(1), 115–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K.D., Flynn M., Abaied J.L., Groot A., Thompson R. (2009). Why is past depression the best predictor of future depression? Stress generation as a mechanism of depression continuity in girls. Journal of Clinical Child and Adolescent Psychology 38(4), 473–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M., Szatmari P., Spinner M., et al. (1995). Predicting the one-year course of adolescent major depression. Journal of American Academy Child Adolescent Psychiatry 34(12), 1618–28. [DOI] [PubMed] [Google Scholar]
- Sheeber, L., Hyman, H., Davis, B. (2001). Family processes in adolescent depression. Clinical Child and Family Psychology Review, 4(1), 19–35. [DOI] [PubMed]
- Silk J.S., Siegle G.J., Lee K.H., Nelson E.E., Stroud L.R., Dahl R.E. (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience 9(11), 1798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Irwin M.R. (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin 140(3), 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Silk J.S. (2002). Parenting adolescents In: Bornstein M.H., editor. Handbook of Parenting: Vol. 1: Children and Parenting, 2nd edn, pp. 103–133, Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Summerfield J.J., Hassabis D., Maguire E.A. (2009). Cortical midline involvement in autobiographical memory. Neuroimage 44(3), 1188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann W.B., Wenzlaff R.M., Tafarodi R.W. (1992). Depression and the search for negative evaluations: more evidence of the role of self-verification strivings. Journal of Abnormal Psychology 101(2), 314–7. [DOI] [PubMed] [Google Scholar]
- Tan P.Z., Lee K.H., Dahl R.E., et al. (2014). Associations between maternal negative affect and adolescent's neural response to peer evaluation. Development of Cognitive Neuroscience 8, 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, R., Calley, C. S., Hart, J., et al. (2012). Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. American Journal of Psychiatry, 169(4), 381–8. [DOI] [PMC free article] [PubMed]
- Whittle S., Yucel M., Forbes E.E., et al. (2012). Adolescents' depressive symptoms moderate neural responses to their mothers' positive behavior. Social Cognitive and Affective Neuroscience 7(1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, C. W., Krishnan, A., Wager, T. D. (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage, 91, 412–9. [DOI] [PMC free article] [PubMed]
- Woods R.P., Mazziotta J.C., Cherry S.R. (1993). MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography 17(4), 536–46. [DOI] [PubMed] [Google Scholar]
- Yang, T. T., Simmons, A. N., Matthews, S. C., et al. (2010). Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child & Adolescent Psychiatry, 49(1), 42–51. [DOI] [PMC free article] [PubMed]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8(8), 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.