Abstract
Previous neuroimaging work has shown that increased reward-related activity following exposure to food cues is predictive of self-control failure. The balance model suggests that self-regulation failures result from an imbalance in reward and executive control mechanisms. However, an open question is whether the relative balance of activity in brain systems associated with executive control (vs reward) supports self-regulatory outcomes when people encounter tempting cues in daily life. Sixty-nine chronic dieters, a population known for frequent lapses in self-control, completed a food cue-reactivity task during an fMRI scanning session, followed by a weeklong sampling of daily eating behaviors via ecological momentary assessment. We related participants’ food cue activity in brain systems associated with executive control and reward to real-world eating patterns. Specifically, a balance score representing the amount of activity in brain regions associated with self-regulatory control, relative to automatic reward-related activity, predicted dieters’ control over their eating behavior during the following week. This balance measure may reflect individual self-control capacity and be useful for examining self-regulation success in other domains and populations.
Keywords: self-control, reward, fMRI, dieting, individual differences
In most situations, human beings can successfully exert control over thoughts, impulses and behaviors. People often take this self-control capacity for granted, until it fails and they succumb to temptation. Psychologists have proposed dual-process models of self-control that include dissociable impulsive and inhibitory components (e.g. Hofmann et al., 2009). A recent theory suggests that the neural basis of self-control involves a balance between automatic processes that represent rewarding qualities of stimuli and controlled activity in prefrontal cortex that regulates this bottom-up activity (Heatherton and Wagner, 2011). Human neuroimaging studies have reliably mapped reward and control systems, but often separately, via cue-reactivity paradigms in which participants passively view or evaluate their responses to appetitive cues, such as high-caloric foods or drug cues (Garavan et al., 2000; Cloutier et al., 2008; Kober et al., 2010; Casey et al., 2011), and inhibitory control tasks in which participants are instructed to inhibit reactions to stimuli that otherwise would elicit automatic responses (Padmala and Pessoa, 2010; Aron et al., 2014; Berkman et al., 2014; White et al., 2014). An unanswered question is the extent to which both reward and control systems are simultaneously recruited when people encounter tempting cues that threaten self-control (Kelley et al., 2015), and whether differential engagement of these systems predisposes people to experience self-control success or failure in real world settings.
Neuroimaging studies assessing reactivity to appetitive food cues have reliably observed activation of reward circuitry, particularly ventral striatum and orbitofrontal cortex (OFC) (Garavan et al., 2000; Knutson et al., 2005; Cloutier et al., 2008; Somerville et al., 2010; van der Laan et al., 2011). Furthermore, increased reward-related activity in these regions is predictive of self-control failure outside of the scanner environment, with higher reward activity associated with greater likelihood to indulge in desires to eat on a daily basis (Lopez et al., 2014) as well as weight gain over a longer time span (Demos et al., 2012). Importantly, these effects were observed in individuals not selected for dieting status and for whom occasional indulgences were unlikely to reflect self-regulatory failure. Restrained eaters, on the other hand, try to maintain ongoing regulatory goals with respect to eating, but this group is prone to self-control lapses and failure (e.g. Herman and Polivy, 1975; Heatherton and Baumeister, 1991). Additionally, paradigms that have experimentally manipulated restriction of food intake, by randomly assigning participants to dieting or non-dieting (control) conditions, have demonstrated that dieting increases cortisol production (Tomiyama et al., 2010) and can backfire by leading to increased consumption (Giuliani et al., 2015).
Common sense suggests that chronic dieters might be especially likely to show heightened reward-related brain activity in response to food cues. Quite paradoxically, however, in several studies restrained eaters show minimal activity in regions associated with reward processing (i.e. ventral striatum and OFC) during passive viewing of food cues in neutral conditions (Demos et al., 2011; Wagner et al., 2013). Indeed, in many circumstances dieters eat considerably less than do non-dieters. After all, by definition they are limiting intake of food. But, dieters are also notorious for self-control failure over eating in many circumstances. Behaviorally, self-regulation can be disrupted by situational contexts that lead to self-regulation failures. Three prominent causes of dietary failure outside of the laboratory are emotional distress, diet violations, and depletion of self-regulatory resources (Kelley et al., 2015). Laboratory studies have revealed consistent evidence that chronic dieters overeat when placed in these contexts (Heatherton, 2011; Wagner and Heatherton, 2015 for reviews).
Importantly, each of these contexts has been studied using neuroimaging and each is marked by increased food cue-reactivity in the reward system. That is, this pattern obtains when diets are broken via a milkshake preload (Demos et al., 2011); when dieters receive a negative mood induction (Wagner et al., 2012); and following effortful exertion of self-control (i.e. depletion; Wagner et al., 2013). Thus, studies using neuroimaging have found increased reward activity for situational contexts where dieters are prone to overeating both inside and outside the lab. One speculation is that absent other demands on self-regulatory resources, dieters can effectively inhibit reward-related responses to food cues. When self-regulatory resources are challenged, however, inhibitory control is reduced and reward responsivity reemerges. In support of this possibility, Wagner et al. (2013) showed that after completing a depleting attention task, dieters experienced a breakdown of functional connectivity between the inferior frontal gyrus (a region in prefrontal cortex reliably associated with inhibitory control) and OFC when viewing appetizing food cues, as well as increased OFC activity.
The balance between reward and self-regulation capacity generalizes beyond eating behavior and may be important for understanding more basic principles of self-regulation. For instance, a recent experiment by Freeman and Aron (2016) demonstrated a depletion effect for an inhibitory control task (i.e. go/no-go). In their study, participants whose self-regulatory resources were taxed committed more errors on no go trials—but only for trials where the no-go stimulus had high-reward value (Freeman and Aron, 2016). Taken together, these findings suggest that self-regulatory depletion manipulations may interfere with processes related to top-down inhibitory control, resulting in increased reward-related activity.
In light of these findings from previous behavioral and neuroimaging work, several key aims motivated the present study. First, we set out to test whether the above neural effects following exertion of self-control would correspond with real world eating behaviors, namely dieters’ constant need to engage in self-control in the face of tempting food cues. To this end, we adapted Wagner et al. (2013) design (i.e. a depleting inhibitory control task requiring effortful self-control exertion, followed by passive viewing of appetizing food cues) with the logic that this paradigm might temporarily simulate dieters’ experience of chronic self-regulatory challenges in their daily lives.
Second, we developed a novel approach to analyzing neural cue reactivity and putative regulatory processes. Previous neuroimaging studies have employed paradigms that standardize intentions to regulate by providing all participants with explicit instructions and/or cues to do so. In these paradigms, a regulatory process of interest (e.g. inhibitory control in Berkman et al., 2014, or cognitive reappraisal in Kober et al., 2010) is elicited and measured with an explicit condition or contrast. In this study, we recruited individuals who share the long-term goal of restricting food intake to maintain or lose weight, under the assumption that these chronic dieters, without explicit instruction, routinely regulate their responses to food. Given their need to regulate responses to food cues in daily life, we propose that any food-cue-related activity observed in regions canonically associated with regulation (e.g. lateral prefrontal cortex) might reflect ongoing motivation to regulate food intake.
Third, we wanted to replicate previous studies that have adopted a brain-as-predictor approach (see Berkman and Falk, 2013), but in a population that is particularly vulnerable to self-regulation failure—restrained eaters. Focusing on this population allowed us to formally test several psychological models of self-control that converge on the same core idea. That is, to best characterize self-control outcomes, both impulsive ‘and’ inhibitory processes should be taken into account, such that the relative engagement of one process vs the other may be maximally predictive of behavior—as opposed to the strength or engagement of either process alone (James, 1890; Lewin, 1951; Kotabe and Hofmann, 2015). The balance model recently proposed by Heatherton and Wagner (2011) makes similar predictions, positing that self-control arises from an interplay of both control and reward-related processes, corresponding to brain systems associated with regulation and reward, respectively. Specifically, for reward-related regions, we focused on activity in ventral striatum and OFC, regions that reliably index the reward value of stimuli (e.g. Diekhof et al., 2012). For regions that support regulation, we examined activity in regions of the frontoparietal (FP) network, previously defined in large-sample resting state functional connectivity studies and has been associated with flexibly exerting control on a moment-by-moment basis (Dosenbach et al., 2007; Power et al., 2011; Petersen and Posner, 2012).
To test the balance model in chronic dieters, we simultaneously examined activity within both sets of brain regions, computing a brain-derived measure that reflects the relative balance of food cue-reactivity in regions associated with reward and self-regulatory control. We hypothesized that dieters may vary in this balance of activity, such that those dieters with a greater relative engagement of regulatory (vs reward) processes would demonstrate greater self-control success in everyday life. We also hypothesized that, following an exertion of self-control, this balance measure would have predictive and ecological validity for capturing real world responses to food temptations. As discussed earlier, this is a prototypical experience among dieters because of the ongoing nature of dieting goals (i.e. any time a dieter faces a tempting food cue, he or she likely has previously exerted self-control to resist desires to eat). To ensure ecological validity of the self-control outcomes we measured, we employed a validated ecological momentary assessment (EMA) protocol in which participants were prompted to report their eating behaviors as they occurred throughout a given day (Hofmann et al., 2012; Lopez et al., 2014). The short measurement windows of EMA make it advantageous over other forms of self-report, which are prone to memory and mis-estimation biases (e.g. see Gorin and Stone, 2001; Stone and Shiffman, 2002).
To summarize, we tested the balance model of self-control, by first simulating a challenging context for chronic dieters (i.e. food cue exposure after previous exertion of self-control) and then assessing food-cue brain activity across both control and reward systems. From this activity we developed a novel brain-based measure reflecting relative engagement of control (vs reward) systems and used it characterize and predict the circumstances under which diets succeed (or fail) in real life.
Methods
Seventy-five females (Mage = 19.38, Range = 18–23) from the Dartmouth community participated in the study. All participants were chronic dieters (as assessed by the Restrained Eating Scale; Herman and Polivy, 1980; Heatherton et al., 1988) and gave informed consent. Participants first underwent a functional magnetic resonance imaging (fMRI) scan, followed by a weeklong period of smartphone EMA of eating behaviors. In the fMRI session, participants first performed a difficult inhibition task that taxes self-control capacity (Wagner et al., 2013). This was immediately followed by a previously validated incidental cue-reactivity task that consisted of various image types, including appetizing, high-calorie foods (Wagner et al., 2013; Lopez et al., 2014). Participants made perceptual (indoor/outdoor) judgments about the images, which ensured that participants remained alert but naive to the purpose of the study. fMRI data were analyzed using the Statistical Parametric Mapping software package (SPM8; Wellcome Department of Cognitive Neurology) and add-on tools for automating and batching (freely available at https://github.com/ddwagner/SPM8w).
Following the fMRI scan, participants completed the smartphone EMA portion of the study, in which they reported different aspects of their eating behaviors, several times a day for 1 week. The EMA protocol closely followed that of our previous work (Hofmann et al., 2012; Lopez et al., 2014). Specifically, all EMA prompts were pre-programmed and scheduled using the SurveySignal survey platform (Hofmann and Patel, 2015). Participants were prompted (via SMS message) seven times a day at random intervals, across a 14-h time window that overlapped with participants’ waking hours. Participants received, on average one EMA prompt every two hours. Each prompt included a link to a survey that included questions about whether participants experienced a food desire currently or recently (i.e. ≤20 minutes), the strength of the desire on a seven-point Likert scale, and whether they gave in to the reported desire and already ate, or not (i.e. desire enactment). Additionally, whenever participants reported having a current or recent desire, they categorized desired food in one of eight categories: (i) grains, breads, cereals; (ii) dairy products; (iii) meat; (iv) fish; (v) junk food; (vi) vegetables; (vii) fruits; and (viii) sweets (e.g. candy, chocolate). Energy density of food items is a potentially important factor to consider in eating and health domains, given that higher density foods (e.g. highly processed and/or high in carbohydrates) are more likely to cause weight gain (Donaldson, 2004). Our EMA protocol did not have any questions about the energy density of the desired food items. However, as a rough proxy we created a dichotomous variable representing whether participants desired junk foods or sweets (vs all other food categories), as junk food and sweets tend to have relatively higher caloric content.
fMRI procedure and analysis
fMRI data were collected with a 3-Tesla Philips Intera Achieva scanner (Philips Medical Systems) equipped with a SENSEitivity Encoding head coil. Stimuli were presented using SuperLab 4.0 (Cedrus Corporation) and projected to an Epson ELP-7000 LCD screen positioned at the end of the magnet bore. Participants were able to view the screen via a mirror mounted on the head coil. While in the scanner, subjects completed an event-related cue reactivity task in which they viewed a series of images and were instructed to make perceptual judgments as to whether each image depicted an indoor or outdoor scene. All judgments were made with a corresponding button press on a Lumina LU-400 fMRI response pad. The indoor/outdoor task incorporated images of food, people, animals and nature scenes, so participants were naïve to the purpose of the study. The cue reactivity task employed a rapid, event-related design, with all design parameters and trial timing following those from previous studies that administered the task (e.g. Lopez et al., 2014).
For each functional (EPI) run, data were corrected for differences in slice-timing and preprocessed to remove sources of artifact and noise. Functional data were realigned within and across runs to correct for head movement and were unwarped to reduce any residual movement-related image distortions. Functional data were normalized into a standard stereotaxic space (3-mm isotropic voxels) based on the SPM8 EPI template that conforms to the ICBM 152 brain template space (Montreal Neurological Institute; MNI) and approximates the Talairach and Tournoux atlas space. Normalized images were then spatially smoothed (6mm full-width-at-half-maximum) using a Gaussian kernel. Six subjects’ data were excluded from further analysis, due to excessive motion-related artifact (defined as more than two instances of movement >2 mm; final n = 69 for all subsequent analyses). For each subject, a general linear model that incorporated task conditions (convolved with a canonical hemodynamic response function) and covariates of non-interest (e.g. six motion parameters from realignment correction, a linear trend to account for drifts in scanner signal) were used to compute t-contrast images (weighted parameter estimates) for the Food > Non-Food Control images comparison at each voxel.
Given our interest in the balance between top-down control and bottom-up reward processes, we conducted an a priori region-of-interest (ROI) analysis by extracting parameter estimates from sets of FP and reward ROIs, respectively. For FP regions, we used coordinates from eight regions previously defined by resting-state functional connectivity studies (Dosenbach et al., 2007; Power et al., 2011; see Figure 1 and Table 1). Next, for reward-related activity, we focused on regions robustly associated with reward processing of appetitive cues across neuroimaging studies, namely ventral striatum and OFC (e.g. see van der Laan et al., 2011 for a meta-analysis). Importantly, dieters have shown food cue-reactivity in both areas (Wagner et al., 2013). We used coordinates centered on peak activation from Wagner et al. (2013) study to define ROIs in left and right ventral striatum (MNI coordinates: ±9, 3, −6) and left OFC (coordinates: −30, 33, −18). For all ROIs (see Figure 1), we used 6-mm seeds to extract parameter estimates reflecting food cue specific activity (in both FP and reward regions) for each participant.
Fig. 1.
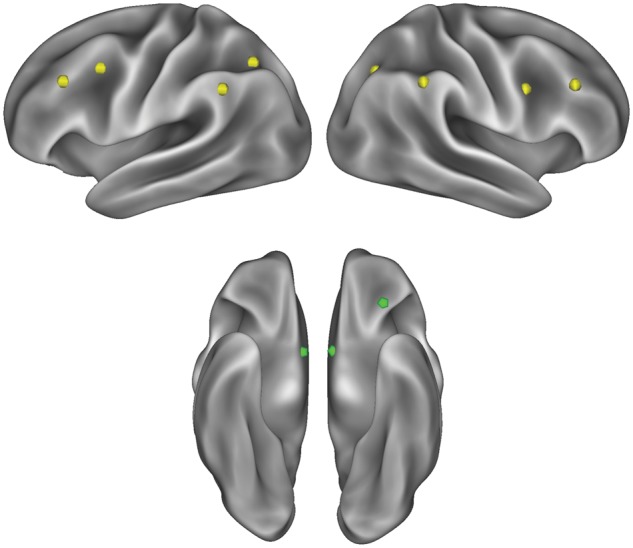
Lateral (top left and right) and ventral (bottom) views on an inflated brain surface depicting ROIs in FP (yellow) and reward (green) systems. ROI spheres have been enlarged to 10 mm for better visualization.
Table 1.
Coordinates of a priori FP and reward ROIs used to create regulation-reward balance scores (all FP ROIs were taken from Dosenbach et al., 2007 and Power et al., 2011, and reward ROIs came from Wagner et al., 2013)
| Region type | MNI coordinates |
Label | |||
|---|---|---|---|---|---|
| X | Y | Z | L/R | ||
| FP | 46 | 28 | 31 | R | Dorsolateral prefrontal cortex |
| FP | −44 | 27 | 33 | L | Dorsolateral prefrontal cortex |
| FP | 44 | 8 | 34 | R | Middle frontal gyrus |
| FP | −42 | 7 | 36 | L | Middle frontal gyrus |
| FP | 54 | −44 | 43 | R | Inferior parietal lobe |
| FP | −53 | −50 | 39 | L | Inferior parietal lobe |
| FP | 32 | −59 | 41 | R | Inferior parietal sulcus |
| FP | −32 | −58 | 46 | L | Inferior parietal sulcus |
| Reward | −30 | 33 | −18 | L | OFC |
| Reward | 9 | 3 | −6 | R | Ventral striatum |
| Reward | −9 | 3 | −6 | L | Ventral striatum |
Computation of brain-based balance scores and outcome measure
To compute brain-based balance scores, we first standardized values within each region, and then took the aggregate mean activity across ROIs in each set. Consequently, each subject contributed one value reflecting mean activity across FP ROIs and one value reflecting mean activity across reward ROIs, with positive values reflecting relative greater or negative values reflecting lesser activity in s.d. units. Importantly, this permitted a simple difference score (control–reward) to be calculated for each subject that reflected relative overall bias towards inhibition (positive values) or impulsivity (negative values). Last, for our behavioral outcome measures, we logged how often subjects gave in to food desires during the 1-week EMA sampling period (i.e. the proportion of times subjects ate after experiencing a current or recent desire for food), as well as how much food they ate when they did give in to desires to eat, measured on a scale from 1 (‘a tiny bit’) to 5 (‘much more than a regular portion–I’m stuffed’).
Results
Participants completed an average of 31.36 EMAs (s.d. = 8.59; range = 8–44) during the sampling period. Overall reported frequency of food desires was relatively low, but revealed some variation (M = 35.3%, s.d. = 15.7%, range = 5.4–71%). When desires were reported, participants gave in to their desire and consumed food 49.6% of the time (s.d. = 22.8%, range = 0–100%). We compared desires and enactment for junk food/sweets vs all other food categories. Following this dichotomous coding of desire instances, 37 out of 69 (53.6%) reported having desires for junk food or sweets during the sampling period. Among these participants, enactment rate for junk food/sweets (48.9%) and non-junk food/sweets (51.0%) did not significantly differ, t(36) = −0.301, P = 0.765. Additionally, to test for the possibility that those dieters who reported having desires for junk food/sweets might struggle in controlling their desires for foods more generally, we compared enactment rate for all other food types in the 37 participants who reported craving junk food/sweets, to that in the 32 participants who didn’t report craving junk food/sweets. The enactment rates of these two subgroups (45.2 and 48.9%, respectively) did not significantly differ, t(67) = −0.625, P = 0.534. Since no differences were observed in these comparisons, we focused on overall enactment rate as our main outcome measure in all subsequent analyses.
In terms of brain activity, food-cue reactivity in either the reward or control networks did not predict giving in to food temptations. That is, neither reward system activity nor FP activity was associated with participants’ enactment of their desires to eat. There were also no significant correlations between activity in any individual reward or FP region and desire enactment (all P’s > 0.10). However, and in support of our main hypotheses, a linear model regressing enactment on regulation-reward balance scores did demonstrate a robust relationship, b = −0.132, r(67) = −0.410, P = 0.0005 (95% bootstrapped CI of correlation coefficient with 5000 iterations: −0.652, −0.215). Specifically, those dieters with higher regulation-reward balance scores successfully resisted their desires to eat more frequently (see Figure 2).1 To account for any variance in enactment associated with activity in the brain regions we selected, we ran two multiple regression models predicting enactment, in which we included regulation-reward balance scores as the main regressor of interest, and parameter estimates from either the three reward regions (model 1) or eight FP regions (model 2) as regressors of no interest. In these two models, balance scores remained robustly associated with less frequent enactment (both P’s < 0.003) with stable regression coefficients (bmodel1 = −0.131, bmodel2 = −0.144).
Fig. 2.
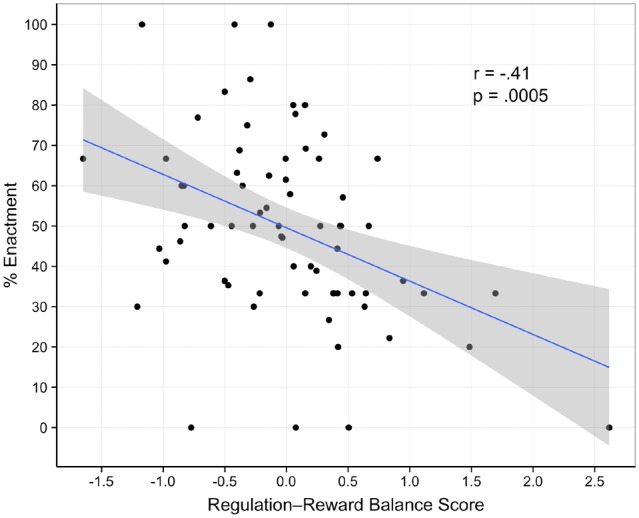
Scatter plot of regulation-reward balance scores regressed on percentage of enacted food desires.
As a further test of our hypothesis that greater engagement of control vs reward systems mitigates enactment, the sample was split into two groups based on positive (i.e. higher control) and negative (i.e. higher reward) balance scores. We then inspected the proportion of desire enactment by group. The group with higher relative engagement of reward regions gave in to desires to eat more often (57.3% of the time) than the group with higher engagement of the FP control system (41.6% of the time), χ2(1) = 8.086, P = 0.005. Additionally, there was a significant negative association between balance scores and amount of food eaten throughout the week, r(67) = −0.256, P = 0.033 (95% bootstrapped CI of correlation coefficient with 5000 iterations: −0.527, −0.025), with those dieters with higher relative FP-vs-reward engagement reporting eating smaller portions when they did give in to desires to eat.
Discussion
Here we show that the balance of dieters’ food cue reactivity between control and reward brain systems is predictive of self-control success and failure. Our findings suggest that when faced with tempting food cues, dieters who recruit FP (control) regions more so than reward regions are more successful in curbing their daily desires to eat. Critically, they provide empirical support for theories that predict that self-control is most likely to occur when regulatory processes that restrain downstream behavior are engaged relative to an impulsive process that, unchecked, would otherwise lead to self-control failure (Hofmann et al., 2009; Heatherton and Wagner, 2011).
In addition to replicating and extending prior research using the brain-as-predictor approach, which correlates brain activity with real world behavioral outcomes (e.g. Berkman et al., 2011), this study makes several contributions. First, we administered a self-regulatory challenge task to evoke activity in both reward and control systems, and then used these evoked patterns of brain activity to predict dieters’ daily eating behaviors. Although prior work has demonstrated brain–behavior relationships in the eating domain (Lopez et al., 2014), the current study linked brain activity to self-regulation success or failure in a population that experiences frequent challenges to control their eating. We were able to operationalize participants’ success or failure as or refraining from or giving in to desires to eat, respectively, since we recruited from a population that is characterized by maintaining the ongoing self-regulatory goal of broadly restricting food intake.
Additionally, in contrast with prior work that has tended to focus separately on either impulsive or control processes and their behavioral correlates, we took a balance model approach (Heatherton and Wagner, 2011) by examining the neural correlates of these processes in tandem, namely the simultaneous, ‘relative’ engagement of brain systems associated with control and reward. The current findings make a new contribution to this literature by demonstrating that the balance of activity may serve as a better neural marker for behavior than activity in either system alone. Importantly, this relative balance of activity was measured as dieters passively viewed appetitive food cues, as opposed to activity elicited by overt instructions to regulate responses to the cues.
The linear relationship observed between balance scores and enactment of food desires promotes speculation that even within the dieting population, there may be: (i) varying levels of (dieting) goal activation following exposure to tempting food cues that may be indicative of one’s underlying intention to regulate; (ii) differential ability or ease when exerting control over food desires; or (iii) some combination of both. Future work can test these hypotheses by designing studies in which these constructs (i.e. intention to regulate and ability/ease when regulating) are explicitly measured and wedded to diet adherence or other outcome measures that index self-control success and failure. Future research might also examine whether the variability in dieters’ recruitment of FP versus reward regions reflects differential priming or activation of dieting goals. The images of food presented during the cue reactivity task may have automatically triggered dieting goals, outside awareness (Aarts, 2007; Neal et al., 2012; Wood and Rünger, 2016), but maybe more so for some dieters in the sample than others.
Another contribution of this study was using a network based approach to aggregate activity within independently defined reward and control networks, rather than discrete brain regions. Kelley et al. (2015) have recently proposed such an approach, arguing that a systems-based approach may be a fruitful avenue to characterize individual differences in self-control capacity—with the FP network a prime candidate as a control system (Kelley et al., 2015). We explicitly tested this by using a priori FP ROIs based on resting state functional connectivity studies (Dosenbach et al., 2007; Power et al., 2011). It is possible, then, to interpret activity in the FP system as indicative of that system’s functional integrity and ability to be recruited across multiple instances of self-control challenges in daily life. This makes sense, given that resting state functional connectivity is thought to reflect statistical patterns of co-activation across regions that develop over time. This approach may inform subsequent neuroimaging work that applies systems-based methods to both resting-state and event-related paradigms to better understand the brain mechanisms underlying an individual’s self-regulatory capacity.
Despite the promise of our analysis approach and the obtained findings, there are several limitations and caveats that need to be acknowledged. First, although the use of EMA in behavioral research is advantageous compared with other survey assessment formats and schedules (e.g. retrospective self-report), EMA responses can be prone to biases or inaccuracies, including in the eating domain (Martin et al., 2002). Additionally, the EMA response rate was somewhat variable, so future studies may implement appropriate incentives to maintain relatively high and consistent response rates. Last, the generalizability of our study’s findings is necessarily constrained, as participants were all female. We recruited only females primarily because women are more likely to restrict food intake than men (Wardle et al., 2004), and also to avoid potential gender confounds (Holm-Denoma et al., 2008). Even so, gender differences—as well as gender–dieting interactions—in the recruitment of regulatory and reward systems should be directly tested in future work.
In summary, this study demonstrated that a balance between cue-induced reward and control activity, observed in defined brain networks, predicted dieters’ daily eating behaviors. The design and analysis of the study entailed three features that may be useful in future work. Specifically, fMRI paradigms can incorporate self-regulatory challenge tasks, like the depleting inhibitory control task, to determine how post-challenge brain activation patterns relate to behavioral outcomes of interest. Second, by taking a balance approach to analyzing neuroimaging data, investigators can test and compare models of self-regulation that simultaneously examine multiple processes that guide behavior to make self-control success more (or less) likely. Last, researchers can test whether system-level correlates of self-regulation success (e.g. FP recruitment and/or interactions between FP and reward systems) are amenable to self-regulation training. Indeed, this approach may yield novel, brain-based indices that can be incorporated into targeted clinical interventions. This would be especially promising for those whose repeated self-control failures pose serious risks for health, such as compulsive over-eating or addiction.
Footnotes
This effect of balance scores on enactment was replicated (r = −0.28, P = 0.02) in this cohort using a large, independent sample (n = 579) of resting-state functional connectivity data to define FP and reward brain systems with graph theory approaches (i.e. community assignment) (Rosvall and Bergstrom, 2008; Power et al., 2011).
Funding
This work was supported by the National Institutes of Health, namely the National Institute on Drug Abuse (R01DA022582) and the National Cancer Institute (1F31CA177203).
Conflict of interest. None declared.
References
- Aarts H. (2007). On the emergence of human goal pursuit: The nonconscious regulation and motivation of goals. Social and Personality Psychology Compass, 1(1), 183–201. [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends in Cognitive Sciences, 18(4), 177–85. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B. (2013). Beyond brain mapping: Using neural measures to predict real-world outcomes. Current Directions in Psychological Science, 22(1), 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B., Lieberman M.D. (2011). In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological Science, 22(4), 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T., Kahn L.E., Merchant J.S. (2014). Training-induced changes in inhibitory control network activity. The Journal of Neuroscience, 34(1), 149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Somerville L.H., Gotlib I.H., et al. (2011). Behavioral and neural correlates of delay of gratification 40 years later. Proceedings of the National Academy of Sciences of the United States of America, 108(36), 14998–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier J., Heatherton T.F., Whalen P.J., Kelley W.M. (2008). Are attractive people rewarding? sex differences in the neural substrates of facial attractiveness. Journal of Cognitive Neuroscience, 20(6), 941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos K.E., Heatherton T.F., Kelley W.M. (2012). Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience, 32(16), 5549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos K.E., Kelley W.M., Heatherton T.F. (2011). Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. Journal of Cognitive Neuroscience, 23(8), 1952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude–an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50(7), 1252–66. [DOI] [PubMed] [Google Scholar]
- Donaldson M.S. (2004). Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutrition Journal, 3, (19), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., et al. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M., Aron A.R. (2016). Withholding a reward-driven action: studies of the rise and fall of motor activation and the effect of cognitive depletion. Journal of Cognitive Neuroscience, 28(2), 237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Pankiewicz J., Bloom A., et al. (2000). Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry, 157(11), 1789–98. [DOI] [PubMed] [Google Scholar]
- Giuliani N.R., Tomiyama A.J., Mann T., Berkman E.T. (2015). Prediction of daily food intake as a function of measurement modality and restriction status. Psychosomatic Medicine, 77(5), 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin A.A., Stone A.A. (2001). Recall biases and cognitive errors in retrospective self-reports: A call for momentary assessments. Handbook of Health Psychology, 23, 405–13. [Google Scholar]
- Heatherton T.F. (2011). Neuroscience of self and self-regulation. Annual Review of Psychology, 62, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Baumeister R.F. (1991). Binge eating as an escape from self-awareness. Psychological Bulletin, 110, 86–108. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Herman C.P., Polivy J., King G.A., McGree S.T. (1988). The (mis)measurement of restraint: An analysis of conceptual and psychometric issues. Journal of Abnormal Psychology, 97(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Wagner D.D. (2011). Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences, 15(3), 132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman C.P., Polivy J. (1975). Anxiety, restraint, and eating behavior. Journal of Abnormal Psychology, 84(6), 666–72. [PubMed] [Google Scholar]
- Herman C.P., Polivy J. (1980). Restrained eating In:Stunkard A.J.editor.Obesity. Philadelphia: Saunders. [Google Scholar]
- Hofmann W., Baumeister R.F., Förster G., Vohs K.D. (2012). Everyday temptations: an experience sampling study of desire, conflict, and self-control. Journal of Personality and Social Psychology, 102(6), 1318–35. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Friese M., Strack F. (2009). Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science, 4(2), 162–76. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Patel P.V. (2015). SurveySignal: a convenient solution for experience sampling research using participants’ own smartphones. Social Science Computer Review, 33(2), 235–53. [Google Scholar]
- Holm-Denoma J.M., Joiner T.E. Jr., Vohs K.D., Heatherton T.F. (2008). The “freshman fifteen” (the “freshman five” actually): predictors and possible explanations. Health Psychology, 27(1S), S3–9. [DOI] [PubMed] [Google Scholar]
- James W. (1890). The Principles of Psychology, Vol. 2 Mineola, NY: Dover. [Google Scholar]
- Kelley W.M., Wagner D.D., Heatherton T.F. (2015). In search of a human self-regulation system. Annual Review of Neuroscience, 38, 389–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Taylor J., Kaufman M., Peterson R., Glover G. (2005). Distributed neural representation of expected value. The Journal of Neuroscience, 25(19), 4806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Mende-Siedlecki P., Kross E.F., et al. (2010). Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America, 107, 14811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotabe H.P., Hofmann W. (2015). On integrating the components of self-control. Perspectives on Psychological Science, 10(5), 618–38. [DOI] [PubMed] [Google Scholar]
- Lewin K. (1951). Field Theory in Social Science: Selected Theoretical Papers. Oxford, England: Harper. [Google Scholar]
- Lopez R.B., Hofmann W., Wagner D.D., Kelley W.M., Heatherton T.F. (2014). Neural predictors of giving in to temptation in daily life. Psychological Science, 25(7), 1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.S., Tapsell L.C., Batterham M.J., Russell K.G. (2002). Relative bias in diet history measurements: a quality control technique for dietary intervention trials. Public Health Nutrition, 5(4), 537–46. [DOI] [PubMed] [Google Scholar]
- Neal D.T., Wood W., Labrecque J.S., Lally P. (2012). How do habits guide behavior? Perceived and actual triggers of habits in daily life. Journal of Experimental Social Psychology, 48(2), 492–8. [Google Scholar]
- Padmala S., Pessoa L. (2010). Interactions between cognition and motivation during response inhibition. Neuropsychologia, 48(2), 558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M., Bergstrom C.T. (2008). Maps of random walks on complex networks reveal community structure. Proceedings of the National Academy of Sciences of the United States of America, 105(4), 1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. (2010). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A.A., Shiffman S. (2002). Capturing momentary, self-report data: A proposal for reporting guidelines. Annals of Behavioral Medicine, 24(3), 236–43. [DOI] [PubMed] [Google Scholar]
- Tomiyama A.J., Mann T., Vinas D., Hunger J.M., DeJager J., Taylor S.E. (2010). Low calorie dieting increases cortisol. Psychosomatic Medicine, 72(4), 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan L.N., de Ridder D.T.D., Viergever M.A., Smeets P.A.M. (2011). The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage, 55(1), 296–303. [DOI] [PubMed] [Google Scholar]
- Wagner D.D., Altman M., Boswell R.G., Kelley W.M., Heatherton T.F. (2013). Self-regulatory depletion enhances neural responses to rewards and impairs top-down control. Psychological Science, 2262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.D., Boswell R.G., Kelley W.M., Heatherton T.F. (2012). Inducing negative affect increases the reward value of appetizing foods in dieters. Journal of Cognitive Neuroscience, 24(7), 1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.D., Heatherton T.F. (2015). Self-regulation and its failure: The seven deadly threats to self-regulation In: Mikulincer M., Shaver P.R., Borgida E., Bargh J.A., editors. APA Handbook of Personality and Social Psychology, pp. 805–42. Washington, DC: American Psychological Association. [Google Scholar]
- Wardle J., Haase A.M., Steptoe A., Nillapun M., Jonwutiwes K., Bellisie F. (2004). Gender differences in food choice: the contribution of health beliefs and dieting. Annals of Behavioral Medicine, 27(2), 107–16. [DOI] [PubMed] [Google Scholar]
- White C.N., Congdon E., Mumford J.A., et al. (2014). Decomposing decision components in the stop-signal task: a model-based approach to individual differences in inhibitory control. Journal of Cognitive Neuroscience, 26(8), 1601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W., Rünger D. (2016). Psychology of habit. Annual Review of Psychology, 67, 289–314. [DOI] [PubMed] [Google Scholar]


