Abstract
The tribal character of the affective link between football fans and their teams is a well-recognized phenomenon. Other forms of love such as romantic or maternal attachment have previously been studied from a neuroimaging point of view. Here we aimed to investigate the neural basis of this tribal form of love, which implies both the feeling of belongingness and rivalry against opposing teams. A pool of 56 participants was submitted to an fMRI experimental design involving the presentation of winning and losing football moments of their loved, rival or neutral teams. We found recruitment of amygdala and reward regions, including the ventral tegmental area (VTA) and substantia nigra (SN), as well as other limbic regions involved in emotional cognition, for ‘positive vs neutral’ and ‘positive vs negative’ conditions. The latter contrast was correlated with neuropsychological scores of fanaticism in the amygdala and regions within the reward system, as the VTA and SN. The observation of increased response patterns in critical components of the reward system, in particular for positive content related to the loved team, suggests that this kind of non-romantic love reflects a specific arousal and motivational state, which is biased for emotional learning of positive outcomes.
Keywords: reward system, non-romantic love, amygdala, ventral tegmental area, substantia nigra, football fan
Introduction
Romantic and maternal love represent well-studied forms of passionate experience, with recognized biological and evolutionary relevance. Both imply a dual relationship in the formation of a bound between individuals (Bartels and Zeki, 2004; Zeki, 2007; Acevedo et al., 2012). Several studies established a coupling between both types of attachment processes and the neural systems underlying the experience of reward. The neural network mediating responses to gratifying stimuli, related to the goals that individuals seek, such as pleasure and motivation, is defined as the reward system. Motivational arousal is largely dependent on dopaminergic modulation (Wise and Rompre, 1989; Wise, 2004). The mesocortical and mesolimbic dopamine systems represent important pathways underlying processing of reward value and motivational function. These pathways stem from dopaminergic cells within the ventral tegmental area (VTA). The mesolimbic dopamine system comprises projections to the nucleus accumbens, but also to the amygdala, hippocampus, orbitofrontal cortex and septum. In the mesocortical dopamine system, medial VTA projects to the medial prefrontal cortex, cingulate gyrus and perirhinal cortex (Wise, 2004). The nigrostriatal pathway provides modulatory influences from substantia nigra (SN) to caudate nucleus and putamen. Both romantic and maternal love have been shown to recruit these core systems underlying reward processing (Bartels and Zeki, 2004; Aron et al., 2005; Zeki, 2007; Xu et al., 2011; Acevedo et al., 2012).
Bonding and identification at the larger scale of a group level is very important in a broader social context. Feelings associated with group belongingness represent a sort of non-romantic love. Such feelings of belongingness can indeed be considered to form the core of a basic human need like food, water and shelter and are also deeply rooted in a human evolutionary context (DeWall et al., 2012). Several studies have demonstrated that loving attachment for in-group members, reflecting the sense of belongingness, is a general phenomenon. It biases empathy, altruistic and helping attitudes among diverse social groups, including race (Mathur et al., 2010; Dovidio et al., 2010; Luo et al., 2015), ethnicity (Bruneau and Saxe 2010), political affiliation (Rand et al., 2009) and even sports team allegiance (Levine et al., 2005; Hein et al., 2010). For reviews on the neuroscience of in-group biases, please see (Molenberghs, 2013) and (Cikara and Bavel, 2014).
A strong example of group identification, feeling of belongingness and loyalty is the passion for a football team, which may therefore be viewed as sort of tribal love. Desmond Morris described in an impactful book this tribal character of the link between, not only football players, coaches and directors, but between the fans and the teams. Football was described as a tribal phenomenon and the author framed in an evolutionary view how the attachment for a preferred football team and nation has achieved such significance for very large audiences (Morris, 1981). The cognitive neuroscience of fan binding to a team is poorly studied from the neuroimaging point of view. Nevertheless, its social importance is irrefutable and the phenomenon of team love is worth deeper scientific investigation.
This issue is indeed relevant in the context of social neuroscience, given that sports have overwhelming socioeconomic impact in large communities worldwide. Concerning football, FIFA’s 2014 financial report summarizes revenues of over 2 billion US dollars in that year (FIFA, 2015). In 2015, the amount spent in transfers of football players in the English premier league, reached 870 million pounds (BBC Sport, 2015). This reflects the enormous amount of time, energy and money that fans spend in their commitment to their beloved teams. This form of passionate behaviour is defined as a strong type of non-romantic love binding the fans to their team and they, as consumers, support the football teams and the leagues, leading to large-scale business models.
Despite the neuroscientific and social relevance of this phenomenon, few studies address its neural underpinnings. The purpose of this work is to study this form of ‘passionate love’. ‘Fanaticism’ is not labelled as a negative term in this context. We rather assess it formally as the intensity of being a fan (as defined by a rating scale), leaving out any negative connotation or any relation to hooliganism or other dysfunctional behaviours.
Football can nurture a range of emotional feelings and behavioural patterns, which are, consciously or unconsciously, perceived by the supporters as needs. We hypothesize that these needs strongly modulate processing in the brain reward system as a function of the coding of the reward value of this particular social context. Reward processing has been studied using fMRI and PET in the study of hedonic responses to stimuli such as food (Goldstone et al., 2009), drugs (van Hell et al., 2010) or love (Bartels and Zeki, 2004; Xu et al., 2011; Takahashi et al., 2015). A critical question is whether the passion for a football team engages similar networks as the love for a person.
To answer this question we implemented a neuroimaging paradigm where participants, with formally defined levels of neuropsychological fanaticism scores, viewed memorable winning and losing football moments where their beloved team competed with rival/neutral teams. We hypothesized that these emotional experiences involve at least part of the same limbic regions that are recruited in other forms of love, and in particular the human reward system. A relevant question was which brain regions are recruited as a function of fanaticism.
Materials and methods
Subjects
A pool of 61 football enthusiasts was recruited from the fan support organization of two Portuguese teams: Futebol Clube do Porto (henceforward labelled as Porto) and Associação Académica de Coimbra (labelled as Académica) as well as citizens that defined themselves as football fans (Porto or Académica supporters), who were evaluated in terms of fanaticism scores. The two teams played in the Portuguese First League at the time of the study, Porto was one of the top ranked teams in the league and Académica ranked at the bottom.
Sixty one subjects were recruited, of which 58 completed the scanning session. In total 2 out of 58 were excluded from this part of the study, given that debriefing revealed that they focused only on the technical aspects of the videos (one was a football player and the other person was a coach). In the functional imaging analysis 56 subjects were included (54 males and 2 females, aged from 21 to 60 years, mean age 34.4 ± 10.7 years). Fifty-five out of 56 used the joystick in the right hand given their handedness. All the subjects had normal or corrected to normal vision. All subjects signed the informed consent of this study, which was approved by the Ethics Committee of the Faculty of Medicine of the University of Coimbra, in accordance with the Declaration of Helsinki.
Acquisition parameters
Structural and functional MRI scans were acquired in a 3T Magnetom Trio Tim MRI scanner (Siemens, Erlangen, Germany), using a 12-channel head coil. A T1-weighted MPRAGE anatomical volume was measured with repetition time (TR) of 2530 ms, echo time (TE) of 3.42 ms, resolution 1 mm3, flip angle of 7°, matrix size 256 × 256, field of view of 256 × 256 and a slice thickness of 1 mm.
Considering the regions of interest (ROIs) that were relevant for the present study, we were aware of the susceptibility artefact. To correct for this source of noise, we acquired gradient-echo field maps before each Echo Planar Imaging (EPI)-blood-oxygen-level dependent (BOLD) sequence to map the distortions. Phase and magnitude field maps were acquired with the same orientation and the same field of view, TR of 3000 ms, TE of 30 ms, echo spacing 0.5 ms, phase resolution 100%, phase encoding direction from anterior to posterior, TE difference of 2.46 ms and bandwidth in the phase direction of 31.25 Hz.
Functional data were acquired using EPI sequences, using slice thickness of 3 mm and voxel size 4 mm2, 36 slices acquired parallel to the AC–PC line, TR 3000 ms, TE 30 ms, flip angle of 90°, matrix size 256 × 256 and FOV of 256 × 256. In the whole session, two out of four runs were acquired for the study we present here. Both runs had 190 volumes.
The videos were rendered through a 698.40 × 392.85 mm LCD monitor (NordicNeuroLab, Bergen, Norway) with a frequency rate of 60 Hz, placed ∼156 cm away from the participants’ head. Audio was provided through MR-compatible headphones. The subject could actively select the response using an MR-compatible joystick (Hybridmojo, San Mateo CA, USA). The participant used this device to move the cursor in the screen and pressed the button on its top to select his/her answer.
Task
Participants underwent fMRI scans while they were watching short video streams of representative negative or positive goal situations in relation to the loved team or a rival team, or neutral (videos of Italian B-series teams, with high probability of being unknown).
To guarantee the consistency of visual content, all videos are exclusively rendered within the same framework of goal situations in football, with explicit exclusion of any images of actors such as fans or coaches and their reactions. All the videos presented a very similar scene content (a football field, movement of football players, which were the only actors, scored goals). All videos were resized to the 1280 × 720 aspect ratio. Despite all efforts made to keep the consistency of the visual content, the nature of the visual stimuli precluded an absolute matching in what refers to e.g. brightness or movement content.
The video collection was tailored to Porto fans and to Académica fans and both included five videos of the favourite team’s winning moments (labelled as +LovedTeam), five videos of winning moments of their favourite team against strong rival teams ( ++LovedTeam), five videos of the favourite team’s losing moments (−LovedTeam), five videos of losing moments of their favourite team against strong rival teams (--LovedTeam), five videos of rival team’s winning moments (+RivalTeam), five videos of rival team’s losing moments (−RivalTeam) and five videos of Italian B-series teams (0Neutral: no fan related or antagonistic content). Henceforward, we will use the abbreviations as used in Table 1 to describe the conditions when specifying the contrasts. The blocks of videos were initiated by TR triggers and could last between 6 and 12 s. The average duration of loved team’s positive videos was 8.8 s, while for negative videos was 9.0 s. For the +RivalTeam, −RivalTeam and 0Neutral, the average duration was respectively 9.2, 8.8 and 7.2 seconds. The duration of each video was considered in the creation of the predictor’s model as detailed further in this section.
Table 1.
Abbreviations and description of videos in each condition and theirs contents
| Abbreviation | Video | Valence on fan’s perspective | Loved team present? | Strong Rival team present? |
|---|---|---|---|---|
| +LovedTeam | Positive for loved team | positive | yes | no |
| ++LovedTeam | Positive for loved team against the rival | positive | yes | yes |
| −LovedTeam | Negative for loved team | negative | yes | no |
| −−Loved Team | Negative for loved team against the rival | negative | yes | yes |
| +RivalTeam | Positive for rival team | negative or neutral | No | yes |
| −RivalTeam | Negative for rival team | positive or neutral | No | yes |
| 0Neutral | Neutral | neutral | no | no |
The participants were asked to classify each video in a - 3 to 3 Likert-like rating scale, right after the video presentation (Figure 1). The participant underwent 2 EPIBOLD runs of 9.5 min each, of 17 and 18 videos, respectively. They contained at least two and up to three trials of each condition per run (overall five trials per condition). In total, 35 videos were presented in a randomized order.
Fig. 1.
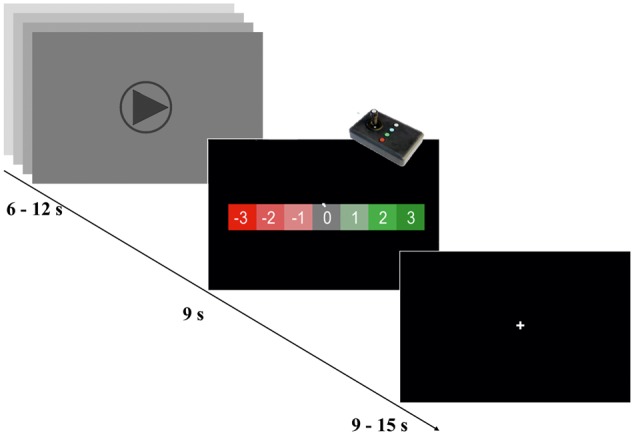
Experimental design. The paradigm had a box-car design. In the first block, the participants were watching video streams between 6 and 12 s of goal situations. Then they were asked to rate the video −3 to 3 scale Likert-like rating scale. Each pair of video/response was followed by a baseline period which duration was jittered in time (randomly between 9 and 15 s). The video presentation order was randomized.
Behavioural data
Before the MRI session, the participants answered a questionnaire and completed the Football Supporter Fanaticism Scale (FSFS) (Wachelke et al., 2008) (in Portuguese, Escala de Fanatismo em Torcedores de Futebol). This is a self-report scale assessing the level of fanaticism for football. This Likert scale was translated from Brazilian Portuguese to Portugal Portuguese by two experienced psychologists and verified by a third element, and used in a one to five ordinal range.
fMRI data analysis
Geometrical distortions were calculated based on the pixelshift algorithm and using Gradient field maps as input in the AnatAbacus v1.1 plugin (Breman et al., 2009) for BrainVoyager QX to undistort the EPI-BOLD images.
Functional data were then pre-processed and analysed using BrainVoyager QX 2.8.2 (Brain Innovation, Maastricht, The Netherlands). Data were corrected for: (i) slice scanning time differences using cubic spline interpolation; (ii) for motion, combining trilinear and sinc function based methods for interpolation in the three axes (the second run was corrected in relation to the first volume of the first run); and (iii) filtered in the time domain using a General Linear Model (GLM) approach with Fourier basis set with two cycles per time course. The anatomical and functional data were co-registered (and manually verified) and then normalized according to the Talairach atlas. After the spatial normalization, spatial smoothing was performed using a Gaussian kernel of 8 mm FWHW. A random effects (RFX) analysis was done at the group level using a GLM approach. The predictor’s model was obtained by convolution of the boxcar time course (considering each video duration individually) with a two-gamma haemodynamic response function. A mask was obtained by averaging all functional files, automatically excluding bone and scalp. Eyes and cerebellum were manually extracted from the mask, using the information from the anatomical files. The RFX-GLM statistical maps were corrected for multiple comparisons using the false discovery rate (FDR) (Benjamini and Heller, 2007) with a fixed P-value lower than 0.01.
Correlation analysis was performed using the same software tool. Beta values, resulting from the RFX-GLM analysis, were correlated with an external covariate (FSFS individual scores). The resulting whole-brain maps containing r-values were corrected for multiple comparisons using cluster threshold levels with a p value of 0.006 and voxel extent, which estimation was based on Monte Carlo simulations (1000 iterations). Significant clusters include at least 78 contiguous voxels.
Volumetric segmentation was performed with FreeSurfer 5.3 software which procedure is documented online (http://surfer.nmr.mgh.harvard.edu). The ROIs segmentation was based on an atlas of probabilistic information computed from a manually labelled dataset (Fischl et al., 2002; Reuter et al., 2012) based on the Duvernoy atlas (Duvernoy, 1999). The obtained ROIs of right and left amygdala, caudate, hippocampus, globus pallidus, putamen and thalamus of 10 subjects in native space were then transformed into Talairach space using the transform files created before in BrainVoyager QX 2.8. The resultant files were combined to extract peak voxels from the RFX statistical maps when contiguous clusters overlapped different anatomical regions.
Results
Behavioural results
Considering the subjects that completed the functional study, 2 out of 56 had missing answers in the FSFS scale. Considering the 54 participants with complete assessments, the mean score was 3.19 ± 0.95 (mean ± s.d., n = 54). For this scale, we saw no differences [t-test, t(52) ≤ 2.01, P < 0.14, non-significant] between the sample of Porto fans (FSFS, mean score 3.38 ± 1.07, n = 28) and the sample of Académica fans (FSFS, mean score 2.99 ± 0.67, n = 26).
Considering the pool of 56 subjects, video classification (in the Likert −3 to 3 scale) yielded the following results (condition mean ± s.d.): +LovedTeam 2.61 ± 0.53; ++LovedTeam 2.62 ± 0.61; −LovedTeam −1.81 ± 1.14; --LovedTeam −2.27 ± 1.14; +RivalTeam −1.01 ± 1.27; -RivalTeam 0.87 ± 1.27; 0Neutral −0.04 ± 0.94. The negative videos (−LovedTeam and --LovedTeam) resulted in different response levels, as revealed by a paired t-test t(55) = 5.1, P < 0.00001. However, the responses in the scale revealed that +LovedTeam and ++LovedTeam conditions were quite similar, as revealed by a paired t-test t(55) = −0.17, P = 0.859, ns.
General random effects analysis
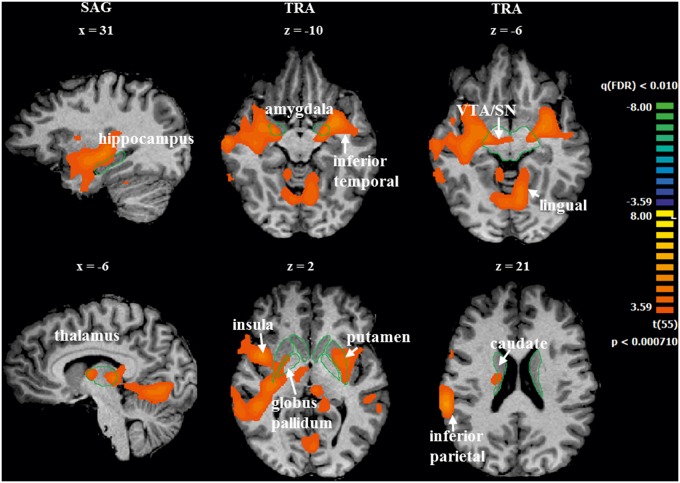
We performed whole-brain group level RFX-GLM analysis [t(55) = 3.59, P < 0.01, FDR corrected] related to the visualization of positive and negative videos. The overall contrast of ‘positive vs negative’ moments ( ++LovedTeam and +LovedTeam vs. --LovedTeam and −LovedTeam) revealed increased activity bilaterally in the inferior temporal lobe, amygdala, hippocampus, insula, putamen, globus pallidus, thalamus, VTA, SN and right caudate. This contrast also showed more activation in the left lingual gyrus and right inferior parietal lobule. The reverse contrast did not show areas of significant activity. See Table 2 and Figure 2 for a complete overview of these results.
Table 2.
Regions significantly activated by the predictor contrast ‘positive vs negative’
| peak |
|||||||
|---|---|---|---|---|---|---|---|
| region | H | x | y | z | t | p | n |
| amygdala | L | −27 | −4 | −10 | 5.4 | 0.000002 | 22 |
| amygdala | R | 24 | −7 | −8 | 5.2 | 0.000003 | 9 |
| hippocampus | L | −18 | −13 | −10 | 4.8 | 0.000013 | 11 |
| hippocampus | R | 21 | −13 | −9 | 4.7 | 0.000016 | 17 |
| caudate | R | 8 | −1 | 10 | 4.3 | 0.000065 | 4 |
| globus pallidus | L | −24 | −7 | −4 | 4.7 | 0.000016 | 11 |
| globus pallidus | R | 25 | −12 | −3 | 4.7 | 0.000020 | 8 |
| putamen | L | −29 | −7 | −5 | 4.9 | 0.000009 | 82 |
| putamen | R | 30 | −17 | −2 | 5.1 | 0.000004 | 48 |
| striatum | R,L | 0 | −4 | 10 | 5.3 | 0.000002 | 7 |
| thalamus | L | −6 | −25 | 7 | 4.9 | 0.000010 | 28 |
| thalamus | R | 3 | −6 | 10 | 4.8 | 0.000012 | 41 |
| VTA and SN | R,L | 20 | −13 | −6 | 5.0 | 0.000006 | 13 |
| inferior parietal lobule (BA 40) | R | 60 | −37 | 19 | 5.8 | <0.000001 | 93 |
| insula | R | 42 | 5 | 4 | 5.3 | 0.000002 | 89 |
| insula | L | −33 | 2 | 16 | 5.0 | 0.000007 | 69 |
| inferior frontal gyrus (BA 44) | R | 61 | 5 | 10 | 5.2 | 0.000003 | 27 |
| lingual | L | −9 | −58 | −8 | 4.8 | 0.000011 | 192 |
| inferior temporal lobe | R | 36 | −19 | −5 | 5.2 | 0.000003 | 220 |
| inferior temporal lobe | L | −33 | −1 | −11 | 5.2 | 0.000003 | 50 |
Regions were identified from a whole-brain RFX-GLM analysis [t(55) = 3.59, P < 0.01, FDR corrected). The clusters are described by their hemisphere (H), peak voxel coordinates in Talairach space, the t and P values in the peak voxel and the number of voxels (n).
Fig. 2.
Significant BOLD activations for the ‘positive vs negative’ contrast. Sagittal and transversal slices show the regions activated in the whole-brain RFX-GLM analysis [t(55) = 3.59, P < 0.01, FDR corrected], overall contrasting ++LovedTeam and +LovedTeam vs –LovedTeam and --LovedTeam conditions. The group results are projected in a single subject’s brain just for visualization purposes. The reverse contrast did not show areas of significant activity. Left = right. Green lines are only present to facilitate localization.
The contrast of ‘positive vs neutral’ moments ( ++LovedTeam and +LovedTeam vs 0Neutral, balanced as [1 1 −2]) showed the involvement of the inferior temporal lobe, right parahippocampus, right hippocampus, amygdala bilaterally, VTA and posterior cingulate. The reverse contrast showed increased activity in the auditory cortex, visual cortex and lingual gyrus [RFX, t(55) = 3.73, P < 0.01, FDR corrected]. Peak voxels are described in Table 3 (Supplementary Figure 1 provided as Supplementary Material).
Table 4.
Regions significantly activated by the predictor contrast ‘negative vs neutral’
| peak |
|||||||
|---|---|---|---|---|---|---|---|
| region | H | x | y | z | t | p | n |
| posterior cingulate (BA 23, 31) | R,L | 0 | −61 | 22 | 8.5 | <0.000001 | 357 |
| midcingulate (BA 24) | R,L | 9 | 20 | 37 | −4.2 | 0.000085 | 15 |
| lingual | R,L | −9 | −79 | −2 | −9.3 | <0.000001 | 332 |
| medial prefrontal (BA 9, 10) | R,L | 3 | 59 | 10 | 4.9 | 0.000008 | 67 |
| middle temporal | R | 45 | −55 | 19 | 4.2 | 0.000093 | 19 |
| middle temporal | L | −39 | −70 | 28 | 6.0 | <0.000001 | 175 |
| auditory cortex | R | 48 | −13 | 4 | −10.5 | <0.000001 | 525 |
| auditory cortex | L | −51 | −19 | 10 | −10.1 | <0.000001 | 504 |
Regions were identified from a whole-brain RFX-GLM analysis [t(55) = 3.57,P < 0.01, FDR corrected]. The clusters are described by their hemisphere (H), peak voxels coordinates in Talairach space, the t and P values in the peak voxel and the number of voxels (n).
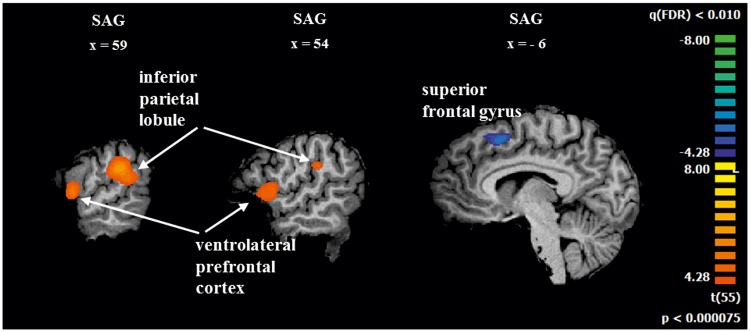
The contrast of ‘negative vs neutral’ moments [--LovedTeam and -LovedTeam vs 0Neutral, balanced as (1 1 -2)] evidenced increased activity in middle temporal gyrus, posterior cingulate and medial prefrontal gyrus. The inverse contrast showed increased activity in the midcingulate, lingual gyrus and auditory cortex [RFX, t(55) =3.57, P < 0.01, FDR corrected]. See details in Table 4 (Supplementary Figure 2 also provided as Supplementary Materials). The contrast of ‘loved team's positive vs rival's negative moments’ (+LovedTeam vs - RivalTeam, considered as directly positive and indirectly positive) showed more activation in the right inferior parietal lobule and right inferior frontal gyrus (See Figure 3 and Table 5). The reverse contrast evidenced increased activity in the left superior frontal gyrus [RFX, t(55)=4.28, P <0.01, FDR corrected].
Table 3.
Regions significantly activated by the predictor contrast ‘positive vs neutral’
| peak |
|||||||
|---|---|---|---|---|---|---|---|
| region | H | x | y | z | t | p | n |
| amygdala | R | 28 | −9 | −11 | 4.4 | 0.000052 | 2 |
| amygdala | L | −27 | −7 | −8 | 4.6 | 0.000026 | 14 |
| VTA | R,L | 0 | −28 | −8 | 3.9 | 0.000287 | 4 |
| hippocampus | R | 30 | −16 | −9 | 5.1 | 0.000005 | 19 |
| lingual | L | −12 | −79 | −5 | −6.9 | <0.000001 | 94 |
| posterior cingulate (BA 23, 26, 29, 30, 31) | R,L | −9 | −55 | 19 | 8.1 | <0.000001 | 490 |
| Inferior parietal lobule (BA 40) | R | 63 | −28 | 31 | 4.3 | 0.000080 | 9 |
| inferior temporal lobe | L | −36 | 17 | −23 | 4.2 | 0.000089 | 8 |
| inferior temp. lobe, parahippocampus | R | 37 | −19 | −8 | 4.8 | 0.000012 | 104 |
| auditory cortex | R | 54 | −10 | 4 | −5.2 | 0.000003 | 61 |
| auditory cortex | L | −51 | −19 | 7 | −6.1 | <0.000001 | 63 |
| visual cortex | R,L | 21 | −88 | −8 | −5.1 | 0.000005 | 13 |
| middle temporal | L | −39 | −67 | 25 | 6.1 | <0.000001 | 45 |
Regions were identified from a whole-brain RFX-GLM analysis [t(55) = 3.73, P < 0.01, FDR corrected]. The clusters are described by their hemisphere (H), peak voxels coordinates in Talairach space, the t and P values in the peak voxel and the number of voxels (n).
Correlation analysis of brain activity patterns and neuropsychological scores
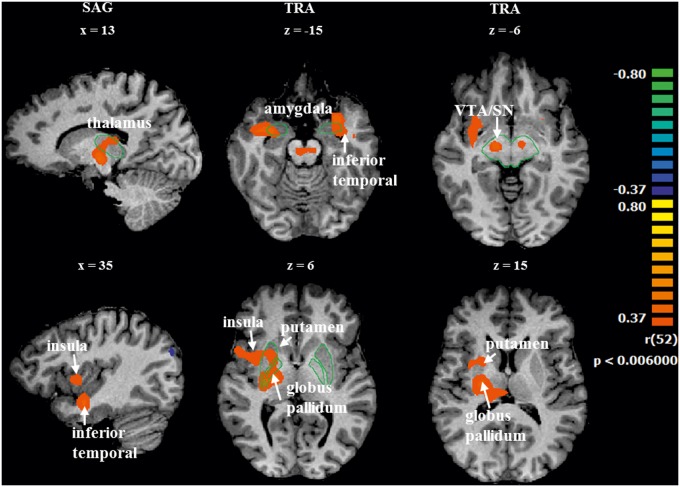
We performed a whole brain correlation analysis between beta values across regions and the FSFS individual scores. The correlation between the contrast of ‘positive vs negative’ moments ( ++LovedTeam and +LovedTeam vs --LovedTeam and −LovedTeam) and the FSFS individual scores showed positive correlations in the inferior temporal lobe, amygdala, right insula, putamen, globus pallidus, thalamus, VTA and SN (r > 0.37 with P < 0.006, corrected). A negative correlation was found in the angular gyrus (BA 39). See Table 6 and Figure 4 for a complete overview of these results.
Table 5.
Regions revealed by the contrast of ‘loved team’s positive vs rival’s negative’ moments
| peak |
|||||||
|---|---|---|---|---|---|---|---|
| region | H | x | y | z | t | p | n |
| x | y | z | |||||
| inferior parietal lobule | R | 61 | −36 | 26 | 7.2 | <0.000001 | 83 |
| Inferior frontal gyrus (BA 44) | R | 54 | 4 | 9 | 5.7 | 0.000001 | 48 |
| superior frontal gyrus | L | −6 | 13 | 50 | −5.7 | <0.000001 | 25 |
Regions were identified from a whole-brain RFX-GLM analysis [t(55) = 4.28, P < 0.01, FDR corrected]. The clusters are described by their hemisphere (H), peak voxels coordinates in the Talairach space, the t and P values in the peak voxel and the number of voxels (n).
Fig. 4.
Correlation maps which were calculated by contrasting ‘positive vs negative’ moments and the fanaticism score. The contrast ++LovedTeam and +LovedTeam vs –LovedTeam and --LovedTeam was correlated with the FSFS individual scores in a whole-brain analysis (r > 0.37 with P < 0.006, corrected). Left = right. Green lines are only present to facilitate localization.
Fig. 3.
Significant BOLD activations for the contrast of ‘loved team’s positive videos vs rival team’s negative videos’ (directly positive to the self because of positive affect vs indirectly positive to the self because of negative affect of rival fans). Sagittal views to show the regions activated in the whole-brain RFX-GLM analysis [t(55) = 4.28, P < 0.01, FDR corrected].
Table 6.
Regions retrieved from the r-map
| peak |
|||||||
|---|---|---|---|---|---|---|---|
| region | H | x | y | z | t | p | n |
| VTA/SN | R, L | 12 | −16 | −6 | 0.44 | 0.000927 | 23 |
| amygdala | R | 27 | −4 | −20 | 0.46 | 0.000409 | 6 |
| amygdala | L | −30 | −4 | −20 | 0.44 | 0.000864 | 8 |
| globus pallidus | R | 18 | −11 | 1 | 0.43 | 0.001259 | 11 |
| putamen | R | 27 | −19 | 7 | 0.44 | 0.000893 | 44 |
| thalamus | R | 14 | −15 | 0 | 0.43 | 0.001148 | 19 |
| inferior temporal lobe | R | 27 | −7 | −20 | 0.48 | 0.000232 | 64 |
| inferior temporal lobe | L | −27 | 8 | −14 | 0.48 | 0.000250 | 91 |
| angular gyrus (BA 39) | R | 45 | −67 | 25 | −0.44 | 0.000791 | 48 |
| insula | R | 36 | 2 | 7 | 0.42 | 0.001705 | 29 |
Regions were identified from the correlation between the FSFS individual scores and the contrast of ‘positive vs negative’ moments ( ++LovedTeam and +LovedTeam vs –LovedTeam and −LovedTeam). R > 0.37 with P < 0.006, corrected for multiple comparisons using cluster threshold levels. The clusters are described by their hemisphere (H), peak voxel coordinates in Talairach space, the r coefficient, P values in the peak voxel and the number of voxels (n).
Discussion
The aim of this work was to address the neural correlates of passionate attachment to a loved team, a sort of modern tribal love that is dissociable from romantic and maternal love. We found strong recruitment of reward regions including the brain stem, VTA and SN, as well as limbic regions involved in emotional cognition. Using neuropsychological team attachment scales we further found a significant correlation between regions that responded to positive states of team love with individual scores of fanaticism.
Reward value and love for the preferred competing team
Reward system. We expected that the positive content in the selected stimuli would activate motivational and reward related circuitry, reflecting the value attributed to the team affective attachment. Reward and motivational arousal are dopamine-dependent (Wise and Rompre, 1989; Wise, 2004) which is well in line with the regions identified in our study. The dopamine-reward system, mediates the link between pleasure and motivation, in other words, the affective goals that individuals seek (Knutson and Cooper, 2005) during emotionally loaded social contexts.
A core network involving the VTA, SN, striatum (caudate, putamen and globus pallidus), insula, hippocampus and amygdala were shown to be activated by the contrast of ‘positive vs negative’ valence conditions. These are critical areas of the reward and affective processing systems mediating the perception of reward as well as for learning and processing of social reinforcement.
Two of the four major dopamine pathways in the brain, the the mesocortical and mesolimbic pathways, are particularly involved in motivational processing and receive strong influence from the dopaminergic VTA (Wise, 2004).
The VTA, amygdala and hippocampus were all activated with both the contrasts of ‘positive vs negative’ and ‘positive vs neutral’ videos. These results suggest the engagement of the mesolimbic pathway during the visualization of positive situations involving the loved team.
Interestingly, the cingulate gyrus and perirhinal cortex (included in the temporal clusters) were activated by both contrasts ‘positive vs neutral’ and ‘negative vs neutral’. This finding may be linked with the postulated suggestion that the mesocortical pathway is involved in reward processing both for conditions entailing positive reward and punishment. Moreover, the medial prefrontal cortex, which has a role in emotion regulation and it is also part of the mesocortical pathway, was activated by the contrast of ‘negative vs neutral’ conditions. This finding corroborates the notion that this region is important for cognitive control and appraisal of negative emotions (Etkin et al., 2011). Overall, these results support the notion that regions within the reward system are strongly involved in the processing of positive and negative valence stimuli in the context of non-romantic ‘tribal’ love for a football team.
Despite the widespread interest in the study of the reward system in the context of social neuroscience, rare neuroimaging studies address the neurobehavioural correlates of sports fanaticism. McLean et al. (2009) studied anticipatory pleasure (conditions: goals; missed chances; open play) in nine football supporters. The authors suggested that the anterior cingulate cortex is involved in cognitive aspects of pleasure processing, while the putamen was implicated in the anticipation of pleasure. Moreover, Cikara et al. found that the anterior cingulate (and also insula) related to the processing of negative stimuli related to the participants’ favourite team. They studied the neural effects of social group identity in sport (baseball fans) and they found the ventral striatum was strongly modulated by the processing of positive stimuli. Interestingly, this effect was found to be correlated with self-reported desire to aggress against outgroup members. Botzung et al. (2010) assessed 23 basketball fans in a study of emotional memory. Before the fMRI session, the fans watched a game with the rival team. Then, while they were scanned, they reviewed short videos which ended in the moment that the ball was released towards the basket. The participants had to recall whether or not the ball went into the basket, as well as the confidence on that answer and the emotional valence of that clip. Results showed that such memory retrieval activated dorsal frontoparietal regions. Memories retrieved with high confidence additionally recruited insula and the medial temporal lobe (Botzung et al. 2010). These studies did not emphasize the affective and social neuroscientific aspects of team love.
Tribal (in-group) vs individual maternal/partner love bonds. Neuroimaging studies directly addressing in-group (tribal) vs out-group behaviour are also sparse in the literature. Moreover, to our knowledge, there is no previous work studying the neural correlates of in-group non-romantic love in relation to the concept of a strong affective link supporting the tendency to favour the in-group (tribal) vs out-group members (Mathur et al., 2010; Luo et al., 2015; Bruneau and Saxe, 2010; Rand et al., 2009; Levine et al., 2005; Hein et al., 2010). The present results are therefore worth discussing within the context of other forms of inter-individual bonding such as maternal and romantic love.
Our results show striking similarities with the neural underpinnings of maternal love and romantic love as described by Bartels and Zeki (2000, 2004). Activation of the insula, striatum (putamen, caudate and globus pallidus), thalamus, SN/VTA were also found in our study. Acevedo et al. (2012) studied long-term romantic love and also identified a similar core network (SN/VTA, caudate, putamen, globus pallidus, thalamus, insula, hippocampus and amygdala). The results suggest that the passionate behaviour experienced by football fans involves a similar core of key neural structures underlying reward and affective processing. The reward value taken from the vivid football moments can act as the motivational ‘glue’ that keeps the in-group cohesion suggested by other studies.
However, we did not find the pattern of deactivation that was identified in studies of romantic and maternal love, namely in the amygdaloid cortex, posterior cingulate, middle temporal cortex and lateral prefrontal (Bartels and Zeki, 2000, 2004). Importantly, in the present study, the amygdala showed increased activity in the contrasts ‘positive vs negative’ and ‘positive vs neutral’, and the posterior cingulate showed increased activity in both ‘positive vs neutral’ and ‘negative vs neutral’ contrasts. The positive involvement of posterior cingulate was expected due to the memory content and limbic processing likely triggered by positive and negative content. The fact that amygdala activation was biased for positive valence stimuli is further discussed in ‘Activation in the reward system is modulated by the level of fanaticism’ section.
Langeslag and colleagues used an oddball task with pictures of the participants’ loved partners and friends (serving as target and distractor stimuli alternately) to examine how attention modulates the response in regions consistently found in studies concerning romantic love. The results suggested that the dorsal striatum is not activated by romantic information per se, but only when such information is attended to (Langeslag et al., 2014). A study of the neural correlates of hate showed that both the insula and putamen are also activated in addition to romantic love studies (Zeki and Romaya 2008). The present paradigm supports the notion of a common architecture of processing of love related information but also important distinctions underlying passionate attachment to a team.
In-group love vs out-group hate. The contrast of ‘loved team’s positive vs rival’s negative’ moments (direct self-oriented positive affect vs indirect positive affect possibly triggered by negative rival outcomes) showed larger activation in the right inferior parietal lobule and right inferior frontal gyrus, which belong to the mirror neuron system. Decreased activity was found in the left superior frontal gyrus. Decreases in the superior frontal gyrus were found to be associated to favourite brand content in a decision task (Deppe, 2005). Mirror neuron system networks located in the inferior parietal lobule (BA 40) and the inferior frontal gyrus (BA44) are thought to support self-relevant perception-action loops (Rizzolatti and Fabbri-destro, 2008; Eres and Molenberghs, 2013). An fMRI study has accordingly shown that group membership can modulate the neural activity in the inferior parietal lobule (Molenberghs et al., 2013). Eres and Molenberghs (2013) suggested that this result is possibly due to the fact that the human brain simulates the actions of in-group members more easily than the out-group members’ actions. Our results of the contrast of ‘loved team’s positive vs rival’s negative’ moments corroborate this view (Table 6).
Outgroup hate is a commonly perceived counterpart of in-group love, and such types of negative feelings are very common in football fans (e.g. Manchester United vs Manchester City). A neurobehavioural response can therefore be triggered, as suggested by our data, by the negative videos for the rival team, which could possibly generate ‘unfair’ positive feelings in football fans.
The role of the amygdala
Amygdala activation for positive videos. The amygdala has been consistently reported to be engaged in situations of negative emotions, fear and aggression. Concerning the studies of love, Bartels and colleague found that the amygdaloid region (left in the whole group and bilateral in the female group) showed evidence for deactivation when contrasting conditions when participants viewed loved partner’s photos vs friends’ photos (Bartels and Zeki, 2000). A similar bilateral deactivating pattern in amygdala was found by others (Aron et al., 2005; Xu et al., 2011), while Acevedo and colleagues suggested that the right amygdala deactivates in early-stage love, while the left amygdala activates in long-term love (Acevedo et al., 2012). A study on maternal love showed a similar bilateral pattern of deactivation when viewing pictures of one’s own child as compared with pictures of a child acquaintance (Bartels and Zeki, 2004). Intriguingly, Barrett et al. (2012) suggested that poorer quality of the maternal experience is related to decreases in the amygdala response, as tested by using a similar paradigm of viewing own and unfamiliar child’s pictures. A racial in-group vs out-group study found differential responses in the amygdala, which showed decreased activity for viewing in-group members’ faces when compared with viewing out-group faces (Hart et al., 2000). Importantly, this difference in amygdala activity was shown to be correlated with implicit measures of racial bias (Cunningham et al., 2004).
A meta-analysis about reward processing in obesity and substance addiction provided clear evidence that the affected participants showed amygdala hyperactivity when processing rewarding stimuli, either general or related with the problematic condition (García-García et al., 2014). Another review confirmed the view that the amygdala is involved in the processing of both appetitive and aversive stimuli (Hayes et al., 2014).
It is also known that amygdala plays a role in the recognition of faces in general (Almeida et al., 2013). The role of the amygdala in affective social cognition and love, either romantic, maternal and in-group love seems therefore to be distinct, raising questions about differential nature of content-related processing biases.
Amygdala, the reward system and emotional memory. The reward system is considered an ancient structure from an evolutionary perspective, due to its fundamental role on the regulation of behaviours that are associated with its value. This implies that the structures that are part of this system engage in a type of processing that, although not purely mnemonic in nature, is crucial for emotional memory, as affective arousal enhances memory consolidation. Interestingly, the contrast results of ‘positive vs negative’ valent conditions showed a pattern of changes largely overlapping the neural network of the human reward system. This result reinforces the notion that the positive videos of football are perceived as positive input stimuli, generating emotional arousal, and are then interpreted as a ‘to repeat’ (reinforced) pattern while it is memorized, engaging the reward system with differential weights concerning positive vs negative stimuli. The amygdala can have a facilitator role in emotional mnemonic processes in the assessment of positive rewarding stimuli, by reinforcing behaviours of positive payoff rather than negative ones. We suggest that, in this study, as it receives input from the mesolimbic dopaminergic pathway, the amygdala plays a role in emotional arousal and learning by processing motivationally relevant information, preferentially in positive valence conditions. The finding of a correlation between the contrast of ‘positive vs negative’ with the fan assessment score (FSFS) in the amygdala, as well as in structures of the reward system, supports this conceptual framework.
Activation in the reward system is modulated by the level of fanaticism
RFX analysis showed that viewing images of intense moments of participants’ loved team engages classical dopaminergic reward regions. Moreover, we found a positive correlation between the contrast of ‘positive vs negative’ with the fanaticism score (FSFS) in the amygdala, inferior temporal lobe, parahippocampus, right insula, putamen, globus pallidus, thalamus, VTA and SN. Hence, the individual FSFS score partially explains the variance of BOLD differences between positive and negative conditions in these regions. So higher the fanaticism score, higher the modulation of the BOLD signal as a function of positive content.
The most fanatic participants, besides reporting their football experiences differently, do actually experience football moments differently as evidenced by this type of analysis.
Conclusion
We investigated the neural basis of the love for a football team as a type of tribal attachment. We found the involvement of the amygdala and reward system regions such as VTA and SN in the processing of this type of affect. The observation of an increased response bias in these areas preferentially to positive stimuli of the loved team suggests that this kind of non-romantic love represents a strong motivational state, with a bias for processing positive content. The amygdala is proposed to have a facilitator role in emotional mnemonic processes in the assessment of positive rewarding stimuli, reinforcing behaviours of positive outcome rather than negative ones. Furthermore, fanaticism scores were strongly associated with individual response differences elicited by positive vs negative affective in-group experiences, thus implicating a weighting bias concerning neural responses for reward vs punishment.
Supplementary data
Supplementary data are available at SCAN online.
Supplementary Material
Acknowledgements
We would like to thank Sónia Costa for her support in the AAC volunteers’ recruitment and for her help in neuropsychological assessment; and to thank all the volunteers for participating in this study. We would like also to thank Nádia Canário for her support in the scale translation.
Funding
This work was supported by, the BRAINTRAIN project FP7-HEALTH-2013-INNOVATION-1–602186, Portuguese Foundation for Science and Technology (FCT) FCT-UID/NEU/04539/2013,—COMPETE, POCI-01-0145-FEDER-007440, Bial Foundation 132/12, 133/12 and 373/14, CENTRO-07-ST24-FEDER-00205.
Conflict of interest. None declared.
References
- Acevedo B.P., Aron A., Fisher H.E., Brown L.L. (2012). Neural correlates of long-term intense romantic love. Social Cognitive and Affective Neuroscience, 7(2), 145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida I., van Asselen M., Castelo-Branco M. (2013). The role of the amygdala and the basal ganglia in visual processing of central vs. peripheral emotional content. Neuropsychologia, 51(11), 2120–9. [DOI] [PubMed] [Google Scholar]
- Aron A., Fisher H., Mashek D.J., Strong G., Li H., Brown L.L. (2005). Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology, 94(1), 327–37. [DOI] [PubMed] [Google Scholar]
- Barrett J., Wonch K.E., Gonzalez A., Ali N., Steiner M., Hll G.B., et al. (2012). Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience, 7(3), 252–68. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. (2000). The neural basis of romantic love. Neuroreport, 11(17), 3829–34. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. (2004). The neural correlates of maternal and romantic love. NeuroImage, 21(3), 1155–66. [DOI] [PubMed] [Google Scholar]
- BBC Sport, 2015. Premier League : Transfer window proves richest ever at £870m. BBC Sport Available http://www.bbc.com/sport/0/football/34118289 [January 2, 2016].
- Benjamini Y., Heller R. (2007). False discovery rates for spatial signals. Journal of the American Statistical Association, 102(480), 1272–81. Available: http://www.jstor.org/stable/27639977. [Google Scholar]
- Botzung A., Rubin D.C., Miles A., Cabeza R., Labar K.S. (2010). Mental hoop diaries: emotional memories of a college basketball game in rival fans. Journal of Neuroscience, 30(6), 2130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman H., Peters J., Ashburner J., Weiskopf N., Goebel R. (2009). Fast fieldmap-based EPI distortion correction with anatabacus: a plugin for BrainVoyager. NeuroImage, 47, S81. [Google Scholar]
- Bruneau E.G., Saxe R. (2010). Attitudes towards the outgroup are predicted by activity in the precuneus in Arabs and Israelis. NeuroImage, 52(4), 1704–11. [DOI] [PubMed] [Google Scholar]
- Cikara M., Bavel J.J.V. (2014). The neuroscience of intergroup relations: an integrative review. Perspectives on Psychological Science, 9, 245–74. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Johnson M.K., Raye C.L., Chris Gatenby J., Gore J.C., Banaji M.R., (2004). Separable neural components in the processing of black and white faces. Psychological Science, 15(12), 806–13. [DOI] [PubMed] [Google Scholar]
- Deppe M. (2005). Nonlinear responses within the medial prefrontal cortex reveal when specific implicit information influences economic decision making. Journal of Neuroimaging, 15(2), 171–82. [DOI] [PubMed] [Google Scholar]
- DeWall C.N., Masten C.L., Powell C., Combs D., Schurtz D.R., Eisenberger N.I. (2012). Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience, 7(2), 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovidio J.F., et al. 2010. Empathy and intergroup relations. In: Prosocial Motives, Emotions, and Behavior: The Better Angels of Our Nature. Washington, D.C.: American Psychological Association. pp. 393–408. [Google Scholar]
- Duvernoy H. 1999. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply, Vienna: Springer-Verleg. [Google Scholar]
- Eres R., Molenberghs P. (2013). The influence of group membership on the neural correlates involved in empathy. Frontiers in Human Neuroscience, 7, (May), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Egner T., Kalisch R. (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences, 15(2), 85–93. Available: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIFA, 2015. FIFA Financial Report 2014, Available: http://resources.fifa.com/mm/document/affederation/administration/02/56/80/39/fr2014weben_neutral.pdf.
- Fischl B., Salat D.H., Busa E., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–55. [DOI] [PubMed] [Google Scholar]
- García-García I., Horstmann A., Jurado M.A., et al. (2014). Reward processing in obesity, substance addiction and non-substance addiction. Obesity Reviews, 15(11), 853–69. [DOI] [PubMed] [Google Scholar]
- Goldstone A.P., Prechtl de Hernandez C.G., et al. (2009). Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience, 30(August), 1625–35. [DOI] [PubMed] [Google Scholar]
- Hart A.J., Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Rauch S.L. (2000). Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport, 11(11), 2351–5. [DOI] [PubMed] [Google Scholar]
- Hayes D.J., Duncan N.W, Xu J., Northoff G. (2014). A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neuroscience and Biobehavioral Reviews, 45, 350–68. [DOI] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C.D., singer T. (2010). Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron, 68(1), 149–60. [DOI] [PubMed] [Google Scholar]
- Knutson B., Cooper J.C. (2005). Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology, 18(4), 411–7. [DOI] [PubMed] [Google Scholar]
- Langeslag S.J.E., van der Veen F.M., Röder C.H. (2014). Attention modulates the dorsal striatum response to love stimuli. Human Brain Mapping, 35(2), 503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Prosser A., Evans D., Reicher S. (2005). Identity and emergency intervention: how social group membership and inclusiveness of group boundaries shape helping behavior. Personality and Social Psychology Bulletin, 31(4), 443–53. [DOI] [PubMed] [Google Scholar]
- Luo S., Li B., Ma Y., Zhang W., Rao Y., Hans S. (2015). Oxytocin receptor gene and racial ingroup bias in empathy-related brain activity. NeuroImage, 110, 22–31. [DOI] [PubMed] [Google Scholar]
- Mathur V.A., Harada T., Lipke T., Chiao J.Y. (2010). NeuroImage Neural basis of extraordinary empathy and altruistic motivation. NeuroImage, 51(4), 1468–75. [DOI] [PubMed] [Google Scholar]
- McLean J., Brennan D., Wyper D., Condon B., Hadley D., Cavanagh J. (2009). Localisation of regions of intense pleasure response evoked by soccer goals. Psychiatry Research, 171(1), 33–43. [DOI] [PubMed] [Google Scholar]
- Molenberghs P. (2013). Neuroscience and biobehavioral reviews the neuroscience of in-group bias. Neuroscience and Biobehavioral Reviews, 37(8), 1530–6. Available: 10.1016/j.neubiorev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Molenberghs P., Halász V., Mattingley J.B., Vanman E.J., Cunnington R. (2013). Seeing is believing : neural mechanisms of action – perception are biased by team membership. Human Brain Mapping, 34, p2055–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. 1981. The Soccer Tribe 1st edn.,London: Jonathan Cape. [Google Scholar]
- Rand D.G., Pfeiffer T., Dreber A., Sheketoff R.W., Wernerfelt N.C., Benkler Y. (2009). Dynamic remodeling of in-group bias during the 2008 presidential election. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. (2012). Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage, 61(4), 1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Fabbri-destro M. (2008). The mirror system and its role in social cognition. Current Opinion in Neurobiology, 18, p179–84. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Mizuno K., Sasaki A.T., et al. (2015). Imaging the passionate stage of romantic love by dopamine dynamics. Frontiers in Human Neuroscience, 9(April), p.Article 191.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hell H.H., Vink M., Ossewaarde L., Jager G., Khan R.S., Ramsey N.F. (2010). Chronic effects of cannabis use on the human reward system: an fMRI study. European Neuropsychopharmacology, 20(3), 153–63. [DOI] [PubMed] [Google Scholar]
- Wachelke J.F.R., De Andrade A.L., Tavares L., Neves J.R.L.L. (2008). Mensuração da identificação com times de futebol: evidências de validade fatorial e consistência interna de duas escalas. Arquivos Brasileiros De Psicologia 60(1), 98–110. [Google Scholar]
- Wise R.A. (2004). Dopamine, learning and motivation. Nature Reviews. Neuroscience, 5(June), p483–94. [DOI] [PubMed] [Google Scholar]
- Wise R.A., Rompre P.P. (1989). Brain dopamine and reward. Annual Review of Psychology, 40, p191–225. [DOI] [PubMed] [Google Scholar]
- Xu X., Aron A., Brown L., Cao G., Feng T., Weng X. (2011). Reward and motivation systems: A brain mapping study of early-stage intense romantic love in Chinese participants. Human Brain Mapping, 32, 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. (2007). The neurobiology of love. FEBS Letters, 581, 2575–9. [DOI] [PubMed] [Google Scholar]
- Zeki S., Romaya J.P. (2008). Neural correlates of hate. PLoS One, 3(10),e3556.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.