1. Introduction
In the 70's, we classified for the first time the blocks at the atrial level into interatrial blocks (IAB),[1] partial and advanced, and other types of atrial blocks including the concept of atrial aberrancy, and atrial dissociation.[2] The concept of IAB, the most frequent and relevant blocks at the atrial level mean that exist a delayed conduction between the right and left atrium (RA and LA). We also divided them using the same concept as at ventricular, sinoatrial, and atrioventricular (AV) level into 1st degree or partial (P-IAB), 3rd degree or advanced (A-IAB), and 2nd degree or intermittent.[3] The location of atrial fibrosis, that in the majority of cases is the responsible of delay of conduction involves much more part of the atria that just the isolated Bachmann bundle.[4]
2. Electrophysiological and ultra-structural mechanism of IAB
Although the delay of conduction may be located in any part of the atria certainly in the case of A-IAB involves, especially the upper part of the interatrial zone around the Bachmann's bundle. The rest of the atria is more or less involved in the presence of A-IAB. This also happens in P-IAB, although in this case, the upper part of the LA is more preserved, and the stimulus may arrive to the LA with delay but though the Bachmann's bundle.
In patients with IAB, there are electrophysiological and ultrastructural changes in atrium myocardium that explain the appearance of atrial fibrosis and the delay of conduction.[5] Reduced conduction velocity present in atrial fibrosis can arise especially from: (1) increased axial resistance and the gap junctions between cardiomyocites,[6],[7] and (2) decreased maximum upstroke velocity of AP due to reduced Na current density.[8]
3. The clinical diagnosis of IAB
The clinical diagnosis of IAB is based in the detection of delay of conduction of the sinus stimulus between RA and LA. The diagnosis of partial of advanced IAB may be performed easily by analyzing the surface ECG (12 and 3 orthogonal leads) (see later). However, we have to remember that it is necessary the presence of three criteria to define an ECG pattern which caused by a block or delay of conduction and all three have been demonstrated that occur in the case of IAB:[9],[10] (1) the ECG pattern may appear transiently, and the pattern may change abruptly and progressively to more advanced forms; (2) the ECG pattern may appear without associated processes such as cardiac chamber enlargement or ischemia, although in many cases, one or more of these conditions may coexist; and (3) the ECG pattern can be experimentally reproduced.[10] There are other electrical and imaging methods to detect the presence of IAB. We will only mention here a brief summary of them. The vectocardiogram (VCG) provide us the possibility to diagnose also the IAB through the changes of the P loop.[9] These changes may probably, if correlated with the presence of atrial fibrosis detected by cardiac magnetic resonance (CMR), give a better information of the degree and localization of atrial fibrosis than changes of the P wave in surface ECG.[11]
The diagnosis of IAB can also be confirmed by invasive and non invasive electro-mapping techniques.[9],[12] Finally, the diagnosis of IAB may also be suggested by non invasive imaging techniques, that includes new echocardiographic methods, specially 3D speckle-tracking echocardiography that allows to measure the LA strain with excellent reproducibility,[13],[14] and by the new delayed enhancement magnetic ressonance imaging (MRI) techniques,[15],[16] that allows visualizing the presence of atrial fibrosis responsible for the delay of conduction in the atria.
4. The diagnosis of IAB by surface 12 leads ECG
If the stimulus is delayed but still can cross the septum through the normal way (Bachmann's bundle), the P-wave duration will be prolonged (≥ 120 ms) and the morphology usually shows a “notch”, representing P-IAB (the so wrongly called “P-mitrale”). However, if the stimulus is completely blocked in the zone of the Bachmann's bundle (upper part of the atria) and the activation of the LA have to follow a caudo-cranial activation (through the coronary sinus), this represents A-IAB. The changes produced in the P-wave, longer duration and “plus/minus” morphology in the inferior leads II, III and aVF, are characteristic.
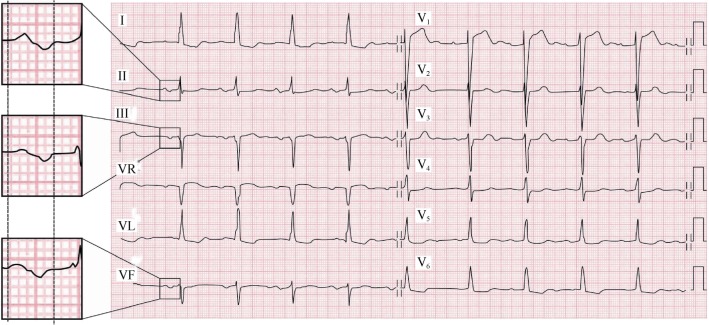
4.1. P-wave duration measurement (Figure 1)
Figure 1. The second part of P wave is isodiphasic in lead II but “positive-negative” in lead III and VF.
In the surface ECG, the measurement of the P-wave and the diagnosis of IAB may be done at a first glance if the ECG recording and the morphology in II, III and aVF is clear and free of artefact or pacing spikes. However, in order to be sure about the measurement in difficult cases and for research purposes, consistency on the measurements is the key. Analyzing digitalized ECG images using amplification is paramount as well as using ECG devices that allow at least three simultaneous channels (6 or 12 channels are ideal). Particular attention should be given to the six leads of the frontal plane at the same time, because it is where we will better identify the changes to the P-wave duration and morphology produced by IAB. However, the horizontal plane leads especially lead V1 for the possible diagnosis of associated LA enlargement (especially the Morris index and the P-terminal force in lead V1) should be also checked.
To perform a good measurement of the P-wave duration, it is important to check and define the interval between the earliest detection of the P-wave (onset) in any lead of the frontal plane and the lead where the end of P wave is detected. Once these two points are defined with lines, the P-wave duration can be measured using semi-automatic callipers, or other more sophisticated methods (Geogebra method).
4.2. The ECG diagnostic criteria
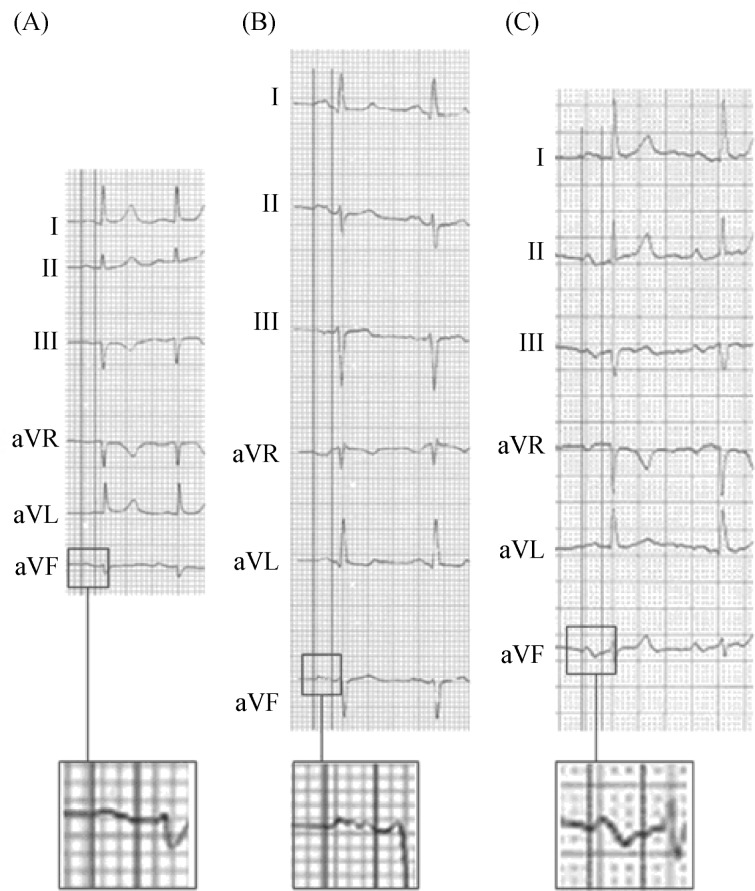
4.2.1. First degree of P-IAB (Figure 2A)
Figure 2. ECG features of normal P wave (A), P wave of P-IAB (B), and P wave of A-IAB (C).
A-IAB: advanced interatrial blocks; P-IAB: partial interatrial blocks.
The P-wave has a normal electrical axis. The electrical impulse is conducted from the RA to LA through the normal propagation route but with a delay at the Bachmann region.
The ECG shows that the P-wave duration is ≥ 120 ms, and usually is bimodal (“notched”) in leads I, II, III, and aVF and sometimes in leads V4-V6.
In the presence of P-IAB, LA enlargement (LAE) is relatively common. In fact, it has been considered that the wide and bimodal P-wave of LAE is better explained because of underlying IAB rather than the longer distance that the impulse has to go across. The morphology of the P-wave in lead V1 allows presuming if there is an associated LAE. In this case, the last part of the activation is directed backwards because the LA enlarges in this direction and then the P wave is going also backwards with a large part of the P wave falling in the negative hemi-field of lead V1 what is recorded in VCG as a figure of “eight” or “zig-zag”. Sometimes, it is possible to record the progressive nature of IAB.
4.2.2. Third degree or A-IAB (Figure 2B and 3)[9],[17]
Figure 3. Typical example of A-IAB with clear P wave “positive-negative” in II, III and VF with a P wave duration > 200 ms.
A-IAB: advanced interatrial blocks.
The diagnosis of A-IAB is made when a P-wave duration is ≥ 120 ms and the morphology of the P-wave in the inferior leads (II, III, aVF) is biphasic or “positive-negative”.
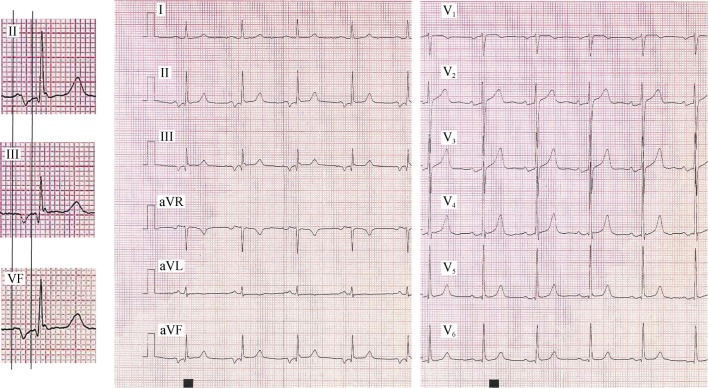
There are different atypical patterns of A-IAB that should be also taken into account: in lead II, the final component of the P-wave can be seen as “isodiphasic” or even “positive-negative-positive”. In this case, in order to be conclusive about the A-IAB diagnosis, the P-wave in leads III and aVF should be clearly “positive-negative” (Figure 1).
In some cases, the ECG morphology can be “positive-negative” in lead II, and completely negative in III and aVF (Figure 4). Sometimes, the initial positive component of the P-wave in all three leads may be very small (and difficult to see without magnifying it) mimicking a completely negative P-wave also in lead II, suggesting a junctional rhythm. However, the P-wave is sinus because the positive polarity in the other leads especially V5-V6, confirms the sinus origin.
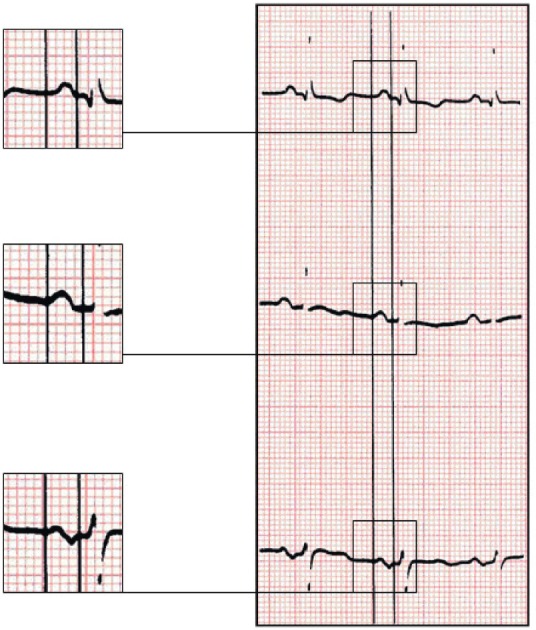
Figure 4. In this case of A-IAB (P = about 160 ms) due to the presence of extensive LA fibrosis, the first part of P wave in III, and VF is isodiphacic, but the onset of P wave in II clearly starts before the negatively of P in III and VF.
A-IAB: advanced interatrial blocks; LA: left atrium.
Finally, in some cases of young patients with high atrial septum aneurysm or septal defect, the stimulus may not be able to cross the upper part of septum but may arrive quickly to LA and depolarize it with some caudo-cranial activation. In these cases, a “positive-negative” morphology may be seen in the inferior leads but the P-wave duration maybe less than 120 ms.
4.2.3. Second-degree interatrial block[9],[18]
Similarly to the conduction block at the AV junction, SA junction, or the ventricles, IAB may occur transiently on a beat-to-beat basis or associated with changes in heart rate (brady- or tachy-dependent) or following pauses induced by premature contractions. Therefore, the P-wave morphology may show transient morphology changes in the same recording.
5. Clinical implications
There is strong evidence that IAB, particularly advanced, predicts atrial fibrillation (AF) and that the longer the P-wave duration, the stronger the association. The association of A-IAB with AF/atrial flutter that was described by our group in 1988,[19] and has been now named Bayés syndrome,[20]–[22] is much more frequent than what we initially thought even in a cohort of normal old people.[23] The presence of some clinical characteristics like advanced age, structural heart disease and ambient arrhythmias make the association even stronger.[24]
It has been also described that patients with IAB may also be at higher risk of stroke,[25] and that in very old people the prevalence of A-IAB is of 25% and is associated with previous stroke and cognitive impairment and dementia.[26]
6. Conclusions
IABs are located in the zone of the high/middle septum especially close to the Bachmann' bundle area but some parts of the right atrium and beyond the interatrial septum in the LA usually is also involved.
In case of first-degree P-IAB, the ECG shows a P-wave duration ≥ 120 ms. If the block is of third degree (A-IAB), the P-wave is also prolonged, and the second part of the P-wave in the inferior leads becomes negative due to retrograde activation of the LA (P ± in leads II, III, and aVF).
IABs are separate entities from left atrial enlargement. However, the two conditions are often associated what can be suspected if negative terminal P-wave forces in lead V1 are present.
VCG is not necessary to diagnose IAB but the morphology of the P loop may probably help to define the intensity and location of atrial fibrosis.
There are important implications related to the presence of IABs. They are frequently associated to supraventricular arrhythmias (specifically AF), stroke, and cognitive impairment.
Footnotes
This article is part of a Special Issue “Atrial fibrillation in the elderly”.
Guest Editors: Manuel Martínez-Sellés & Antoni Bayés de Luna
References
- 1.Bayés de Luna A. Bloqueo a nivel auricular. Rev Esp Cardiol. 1979;39:5–10. [PubMed] [Google Scholar]
- 2.Chung EK. Aberrant atrial conduction. Unrecognized electrocardiographic entity. Br Heart J. 1972;34:341–346. doi: 10.1136/hrt.34.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayés de Luna A. Fouth Edition. Oxford UK: Wiley-Blackwell; 2012. Clinical Electrocardiography. [Google Scholar]
- 4.Kottkamp H. Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013;34:2731–2738. doi: 10.1093/eurheartj/eht194. [DOI] [PubMed] [Google Scholar]
- 5.Tse G, Yeo JM. Conduction abnormalities and ventricular arrhythmogenesis: the roles of sodium channels and gap junctions. Int J Cardiol Heart Vasc. 2015;9:75–82. doi: 10.1016/j.ijcha.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas SP, Kucera JP, Bircher-Lehmann L, et al. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Cir Res. 2003;92:1209–1216. doi: 10.1161/01.RES.0000074916.41221.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardic tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on micro-conduction. Circ Res. 1998;83:781–794. doi: 10.1161/01.res.83.8.781. [DOI] [PubMed] [Google Scholar]
- 8.Nattel S. Effects of heart disease on cardiac ion current density versus current amplitude: important conceptual subtleties in the language of arrhythmogenic ion channel remodelling. Circ Res. 2008;102:1298–1300. doi: 10.1161/CIRCRESAHA.108.178087. [DOI] [PubMed] [Google Scholar]
- 9.Bayés de Luna A, Platonov P, García-Cosio F, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012;45:445–451. doi: 10.1016/j.jelectrocard.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Waldo A, Hurry L, Bush J, et al. Effects on the canine P waves of discrete lesions in the specialized atrial tracts. Circ Res. 1971;29:452. doi: 10.1161/01.res.29.5.452. [DOI] [PubMed] [Google Scholar]
- 11.Baranchuk A. In: Interatrial block and supraventricular arrhythmias: clinical implications of Bayes syndrome. 1st edition. Baranchuk A, editor. Cardiotext Publising; 2017. [Google Scholar]
- 12.Bayés de Luna A. Textbook of clinical electrocardiography. Oxford, UK: Wiley-Blackwell; 2012. [Google Scholar]
- 13.Longobardo L, Todaro MC, Zito C, et al. Role of imaging in assessment of atrial fibrosis in patients with atrial fibrillation: state-of-the-art review. Eur Heart J. 2014;15:1–5. doi: 10.1093/ehjci/jet116. [DOI] [PubMed] [Google Scholar]
- 14.Lacalzada Almeida J, García-Niebla J, Bayés de Luna A. Speckle-tracking echocardiography and advanced interatrial block. Rev Esp Cardiol (Engl Ed) Published Online First: Dec 8, 2016. DOI: 10.1016/j.rec.2016.11.0142016. [DOI] [PubMed]
- 15.Marouche NF, Wilber D, Hindricks G. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation. The DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 16.Benito EM, Bayés de Luna A, Baranchuk A, Mont L. Extensive atrial fibrosis assessed by late gadolinium enhancement cardiovascular magnetic resonance associated with advanced interatrial block electrocardiogram pattern. Europace. 2017 doi: 10.1093/europace/euw294. DOI:10.1093/europace/euw294. [DOI] [PubMed] [Google Scholar]
- 17.Bayés de Luna A, Fort de Ribot R, Trilla E, et al. Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol. 1985;18:1. doi: 10.1016/s0022-0736(85)80029-7. [DOI] [PubMed] [Google Scholar]
- 18.Conde D, Baranchuk A, Bayés de Luna A. Advanced interatrial block as a substrate of supraventricular tachyarrytymias: a well recognized syndrome. J Electrocardiology. 2015;48:135–140. doi: 10.1016/j.jelectrocard.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Bayés de Luna A, Cladellas M, Oter R, et al. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112–1118. doi: 10.1093/oxfordjournals.eurheartj.a062407. [DOI] [PubMed] [Google Scholar]
- 20.Conde D, Baranchuk A. What a cardiologist must know about Bayés' Syndrome. Rev Argent Cardiol. 2014;82:220–222. [Google Scholar]
- 21.Conde D, Baranchuk A. Bloqueo interauricular como sustrato anatómico-eléctrico de arritmias supraventriculares: Sindrome de Bayés. Arch Cardiol Mex. 2014;84:32–40. doi: 10.1016/j.acmx.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Bacharova L, Wagner GS. The time for naming the interatrial block Syndrome: Bayes Syndrome. J Electrocardiol. 2015;48:133–134. doi: 10.1016/j.jelectrocard.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 23.O'Neal WT, Hooman K, Zhu-Ming Z, et al. Advanced interatrial block and ischemic stroke. Neurology. 2016;87:1–5. [Google Scholar]
- 24.Martínez-Sellés M, Fernandez Lozano I, Baranchuk A, et al. Should we anticoagulate patients at high risk of atrial fibrillation? Rev Esp Card. 2016;69:374–376. doi: 10.1016/j.rec.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Ariyarajah V, Puri P, Apiyasawat S, Spodick DH. Interatrial block: a novel risk factor for embolic stroke? Ann Noninv Electrocardiol. 2007;12:15–20. doi: 10.1111/j.1542-474X.2007.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martínez-Sellés M, Massó-van Roessel A, et al. Interatrial block and atrial arrhythmias in centenarians: prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645–651. doi: 10.1016/j.hrthm.2015.10.034. [DOI] [PubMed] [Google Scholar]