1. Introduction
It was not until 1979 that Bayés de Luna described atrial conduction blocks and classified them as interatrial and intraatrial.[1] Interatrial blocks refer to conduction disorders located between the atria, while intraatrial blocks occur within the same atrium. This work motivated several authors, including Bayés himself, to further investigate atrial conduction and interatrial block (IAB). In the following years, the anatomy of the interatrial electrical system was studied in depth. A particular emphasis was placed on the Bachmann's region, as the pathophysiology of IAB is directly related to a block in this area. In a recent consensus, various authors agreed on the definitions of IAB, emphasizing its epidemiology and prevalence.[2]
In their work, Bayés and other authors found IAB to be a substrate for the development of supraventricular arrhythmias [mostly atrial fibrillation (AF)]; a condition that was subsequently referred to as Bayés syndrome.[3] The role of IAB in the elderly will be extensively discussed in this volume, so we will go broader in this article to help the reader to understand the role of IAB in predicting AF in different clinical scenarios regardless of the age of the patient. In this review, we will highlight the association between IAB and AF in different clinical conditions and the ability of IAB to predict “de novo” AF or AF recurrence.
2. IAB in different clinical scenarios
2.1. Post pharmacological cardioversion
IAB has been associated with the development of AF in several different clinical scenarios. In 2014, Enriquez, et al.,[4] conducted a study to determine if IAB predicted AF recurrence following pharmacological cardioversion. They included 61 patients with recent onset AF and no structural heart disease that recently underwent cardioversion with one of two anti-arrhythmic drugs. Thirty-one patients received a single oral dose of propafenone while 30 patients received vernakalant (i.v.). A 12-lead ECG following conversion was evaluated for the presence of partial or advanced IAB. Clinical follow-up and electrocardiographic recordings were performed for a 12-month period. Mean age was 58 years. Advanced IAB was present in 18% of the population and partial IAB in 16.4% of the population. Overall AF recurrence was 36%, with a 90.9% recurrence in patients with advanced IAB versus 70% in those with partial IAB. In patients without IAB, there was a 12.5% recurrence rate (P = 0.001). A multivariate analysis found IAB to be independently associated with AF recurrence [odds ratio (OR): 18.4]. The study confirms that advanced IAB is strongly associated with a higher risk of AF recurrence one year following pharmacological cardioversion; independent of the antiarrhythmic drug used.[4]
2.2. After successful pulmonary vein isolation
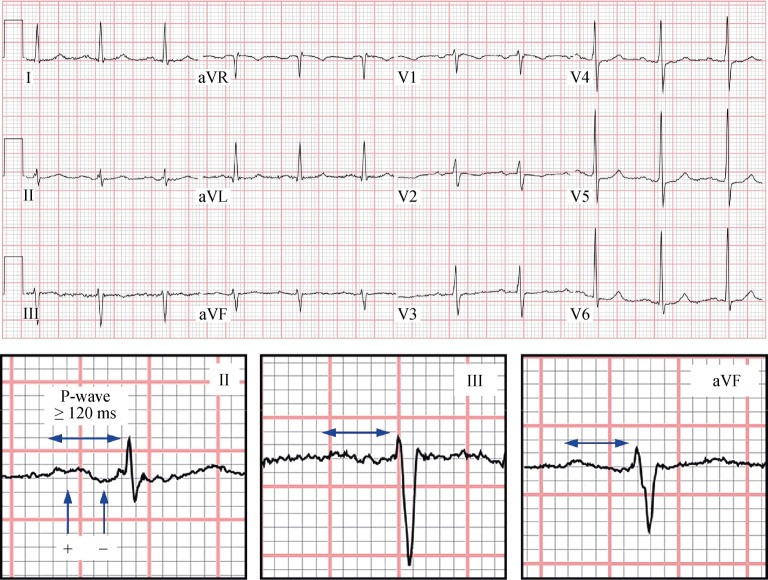
Pulmonary vein isolation in patients with paroxysmal AF is successful in approximately 70%–80% of cases. This variability suggests that underlying electrical abnormalities may predispose patients to persistent AF following isolation. In IAB, delayed interatrial conduction may generate interatrial dyssynchrony with resultant left atrial electrical heterogeneity. This may contribute to the maintenance of AF (Figure 1). For this reason, Caldwell, et al.,[5] performed a study to confirm the hypothesis that IAB predisposes patients with paroxysmal AF to recurrence after pulmonary vein isolation. They analyzed a cohort of 114 patients with paroxysmal AF undergoing pulmonary vein isolation. In all patients, a 12-lead ECG was performed to evaluate for the presence of IAB, P-wave dispersion and AF recurrence. Mean age was 59 years and the prevalence of diabetes was 12%. IAB was found in 37% of patients. The presence of advanced IAB was significantly associated with a higher risk of AF recurrence: 66.6% in the group with IAB vs. 40.3% in the group without block (P < 0.05). A higher P-wave dispersion was also associated with AF recurrence. As the authors had suspected, the presence of pre-existent advanced IAB is associated with a higher risk of AF recurrence post ablation for paroxysmal AF.[5]
Figure 1. 12-lead ECG of a patient with advanced IAB.
Note the prolonged P-wave duration and distal negative component in the inferior leads. IAB: interatrial block.
2.3. After catheter ablation for atrial flutter
Enriquez, et al.,[6] also focused on advanced IAB as a possible predictor of AF following cavo-tricuspid isthmus ablation. They included 122 patients with typical atrial flutter and no history of AF who were scheduled to undergo cavo-tricuspid isthmus ablation. Post ablation 12-lead ECG in sinus rhythm was evaluated in patients for the presence of advanced IAB. Mean age was 67 years and most of the patients were men. Advanced IAB was present in 23% of patients. After a mean follow-up of 30 months, 46.7% of the population had developed new-onset AF. The incidence of AF was significantly higher in patients with advanced IAB compared to those without (71.4% vs. 39.4%, respectively; P = 0.003). A multivariate analysis identified advanced IAB as an independent predictor of AF (OR: 2.9, 95% CI: 1.02–8.6; P < 0.04). In short, advanced IAB is a predictor of new-onset AF after successful cavo-tricuspid isthmus ablation in patients with typical atrial flutter and no history of AF.[6]
2.4. In patients with dual chamber pacemakers
Detection of atrial high rate episodes (AHRE) by pacemakers provides clinicians the ability to suspect AF as the underlying rhythm. Several studies have proved a good correlation between AHRE and AF, and some are already considering it a surrogate. Recently, Tekkesin, et al.,[7] have analyzed a population of 367 patients who were implanted with dual chamber pacemaker due to sinus node dysfunction during a 12-months period. Surface ECG was used to diagnose IAB before pacemaker implantation. Six months after the implant, pacemakers were interrogated to detect AHRE. Patients were divided into two groups according to the presence or absence of AHRE.
AHRE were detected in 107 (30.1%) patients during their device interrogation. IAB was found in 115 (32.4%). Sixty-seven (27%) patients in AHRE (-) group had IAB while 48 (44.9%) patients in the AHRE (+) group (P < 0.01).[7] This initial experience suggests that IAB could predict new onset AF in patients receiving dual chamber pacemakers, and this could be used for a closer follow-up in this population.
2.5. In Chagas' disease
Chagas' cardiomyopathy is an endemic disease in Latin America. These patients usually present with a low ejection fraction and some of them will have an ICD device implanted for primary or secondary prevention. A significant proportion of these patients develop AF, which may result in stroke, embolism and inappropriate ICD shock. This is associated with increased morbidity and/or mortality. Enriquez, et al.,[8] investigated IAB as a possible predictor for AF in this population. They conducted a retrospective study of patients with Chagas' cardiomyopathy and ICDs from 14 centers in Latin America. The presence of advanced or partial IAB was identified and the patients were followed over a 33 month period to evaluate for new-onset AF. Mean age was 54.6 years, mean ejection fraction was 49% and the presence of IAB was 18.8% (10% advanced, 8.8% partial). During the follow-up period, AF occurred in 13.8% of patients and was significantly greater in the group with IAB (advanced and partial) compared with the group without IAB (73.3% vs. 0, respectively, P < 0.0001). The number of inappropriate ICD shocks was significantly higher in the group with IAB (P = 0.014).[8] In conclusion, IAB predicts new-onset AF in patients with Chagas' cardiomyopathy and ICDs.
2.6. After a successful TAVR procedure
Aortic stenosis (AS) is a the most common valvular disease in the United States and Europe, affecting more than 9.8% of patients aged 80 to 89 years. Left untreated, severe AS carries a dismal prognosis.[9] The transcatheter aortic valve replacement (TAVR) procedure has become a key procedure for those deemed too high risk for traditional aortic valve replacement using a conventional sternotomy, cardioplegia and cardiopulmonary bypass. AF is a common post-TAVR complication; however, identification of risk factors remains a challenge. Alexander, et al.,[10] have recently conducted a retrospective study of patients who underwent a successful TAVR at one hospital in Canada and one hospital in Spain. Prior history of AF, intraoperative conversion to surgical aortic valve replacement, and atrial pacing were used as exclusion criteria. A total of 62 patients were included in the analysis. The study population had a mean age of 83 ± 6 years; mean aortic valve area of 0.7 ± 0.2 cm2 and a mean aortic pressure gradient of 81.1 mmHg. Advanced IAB was determined to be present in 14/62 patients (23%). Post TAVR new-onset AF occurred in 17/62 patients (27%). Post-TAVR AF had a higher incidence in the advanced IAB population compared to the non advanced IAB population [6/14 (42.9%) vs. 11/48 (22.9%); P = 0.14]. The mean P-wave duration of patients who developed AF was longer than those who did not (130 vs. 121 ms; P = 0.087). Post-TAVR AF was also positively associated with a P-wave duration greater than 130 ms [9/17 (52.9%) vs. 13/35 (28.9%); P = 0.077].[10]
A simple an inexpensive surface 12-lead ECG can help in identifying patients at higher risk of developing post-TAVR AF. If this is confirmed in larger populations, the ECG should be integrated into a clinical model for assessment of stroke risk after a TAVR procedure.
2.7. In obstructive sleep apnea
Obstructive sleep apnea (OSA) is a common disorder that affects 5% of the North American adult population. It is associated with AF; however, the pathophysiology of this association remains controversial. IAB could be a factor associated with AF in these patients. Baranchuk, et al.,[11] conducted a study to identify the factors associated with IAB in patients with OSA. They included 180 consecutive patients referred for a poly-somnography study. The severity of OSA was determined by the apnea-hypopnea index. The presence of IAB was determined by 12-lead electrocardiography, evaluating only the P-wave duration and using the definition of partial IAB. Moderate to severe OSA was present in 144 patients (OSA group), and the remaining 36 had mild OSA or no OSA (control group). The prevalence of IAB was significantly higher in the OSA group compared with the control group (34.7% vs. 0; P < 0.001). In a linear regression analysis, age and moderate to severe OSA were independent predictors on P-wave duration (P = 0.001 and P < 0.001, respectively). In conclusion, advanced age and severe OSA are predictors of IAB in these patients. This feature could explain the high prevalence of supraventricular arrhythmias in patients with OSA.[11]
2.8. Heart failure patients with resynchronization therapy
Sadiq, et al.,[12] further evaluated IAB in patients with advanced heart failure requiring Cardiac Resynchronization Therapy (CRT). They sought to determine whether IAB could predict new onset AF in this population. The study included 112 patients with severe heart failure receiving an implanted cardiac resynchronization device. Patients had no previous history of AF and 65% of heart failure was due to ischemic heart disease. Mean age was 67 years and 37.2% of patients had advanced IAB. After 30 months of follow-up, new onset AF occurred in 29% of patients. The prevalence of AF was significantly higher in the group with advanced IAB compared to the group without (50% vs. 17%, respectively; P < 0.001). In a multivariate analysis, older age (OR: 1.06, 95% CI: 1.002–1.130; P = 0.04) and advanced IAB (OR: 4.91, 95% CI: 2.06–11.69; P ≤ 0.001) were independent predictors of AF occurrence.[12] In summary, IAB was detected in more than one third of this population. It was an independent predictor of AF in patients with severe heart failure undergoing cardiac resynchronization device implantation with no history of AF.[12]
2.9. In the hospitalized population
Bayés de Luna was one of the first authors to describe the prevalence of IAB in the hospital population. In 1985, Bayés and his collaborators analyzed 81,000 ECGs and found that 83 of them fulfilled criteria for advanced IAB.[13] These were subsequently compared with two control groups: one with structural heart disease and another without structural heart disease. The prevalence of IAB was nearly 1% in the general population and 2% among patients with valvular heart disease. The study demonstrated an important association between IAB and structural heart disease. In other articles of this series, the current knowledge on epidemiological issues will be discussed in detail.
2.10. In patients with stroke
The group led by Spodick has made major contributions on this field.[14],[15] In their studies, among 293 neurological admissions over two years, 85 patients were diagnosed with embolic strokes and 208 with non-embolic strokes. Patients were then matched for stroke risk factors and evaluated for IAB. Eighty-eight percent of probable embolic stroke patients showed sinus rhythm, demonstrating a 61% IAB prevalence. Only hypertension (P < 0.001; r = 0.3) and IAB (P < 0.006; r = 0.2) were significant and directly correlated. This has triggered the interest to not only study IAB as a predictor for AF, but also as a predictor of stroke and cognitive impairment. This is particularly relevant in the elderly population, where non-invasive tests could help in deciding early anticoagulation.[16]–[18]
3. Future directions
After reviewing the results of the studies, we conclude that IAB is associated with AF in multiple clinical scenarios. However, the role of IAB as predictor of arrhythmias in many other situations has not yet been evaluated. For this reason, six studies are currently being conducted to further evaluate the association of IAB with AF. The studies will evaluate patients following electrical cardioversion, aortic valve replacement, mitral valve surgery and significant carotid disease. Studies evaluating IAB in the context of advanced coronary artery disease are finalized and data is being processed. Finally, an International Registry has been launched in October 2016 with almost 40 centers from all around the world looking after almost 700 patients.[19] This work will provide further insight into the ability of IAB to predict AF (in elderly populations with structural heart disease) using a simple and economical method such as 12-lead ECG.[19]
Footnotes
This article is part of a Special Issue “Atrial fibrillation in the elderly”.
Guest Editors: Manuel Martínez-Sellés & Antoni Bayés de Luna
References
- 1.Bayés de Luna A. Bloqueo a nivel auricular. Rev Esp Cardiol. 1979;39:5. [PubMed] [Google Scholar]
- 2.Bayés de Luna A, Platonov P, Cosio FG, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012;45:445–451. doi: 10.1016/j.jelectrocard.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Conde D, Baranchuk A. Bloqueo interauricular como sustrato anatómico-eléctrico de arritmias supraventriculares: síndrome de Bayés. Arch Cardiol Mex. 2014;84:32–40. doi: 10.1016/j.acmx.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez A, Conde D, Hopman W, et al. Advanced interatrial block is associated with recurrence of atrial fibrillation post pharmacological cardioversion. Cardiovasc Ther. 2014;32:52–56. doi: 10.1111/1755-5922.12063. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell J, Koppikar S, Barake W, et al. Advanced interatrial block is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J Electrocardiol. 2013;46:e1. doi: 10.1007/s10840-013-9851-1. [DOI] [PubMed] [Google Scholar]
- 6.Enriquez A, Sarrias A, Villuendas R, et al. New-onset atrial fibrillation after cavotricuspid isthmus ablation: identification of advanced interatrial block is key. Europace. 2015;17:1289–1293. doi: 10.1093/europace/euu379. [DOI] [PubMed] [Google Scholar]
- 7.Tekkesin AI, Çinier G, Cakilli Y, et al. Interatrial block predicts atrial high rate episodes detected by cardiac implantable electronic devices. J Electrocardiol. DOI: 10.1016/j.jelectrocard.2016.09.004. Published online first: Sep 8, 2016. [DOI] [PubMed]
- 8.Enriquez A, Conde D, Femenia F, et al. Relation of interatrial block to new-onset atrial fibrillation in patients with Chagas cardiomyopathy and implantable cardioverter defibrillators. Am J Cardiol. 2014;113:1740–1743. doi: 10.1016/j.amjcard.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 9.Eveborn GW, Schirmer H, Heggelund G, et al. The evolving epidemiology of valvular aortic stenosis. The Tromsø study. Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 10.Alexander B, Rodriguez C, Perez de la Isla L, et al. The impact of advanced interatrial block on new-onset atrial fibrillation following TAVR procedure. Int J Cardiol. 2016;223:672–673. doi: 10.1016/j.ijcard.2016.08.083. [DOI] [PubMed] [Google Scholar]
- 11.Baranchuk A, Parfrey B, Lim L, et al. Interatrial block in patients with obstructive sleep apnea. Cardiol J. 2011;18:171–175. [PubMed] [Google Scholar]
- 12.Sadiq Ali F, Enriquez A, Conde D, et al. Advanced interatrial block is a predictor of new onset atrial fibrillation in patients with severe heart failure and cardiac resynchronization therapy. Ann Noninv Electrophysiol. 2015;20:586–591. doi: 10.1111/anec.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayes de Luna A, Fort de Ribot R, Trilla E, et al. Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol. 1985;18:1–13. doi: 10.1016/s0022-0736(85)80029-7. [DOI] [PubMed] [Google Scholar]
- 14.Ariyarajah V, Apiyasawat S, Najjar H, et al. Frequency of interatrial block in patients with sinus rhythm hospitalized for stroke and comparison to those without interatrial block. Am J Cardiol. 2007;99:49–52. doi: 10.1016/j.amjcard.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 15.Ariyarajah V, Puri P, Apiyasawat S, Spodick DH. Interatrial block: A novel risk factor for embolic stroke? Ann Noninvasive Electrocardiol. 2007;12:15–20. doi: 10.1111/j.1542-474X.2007.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Selles M, Baranchuk A, Elosua R, Bayes de Luna A. Advanced interatrial block, risk of ischemic stroke and anticoagulation. Neurology. 2016;87:2499. doi: 10.1212/WNL.0000000000003445. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Selles M, Escobar LA, Baranchuk A. Interatrial block and the risk of ischemic stroke. J Atheroscler Thromb. 2017;24:185–186. doi: 10.5551/jat.37242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martínez-Sellés M, Fernández Lozano I, Baranchuk A, et al. Should patients at high risk of atrial fibrillation receive anticoagulation? Rev Esp Cardiol. 2016;69:374–376. doi: 10.1016/j.rec.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Sellés M, Baranchuk A, Elousa R, Bayes de Luna A. Rationale and design of the BAYES (interatrial Block And Yearly EventS) Registry. Clin Cardiol. DOI: 10.1002/clc.22647.2016. Published online first: Nov 24, 2016. [DOI] [PMC free article] [PubMed]