Abstract
Drug-resistant tuberculosis (TB) is emerging as an important health problem in Togo. From sputum of previously treated TB patients, multidrug-resistant (MDR) TB was diagnosed in 24% (10/42) patients via GeneXpert MTB/RIF compared to 25% (6/24) patients via conventional drug susceptibility testing (BACTEC MGIT 960 system). The agreement between these two methods to detect MDR-TB is excellent. However, GeneXpert MTB/RIF offers the advantage of rapidly detecting Mycobacterium tuberculosis complex in sputum samples in instances where the cultures are negative (33%, 14/42) or contaminated (9.5%, 4/42). GeneXpert MTB/RIF permitted us to estimate the prevalence of MDR-TB in previously treated TB patients and to improve TB diagnostics among HIV-positive and -negative patients in Togo, where culturing M. tuberculosis complex from sputum samples is challenging.
Introduction
Mycobacterium tuberculosis is one of the world's most devastating human pathogens. Each year more than nine million people develop active TB; two million die from this disease, and a third are associated with HIV coinfection [1]. The current vaccine for tuberculosis (TB), bacillus Calmette-Guérin, has variable (mostly poor) efficacy in protecting against pulmonary TB in children and adults [2], [3]. TB control is further threatened by the emergence of multidrug-resistant (MDR), extremely drug-resistant and totally drug-resistant strains of M. tuberculosis [4]. In new TB cases the proportion of MDR-TB (isolates that exhibit resistance to at least rifampicin (RIF) and isoniazid (INH)) ranged from 0 to 22.3%. The highest proportion of MDR-TB reported was 60% among previously treated cases [4], [5].
Using accurate and early detection tests to manage drug-resistant TB cases is of great urgency for countries facing high proportions of drug resistance and countries with a population heavily coinfected with HIV [5], [6]. The prevalence of MDR-TB has been estimated to be low in sub-Saharan Africa, where surveillance of drug resistance is notably limited [1]. In Togo little work has been done to determine the exact prevalence of MDR-TB. The recently introduced GeneXpert MTB/RIF assay detects the presence of M. tuberculosis complex DNA and its susceptibility to RIF in a single reaction. Ninety-five per cent of RIF-resistant isolates also exhibit resistance to INH [7], [8], [9]. Therefore, the detection of RIF resistance may serve as a surrogate marker for MDR M. tuberculosis [8], [9], [10].
The objective of this study was to determine the prevalence of MDR among previously treated HIV-positive and -negative patients and to evaluate the performance of GeneXpert MTB/RIF in detecting MDR cases in Togo.
Materials and methods
Sample and selection criteria
This study was conducted in Togo in the mycobacteria reference laboratory from January 2013 to June 2013. Ethical approval was obtained from the Université de Lomé institutional review board. All participants provided written informed consent for the collection of samples and subsequent analyses. Sputum samples were collected from previously TB-treated HIV-positive and -negative patients who had TB according to the definition established by the World Health Organization (WHO): relapse, failure or default (https://www.cap-tb.org/resources/tb-case-definitions-revision-6-may-2011). Samples were obtained from patients all over the country and were sent to the reference laboratory in a cooler box (4–8°C) within 24 hours of sample collection for the closest sites and stored in a refrigerator for microscopy, culture, Mycobacteria Growth Indicator Tube (MGIT) drug susceptibility testing (DST) and GeneXpert MTB/RIF assay.
HIV testing
Alere Determine HIV-1/2 (Alere, Yavne, Israel) and HIV Tri-dot (J. Mitra, New Delhi, India) tests were used to screen for HIV-1 and HIV-2 infection.
Microscopy
Microscopy was performed on all collected samples using Ziehl-Neelsen staining, which is available in all peripheral health care centre laboratories in Togo.
Culture
Both the BACTEC MGIT 960 system (Becton Dickinson, Sparks, MD, USA) (liquid media) and Löwenstein-Jensen agar were systematically used for sputum culture samples, irrespective of smear microscopy result. Positive growth without contaminant was confirmed by Ziehl-Neelsen staining and by the rapid test confirmation kit (i.e. MPT64 protein detection-based immunochomatographic test; SD Bioline Kit, Standard Diagnostics, Yongin-si, Korea) [11]. The interpretation of results was based on the manufacturer's instructions. Positive results were considered as belonging to M. tuberculosis complex. Negative results were regarded as atypical mycobacteria and were not taken into account in this study.
Drug susceptibility testing
Conventional DST using the BACTEC MGIT 960 system [12] and GeneXpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) [13], [14] were used simultaneously to screen all cases for any resistance pattern present. The interpretations of results were based on the manufacturer's instructions.
Statistical analysis
Agreement, sensitivity and specificity, and values of the BACTEC MGIT 960 system for DST compared to GeneXpert MTB/RIF were calculated for RIF. The agreement between the two methods was determined by the κ statistic. The κ value, a measure of test reliability, was interpreted as follows: <0.2, poor; 0.21 to 0.4, fair; 0.41 to 0.6, moderate; 0.61 to 0.8, good; and ≥0.81, excellent [15].
Results
A total of 42 sputum samples were collected from previously TB-treated patients in 15 peripheral health care centres: CMS Adakpame (n = 3), CMS Nukafu (n = 1), CMS Kodjoviakope (n = 1), CMS Amoutive (n = 1), CMS Be (n = 1), CMS Guerin-kouka (n = 2), CHU Sokode (n = 17), Hopital District 2 (n = 1), Hopital Be (n = 1), CLT Zio (n = 2), CDT Bassar (n = 1), Polyclinic Tsevie (n = 2), Polyclinic Aneho (n = 1), Polyclinic Kara (n = 3) and Hopital Tabligbo (n = 3). There were 27 (64%) male and 15 (36%) female subjects for a male/female sex ratio of 1.8:1. One patient was younger than 15 years old, 32 were aged between 15 and 45, eight were aged 45 to 65 and one was older than 65. The average age was 34.5 years; most patients fell between the ages of 15 and 45.
According to the WHO TB case definition, of the 42 cases, 22 were relapse cases (52.38%), 14 were failure cases (33.34%) and six were default cases (Table 1). Only 31 (73.8%) of 42 had consented to undergo HIV testing. Seven (22.6%) of these 31 patients were positive for HIV-1; no cases of HIV-2 were detected. Three of these seven were relapse cases and four were failure cases. Fourteen of the 42 total cases were culture negative, and four samples were contaminated.
Table 1.
Distribution of type of retreatment cases and resistance profile
| Resistance | n (%) | GeneXpert MTB/RIF, n (%) |
|---|---|---|
| Relapse | 22 (52.38) | 6 (27.27) |
| Failure | 14 (33.34) | 2 (14.28) |
| Default | 6 (14.28) | 2 (33.34) |
| Total | 42 (100) | 10 (25) |
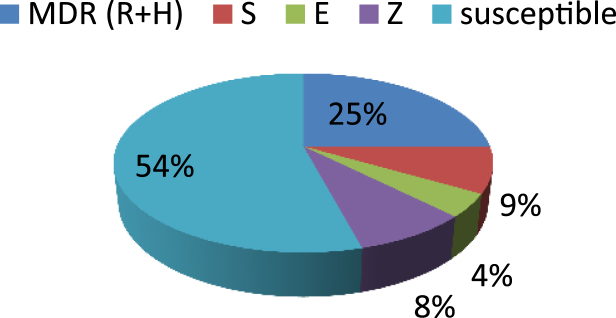
Using the BACTEC MGIT 960 system for DST, 13 (54%) of 24 were susceptible to all the drugs, six (25%) were resistant to at least RIF and INH combined but not to other drugs and five (21%) were monoresistant, as follows: streptomycin (n = 2), ethambutol (n = 1) and pyrazinamide (n = 2) (Fig. 1). Among the 42 sputum samples, the sensitivity and specificity of the BACTEC MGIT 960 system for detecting MDR-TB were 60% and 100%, respectively.
Fig. 1.
Distribution of drug susceptibility testing profile based on BACTEC MGIT 960 system.
GeneXpert MTB/RIF detected M. tuberculosis complex from the 42 sputum samples even though culture results were negative (n = 14) or contaminated (n = 4). Among the 42 samples ten (24%) exhibited resistance to RIF. Two of ten instances of RIF resistance were detected by GeneXpert MTB/RIF and were positive for HIV (Table 2), one with relapse and the other with failure. Overall, the six MDR-TB cases detected by the BACTEC MGIT 960 system were confirmed by GeneXpert MTB/RIF. There was excellent agreement between the BACTEC MGIT 960 system and GeneXpert MTB/RIF for DST (κ = 1).
Table 2.
Detection of rifampicin resistance by GeneXpert MTB/RIF
| GeneXpert MTB/RIF | HIV result | n |
|---|---|---|
| Total | 42 | |
| Rifampicin resistance | Negative | 8 |
| Positive | 2 |
Discussion
Although TB remains one of the most difficult infections to treat, the current Direct Observed Treatment Short regimen, which usually takes 6 to 9 months of continuous chemotherapy, is quite effective in treatment of drug-susceptible TB. Mutations in drug target genes have contributed to the evolution of M. tuberculosis strains that are resistant to the currently available first-line TB antibiotics [9].
In this study, first-time diagnosed TB patients received chemotherapy (RIF, INH, ethambutol and pyrazinamide). Patients who improved after 2 months of intensive-phase therapy then continued with only RIF and INH therapy for 4 months. Treatment was considered a failure if the patient's sputum smear or culture remained positive at 5 months or later during treatment; the case was considered a relapse when a previously treated patient developed another episode of TB after a completed treatment and had a negative smear and/or culture result [5], [6], [11], [16], [17]. Forty-two previously treated patients were enrolled onto this study; subjects had an HIV prevalence of 22.58% (only 31 consented to an HIV test). Most affected patients were within the active population range of 15 to 45 years.
The HIV epidemic caused a dramatic increase in TB incidence in sub-Saharan countries. In South Africa the HIV-driven rise in TB incidence was accompanied by escalating rates of TB drug resistance [18]. In Togo a previous study found that the prevalence of TB-HIV coinfection was 23.7% in TB patients [19]. In our study more than half of the participants were relapse cases (52.4%), followed by failure cases (14%). The overall prevalence of MDR-TB based on the BACTEC MGIT 960 system for DST was 25% (6/24) and was confirmed by GeneXpert MTB/RIF (24%, 10/42) among retreatment cases.
According to a 2008 WHO report [20] Estonia reported 52.1% MDR-TB among previously treated cases, Azerbaijan (Baku City) reported 55.8%, Uzbekistan (Tashkent) reported 60.0% and Lebanon reported 62.5%. The Russian Federation reported data on retreatment cases in Orel Oblast only. Sixteen settings reported MDR-TB of 25% or higher among previously treated cases [5]. These data correlate with our findings in Togo, with the majority found among relapse cases, followed by failure cases. Repeated misuse or intense use of these few drugs has led to the sequential accumulation of mutations, resulting in the emergence of strains simultaneously resistant to multiple existing TB drugs [9], [10], [21]. Unlike the situation in other bacteria, where acquired drug resistance is generally mediated through horizontal transfer by mobile genetic elements such as plasmids, transposons or integrons, in M. tuberculosis acquired drug resistance is caused mainly by spontaneous mutations in chromosomal genes, producing the selection of resistant strains during suboptimal drug therapy [10]. In our study, only 24 were detected as culture positive due to the antibiotic pressure on the physiology of the bacteria (when the patient was receiving treatment), or in the paucibacillary sample for relapse cases. DST was performed only on these 24 isolates, and 25% (n = 6) were MDR. Compared with the BACTEC MGIT 960 system for DST, GeneXpert correctly identified 98.2% (95% confidence interval, 91.5–99.9) of RIF-resistant and 95.5% (95% confidence interval, 85.8–99.2%) of RIF-susceptible specimens [22]. In our study all 42 cases detected as M. tuberculosis complex had 100% sensitivity.
The MTB/RIF test is a simple method, and routine staff with minimal training can use the system. The test appeared to be as sensitive as culture with smear-positive specimens but less sensitive with smear-negative pulmonary and extrapulmonary specimens that included low numbers of bacilli [14]. The rpoB gene represents a much better molecular target for the simultaneous detection of TB and the key form of drug resistance [23]. The GeneXpert assay was highly effective for TB diagnosis and identification of RIF-resistant strains in smear-negative samples [8], [13]. All ten MDR cases detected as RIF resistant with GeneXpert were confirmed by MGIT DST testing with an association to at least INH resistance. This demonstrates the accuracy of GeneXpert to detect MDR cases. The detection of RIF resistance may therefore serve as a surrogate marker for MDR M. tuberculosis [8], [9].
Conflict of interest
None declared.
Acknowledgements
We thank the patients for their participation in the study. This work was supported in part by an ASM-UNESCO VRP grant (to TA) and an Emory-CFAR03 developmental grant 00044613 (to TA) from P30AI050409 (to Emory University). The content is solely the responsability of the authors and does not necessarily represent the official views of ASM/UNESCO and Emory-CFAR.
References
- 1.World Health Organization . World Health Organization; Geneva, Switzerland: 2012. Global tuberculosis report, 2012.http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf Available at: [Google Scholar]
- 2.Verhoef J. The BCG controversy. Int J Antimicrob Agents. 1994;4:291–295. doi: 10.1016/0924-8579(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 3.Gu C. 1996. Mycobacterium tuberculosis: BCG et stratégies vaccinales; pp. 107–116.http://www.ipubli.inserm.fr/bitstream/handle/10608/208/?sequence=17 Available at: [Google Scholar]
- 4.Zhang Y., Yew W. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13:1320–1330. [PubMed] [Google Scholar]
- 5.WHO/HTM/TB . 2008. Anti-Tuberculosis Drug. [Google Scholar]
- 6.Nagaraja S.B., Satyanarayana S., Chadha S.S., Kalemane S., Jaju J., Achanta S. How do patients who fail first-line TB treatment but who are not placed on an MDR-TB regimen fare in South India? PLoS One. 2011;6:e25698. doi: 10.1371/journal.pone.0025698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marlowe E.M., Novak-Weekley S.M., Cumpio J., Sharp S.E., Momeny M.A., Babst A. Evaluation of the Cepheid Xpert MTB/RIF assay for direct detection of Mycobacterium Tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011;49:1621–1623. doi: 10.1128/JCM.02214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannidis P., Papaventsis D., Karabela S., Nikolaou S., Panagi M., Raftopoulou E. Cepheid geneXpert MTB/RIF assay for Mycobacterium tuberculosis detection and rifampin resistance identification in patients with substantial clinical indications of tuberculosis and smear-negative microscopy results. J Clin Microbiol. 2011;49:3068–3070. doi: 10.1128/JCM.00718-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manuscript A. NIH Public Access. Changes. 2012;29:997–1003. [Google Scholar]
- 10.Almeida Da Silva P.E.A., Palomino J.C. Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J Antimicrob Chemother. 2011;66:1417–1430. doi: 10.1093/jac/dkr173. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V.G., Urs T.A., Ranganath R.R. MPT 64 antigen detection for rapid confirmation of M. tuberculosis isolates. BMC Res Notes. 2011;4:79. doi: 10.1186/1756-0500-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe C., Aono A., Hirano K. [Evaluation of the BACTEC MGIT 960 system for drug susceptibility testing of Mycobacterium tuberculosis isolates compared with the proportion method on solid media] Kekkaku. 2001;76:657–662. [PubMed] [Google Scholar]
- 13.Hillemann D., Rüsch-Gerdes S., Boehme C., Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol. 2011;49:1202–1205. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeka A.N., Tasbakan S., Cavusoglu C. Evaluation of the GeneXpert MTB/RIF assay for rapid diagnosis of tuberculosis and detection of rifampin resistance in pulmonary and extrapulmonary specimens. J Clin Microbiol. 2011;49:4138–4141. doi: 10.1128/JCM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Rattan A., Kalia A., Ahmad N. Multidrug-resistant Mycobacterium tuberculosis: molecular perspectives. Emerg Infect Dis. 1998;4:195–209. doi: 10.3201/eid0402.980207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Thoracic Society; US Centers for Disease Control and Prevention; Infectious Diseases Society of America Treatment of tuberculosis. MMWR Morb Mortal Wkly Rep. 2003;52(RR11):1–77. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5211a1.htm Available at: [Google Scholar]
- 18.Mboma S.M., Houben R.M.G.J., Glynn J.R., Sichali L., Drobniewski F., Mpunga J. Control of (multi)drug resistance and tuberculosis incidence over 23 years in the context of a well-supported tuberculosis programme in rural Malawi. PLoS One. 2013;8:e58192. doi: 10.1371/journal.pone.0058192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagnra A.Y., Adjoh K., Tchaptchet Heunda S., Patassi A.A., Sadzo Hetsu D., Awokou F. [Prevalence of HIVTB co-infection and impact of HIV infection on pulmonary tuberculosis outcome in Togo] Bull Soc Pathol Exot. 2011;104:342–346. doi: 10.1007/s13149-010-0079-3. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . World Health Organization; Geneva, Switzerland: 2008. World health statistics, 2008.http://www.who.int/gho/publications/world_health_statistics/EN_WHS08_Full.pdf Available at: [Google Scholar]
- 21.Barbosa T.M., Levy S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat. 2000;3:303–311. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- 22.Kurbatova E.V., Kaminski D.A., Erokhin V.V., Volchenkov G.V., Andreevskaya S.N., Chernousova L.N. Performance of Cepheid® Xpert MTB/RIF® and TB-Biochip® MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis. 2013;32:735–743. doi: 10.1007/s10096-012-1798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawn S.D., Nicol M.P. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]