The remarkable plasticity of adult stem cells within the gastrointestinal tract has been further highlighted by a recent paper from the Clevers’ group published in Cell Stem Cell this year (1). This study reports on some fundamental aspects of organoid biology that are of general interest as well as putting a focus on how differentiation is regulated in one of the remaining under-explored cell lineages—enteroendocrine cells. Enteroendocrine cells play diverse roles serving as factories for hormone secretion but as they comprise a minor fraction of cell types in the epithelium and are not easily identified under light microscopy via morphological criteria alone, there have been less studies on the molecular mechanisms that control their cell fate. It is important to note that despite the relatively low cellular abundance the intestine is arguably the largest endocrine organ in the body (2). In organoids, enteroendocrine cells also represent a relatively small fraction of the cell types present and are inherently heterogeneous so they have been challenging to study. The recent paper by Onour Basak and colleagues made use of the organoid system but instead of driving proliferation of stem/progenitor cells, they exploited the consequences of induced quiescence and revealed the expansion of enteroendocrine progenitors when the organoids were reactivated.
Before the landmark studies driven by Toshiro Sato (3) and Nick Barker (4) within Hans Clever’s group, our extensive understanding of the stem/progenitor cells within the intestinal crypt was informed by exacting labelling and observational studies, kidney capsule transplant models, mouse transgenic/knock-out genetics and extrapolation from investigation of transformed cell lines. The reliable isolation and expansion of Lgr5+ve cells and the recognition of WNT potentiating R-spondin as an important driver of stem cell proliferation were game changers and set the foundations that has allowed laboratories around the world to grow organoids initially from the small intestine of mice to ultimately most epithelial tissues from humans organs. Now the field has a source of primary material that is renewable, amenable to live cell imaging and can be manipulated by external signals.
Three core signalling pathways orchestrate intestinal stem/progenitor cell survival, expansion and cell fate—WNT, EGFR and NOTCH. This reductionist view will serve its purpose for this editorial but does so by ignoring a range of many other factors and signalling networks that influence and fine-tune these processes, particularly in vivo. We now have the ability to generate the representative cell lineages of the crypt, namely enterocytes, goblet cells, enteroendocrine cells and Paneth cells from a single stem cell. This cell typically expresses robust levels of Lgr5 in a cocktail of factors that engage these three pathways, within the physical cradle of matrix plus the nuance of blockade of anoikis signalling sets the scene for the Basak et al. paper. The key experiment upon which the pertinent observations about enteroendocrine cells followed was the removal or blockade of EGFR signalling.
Epidermal growth factor (EGF) belongs to a family of growth factors that drive proliferation in many tissues. Transformed cells such a colorectal cancer cells will often form tumour organoids without EGF but normal intestinal organoids require EGF for their propagation. The ability to monitor Lgr5 expression with reference to GFP expression in reporter mice which labels the stem cell population allowed Basak et al. to explore the effects on EGF withdrawal. As many in the field would appreciate, such a removal of EGF leads to smaller organoids with apoptotic cells. Further investigation of this by the authors, demonstrated that proliferation and cell cycle progression ceased but unexpectedly the relative proportion of Lgr5+ve cells increased (Figure 1). This was particularly interesting because Lgr5+ve cells have been appreciated as highly proliferative stem cells. Indeed the community of intestinal stem cell researchers around the world have robustly discussed the definition, location and potential of quiescent stem cells and this study has now described another potential cell type to explore. Importantly, although it appeared that stem cell quiescence had been induced by inhibition of EGF, this state could be reversed with the potential to generate all cellular lineages retained. Notwithstanding this preserved multi-lineage potential, qPCR analyses suggested that chromogranin A encoding (ChgA) mRNA expression was restored to a greater extent upon reactivation than genes that mark other cell lineages. For instance, the proportion of lysozyme positive Paneth cells bounced back to normal levels upon EGFR reactivation which is particularly instructive in that Paneth cells “nurse” Lgr5+ve stem cells on their journey. Paneth cell help to establish complete organoids providing locally available ligands/membrane bound growth factor receptors, most notably stimulating NOTCH, WNT and EGF signalling. Even so the cultures still depend on addition of exogenous R-spondin which facilitates WNT signalling and provision of NOTCH ligands. NOGGIN polypeptide is additionally needed to inhibit tumour growth factor beta receptor (TGFBR) activation. So there is a complex network of signalling taking place in these structured clusters of cells called “organoids”.
Figure 1.
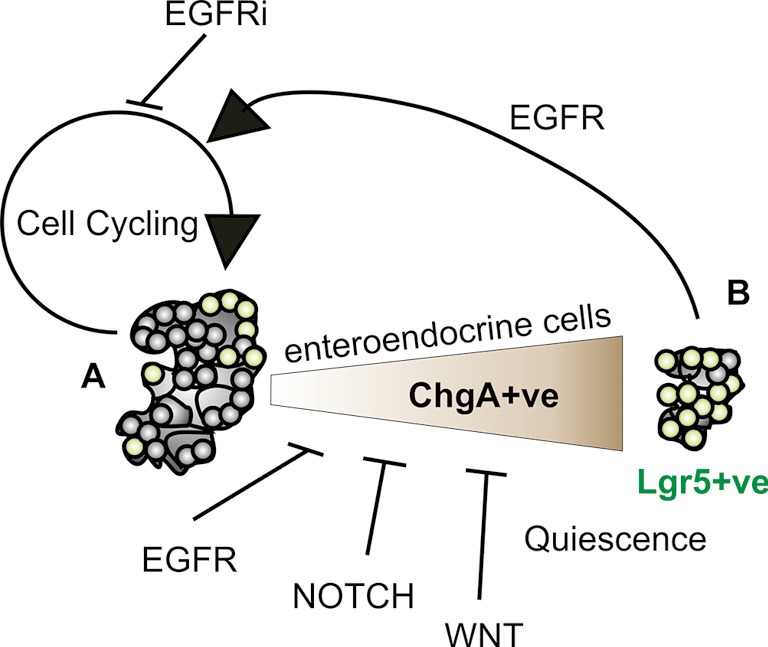
Intestinal organoids are readily generated from the small intestine in the presence of EGFR, NOTCH and WNT stimuli and have the capacity to generate multiple cell lineages. These cell lineages arise from a stem cell population identified by robust expression of R-spondin receptor, Lgr5. A, when EGFR signalling is blocked (EGFRi) cells withdraw from the cell cycle. Under these conditions expression of the enteroendocrine lineage marker chromogranin A (ChgA) is increased slightly and is increased further by subsequent withdrawal of NOTCH and WNT stimuli. B, under these conditions of growth factor blockade an expanded proportion of quiescent Lgr5+ve cells populate the smaller organoids. These however retain multi-lineage potential as well as a source of enriched heterogeneous enteroendocrine cells.
It is interesting to reflect on how stem cells can transit between quiescence and a state of active proliferation. The results of this study show stem cell quiescence in intestinal crypts demonstrate considerable plasticity. It is also instructive to consider that stem cells in general may have a flexibility similar to a “rubber band” in that the “state of quiescence” varies in terms of stem cell turn-over, time frame, environmental stimuli and finally tissue source. For instance, earlier studies of murine bone marrow stem cells with genuine long-term repopulating ability suggested cellular turn over in the order of 30+ days (5) while the Lgr5+ve population in the mouse intestinal crypt is more generally considered to cycle in the order of 21 hours. In the gut it is not as simple as contemplating as a single homogeneous stem cell population as previous edifying study from Doug Winton’s group revealed (6). They identified quiescent stem cells using a histone tagging method that also robustly expressed Lgr5 at a much higher frequency than calculated in the past using radiolabeled nucleotides. It appears that essentially high turnover and quiescent Lgr5+ve stem cells co-exist with the latter called upon under stress or injury scenarios. The interesting question raised by these observations of two categories of stem cells is one of lineage potentiality.
The ability of Basak et al. to induce cell cycle exit over days by withdrawal of EGFR signalling while retaining the capacity of the Lgr5+ve stem cells to be reactivated with multi-potency adds another attribute to the credentialing of these as true stem cells. The authors explored multiple approaches, notably gene expression, phospho-proteomic and inhibitor studies to show that blocking EGFR signalling via the MAPK pathway was required to set up the Lgr5+ve enrichment of the quiescent population. Buczacki et al. (6) noted that the subpopulation of quiescent stem cells defined by label retention that they characterized also express Lgr5 and have a propensity towards the secretory lineage. Paneth cell differentiation was favoured and a classic enteroendocrine gene expression signature was also enriched. With the observation that secretory lineage signatures come up in quiescent stem cells one wonders how far can this can be exploited?
To follow up this trend towards enteroendocrine cell gene expression when iEGFR was employed, Basak et al. then progressively removed further stimuli by inhibiting NOTCH and WNT signalling. As expected, iNOTCH treatments expanded Paneth cell numbers while iWNT eliminated Paneth cells but induced the generation of other secretory lineage cells; goblet and enteroendocrine cells. At this point, the combined signalling blockade unmasked not only the propensity to generate enteroendocrine cells but also the diversity within this class of cell. The standout products expressed by these cells were chromogranin A and serotonin to the extent that a third of cells within an organoid were found to be CrgA+ve by immunofluorescence. A more focused analysis where single cell RNA expression profiles were examined, identified a broad spectrum of hormone gene expression amongst cells. This raises the question about how this diversity comes about; is this stochastic or a result of inductive signals from other cells?
It is important to recognise that advances in our understanding of intestinal crypt biology that have come to the field via organoid propagation need to be viewed through the prism that organoids are essentially a rapid regeneration system rather than a model of homeostasis. The work from Winton’s group explored organoids to some extent but their focus was on in vivo systems (6). The other consideration is that organoid culture protocols are designed to optimise expansion removing the subtleties of signalling thresholds which we know are required for normal development and may be more evident in vivo. There is no reason to think that threshold effects will not be involved in crypt biology, toggling signalling of individual pathways and pathway cross talk with the ability to respond to stress and injury which clearly destabilises homeostasis in profound ways.
While Lgr5-based reporter models are extremely valuable in visualising the behaviour of stem cells and lineage tracing they have notable limitations. Other approaches that employ judicious choices of cell surface markers can be used to enrich for stem cells with equivalent and perhaps superior potential (7). As with much of the stem cell research field markers are at best surrogates for functional potential. In the intestinal stem cell field, the antibodies to LGR5 remain problematic and the Lgr-GFP reporter (4) that has served research so well is influenced by haploinsufficiency via the knock-in of the gfp gene cassette into one Lgr5 allele. It is noteworthy to remember that Lgr5 serves as the R-spondin receptor and in the reporter mouse its expression is compounded by mosaic distribution. A further puzzle is what is keeping Lgr5 expression turned-on when WNT and NOTCH signalling is ablated. It appears that activation of NOTCH under conditions of constitutive WNT signalling in organoids further induces Lgr5 expression but that removal of a single allele (8) or expression of hypomorphic versions of Lgr5 co-regulator Myb are enough to substantially shut down Lgr5 expression (8-11).
So what are the broader implications of these studies to human biology and disease? The ability to expand the enteroendocrine population in mouse organoids will inevitably be replicated in human intestinal organoids providing an opportunity to compare these cells across species. Perhaps more interesting will be to explore the plasticity of individual enteroendocrine cells and how the repertoire of more than 30 hormones are produced (2). Another implication of this work is one of endocrine cancers of the gut. So far the mutational profiling has not highlighted clinically employed targeted therapies, although tyrosine kinase, SRC and defective TGF-beta signaling do offer possibilities (12). The developments in targeting chromogranin+ve endocrine tumours that produce serotonin and co-express its receptor is in rapid development.
New approaches that employ isotopes that target cytotoxic insult to the tumour while also permitting PET imaging is an exciting development in oncology (13,14). Nevertheless, this study by Basak et al., indicating that enteroendocrine cells arise from Lgr5+ve cells that can be in a quiescent state may also inform future approaches and the opportunity to model this biology using organoids. The final consideration that comes to mind is the potential of combining anti-EGFR therapies that might modulate the enrichment of enteroendocrine cells within tumours which may provide improved opportunities to kill these cancer cells.
In summary, the advances to our understanding of intestinal stem cell biology through the wide spread adoption of “organoid” technology have been remarkable but such developments still require biological insights to exploit observations that arise through manipulating organoids under a plethora of culture conditions. This paper is a good example of taking an observation and dissecting its implications. When combined with deeply considered in vivo studies pioneered by Potten, Cheng and Leblond beginning decades ago, we are now enriched with extraordinary detail on how amazingly plastic the intestinal crypt is.
Acknowledgements
We thank Dr. Vignesh Narasimhan for critical reading of this manuscript.
RG Ramsay and HE Abud receive research funding from the National Health and Medical Research Council of Australian and the Victorian Government, Australia.
Provenance: This is an invited Editorial commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Basak O, Beumer J, Wiebrands K, et al. Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell 2017;20:177-90 e4. [DOI] [PubMed]
- 2.Ahlman H, Nilsson. The gut as the largest endocrine organ in the body. Ann Oncol 2001;12 Suppl 2:S63-8. 10.1093/annonc/12.suppl_2.S63 [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262-5. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- 4.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003-7. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- 5.Bradford GB, Williams B, Rossi R, et al. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol 1997;25:445-53. [PubMed] [Google Scholar]
- 6.Buczacki SJ, Zecchini HI, Nicholson AM, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013;495:65-9. 10.1038/nature11965 [DOI] [PubMed] [Google Scholar]
- 7.Nefzger CM, Jarde T, Rossello FJ, et al. A Versatile Strategy for Isolating a Highly Enriched Population of Intestinal Stem Cells. Stem Cell Reports 2016;6:321-9. 10.1016/j.stemcr.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Germann M, Xu H, Malaterre J, et al. Tripartite interactions between Wnt signaling, Notch and Myb for stem/progenitor cell functions during intestinal tumorigenesis. Stem Cell Res 2014;13:355-66. 10.1016/j.scr.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Cheasley D, Pereira L, Lightowler S, et al. Myb controls intestinal stem cell genes and self-renewal. Stem Cells 2011;29:2042-50. 10.1002/stem.761 [DOI] [PubMed] [Google Scholar]
- 10.Malaterre J, Carpinelli M, Ernst M, et al. c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc Natl Acad Sci U S A 2007;104:3829-34. 10.1073/pnas.0610055104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampurno S, Bijenhof A, Cheasley D, et al. The Myb-p300-CREB axis modulates intestine homeostasis, radiosensitivity and tumorigenesis. Cell Death Dis 2013;4:e605. 10.1038/cddis.2013.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banck MS, Kanwar R, Kulkarni AA, et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest 2013;123:2502-8. 10.1172/JCI67963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong G, Thompson M, Collins M, et al. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging 2014;41:1831-44. 10.1007/s00259-014-2788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taieb D, Hicks R, Pacak K. PET imaging for endocrine malignancies: from woe to go. J Nucl Med 2017. [Epub ahead of print]. 10.2967/jnumed.117.189688 [DOI] [PubMed] [Google Scholar]


