Abstract
The aortic valve (AV) experiences a complex mechanical environment, which includes tension, flexure, pressure, and shear stress forces due to blood flow during each cardiac cycle. This mechanical environment regulates AV tissue structure by constantly renewing and remodeling the phenotype. In vitro, ex vivo and in vivo studies have shown that pathological states such as hypertension and congenital defect like bicuspid AV (BAV) can potentially alter the AV’s mechanical environment, triggering a cascade of remodeling, inflammation, and calcification activities in AV tissue. Alteration in mechanical environment is first sensed by the endothelium, which in turn induces changes in the extracellular matrix, and triggers cell differentiation and activation. However, the molecular mechanism of this process is not understood very well. Understanding these mechanisms is critical for advancing the development of effective medical based therapies. Recently, there have been some interesting studies on characterizing the hemodynamics associated with AV, especially in pathologies like BAV, using different experimental and numerical methods. Here, we review the current knowledge of the local AV mechanical environment and its effect on valve biology, focusing on in vitro and ex vivo approaches.
Keywords: Aortic valve, Mechanobiology, Shear stress, Pressure, Stretch, Bicuspid, Calcification
AORTIC VALVE (AV): STRUCTURE AND HEMODYNAMICS
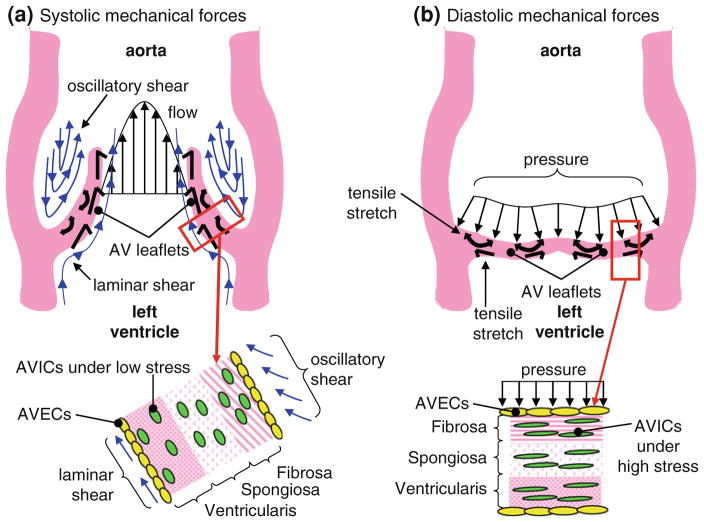
The AV regulates the flow between left ventricle and aorta. It is comprised of three semilunar cusps attached at commissures. Directly behind each cusp is an elliptical depression called the Sinus of Valsalva. The semilunar cusps are attached to the fibrous annulus ring at the base. Each aortic leaflet cusp is comprised of three layers: fibrosa—facing the aorta; ventricularis—facing the left ventricle; and spongiosa—the layer between the fibrosa and ventricularis (Fig. 1). These three layers vary in their organization of extracellular matrix(ECM) components (collagen and elastin), especially along the radial direction. The fibrosa is predominantly made of type I fibrillar collagen that is arranged circumferential direction in parallel bundles, surrounded by a matrix of elastins. The ventricularis is mostly comprised of elastin fibers oriented along the radial direction, while the spongiosa is primarily made up of glycosaminoglycans, which act as shock absorbers and provide the deformability function of the valve leaflets.89
FIGURE 1.
Illustration showing the different mechanical stimuli experienced by the AV endothelial and interstitial cells during a cardiac cycle: (a) Systolic mechanical forces and (b) diastolic mechanical forces.8
The AV cusps are composed of two cell types—interstitial cells (ICs) which make up the majority of the valve and endothelial cells (ECs) which line the AV along the fibrosa as well as the ventricularis. 22 The ECs form single cell monolayers, express Von Willebrand factor, produce vasoactive agents such as endothelin-1 and nitric oxide, and exhibit prostacyclin activity. The ECs are oriented circumferentially and perpendicular to the direction of blood flow. The ICs are a heterogenous (three phenotypes: myofibroblasts, fibroblasts, smooth muscle cell like phenotype) and dynamic population of cells responsible for the constant renewal of ECM. The ICs play a critical role in normal functioning of the valve and in the initiation and progression of valve pathology. The phenotype of valve cells is influenced by both the complex genetic programming30,93,94 as well as the local hemodynamic factors such as leaflet stretch and surface shear stresses.56 Although valvular ECs share some similarities with vascular ECs in terms of responses to mechanical stimuli, they are genetically different120 and also have a higher propensity to undergo endothelial to mesenchymal transition.15 Moreover, recent research also suggests that AV leaflet ECs on either side of the leaflets show differential gene expression profiles, which could be attributed to the conditioning due to different microenvironments on either side.15
The AV opens during systole, which lasts for about 330 ms at a heart rate of 70 beats/min.14 Blood rapidly accelerates through the AV and reaches a peak velocity of approximately 1.2 m/s after the leaflets have fully opened. The flow begins to decelerate rapidly after peak velocity is reached.88 The pressure gradient that is developed affects the low momentum fluid near the wall of the aorta more than that at the center, causing reverse flow in the sinus region.84 During systole the pressure difference required to drive the blood through the AV is of the order of only a few millimeters of mercury; however, the pressure difference aortic and ventricular side of the valve during diastole reaches 80–90 mmHg in normal individuals at rest. The valve closes near the end of the deceleration phase of systole with very little reverse flow through the valve. The annulus reaches its minimum size at the end of systole and its maximum size at the end of diastole. During systole, vortices develop in all three sinuses behind the leaflets of the AV. These vortices help to close the AV efficiently and quickly. The closing volume, or backflow during closure, has been estimated to be less than 1% of the forward flow.13
Impact of AV Diseases
AV disease is a significant source of morbidity and mortality. Worldwide, AV disease is a serious clinical condition, resulting in over 300,000 valve replacements per year, a number which is expected to triple by the year 2050.124 It is also a strong risk factor for other cardiac-related deaths.70,76 In the developed world, 25% of patients 65 years old or older have AV sclerosis, a characteristic of AV stenosis. AV calcification is the most common cause of AV stenosis.63,85 Typically, AV diseases are characterized by inflammation, fractured matrix fibers, thrombus formations, sclerotic, and calcific lesions107 and manifests as stenosis, regurgitation or both.16 AV stenosis is caused by both age-related progressive calcification and congenital malformations such as bicuspid AV (BAV). The decreased valvular opening in stenosis causes an increase in pressure gradient across the valve, which increases with severity of stenosis. Aortic regurgitation (AR) or insufficiency is a condition where there is backflow of blood from the aorta into the ventricle. Around 50% of the cases of aortic insufficiency are due to dilatation of the aortic root, which is idiopathic in most instances. In about 15% of regurgitation cases, the cause is innate BAV, while another 15% of the cases are due to retraction of the cusps as part of post-inflammatory processes of endocarditis in rheumatic fever and various collagen vascular diseases.74
Bicuspid Aortic Valves
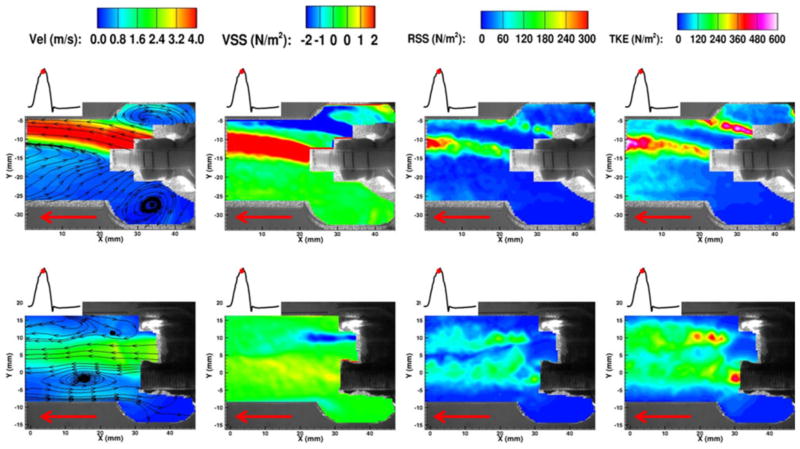
BAV is a congenital condition occurring in 1–2% of all live births. BAVs are associated with significant AS and AR requiring surgical intervention in most cases. Even though genetic factors have been implicated as primary cause for BAVs, the role of hemodynamics in accelerating complications has not been ruled out. BAVs are characterized by eccentric jet, abnormal systolic flow, persistent folding of leaflet tissue, and extended areas of leaflet contact.52,86,118 Turbulent flow structures are seen in the ascending aorta of BAV patients. Hope et al. have observed helical flow in BAV patients with or without dilated aorta using time-resolved phase-contrast magnetic resonance imaging. They indicate that the degree and direction of flow jet eccentricity may determine the segmental aneurysm formation in BAV patients. Altered hemodynamics in BAVs, in comparison with normal tricuspid AV (TAV), has also been studied using numerical models. 54,118 Recently, Saikrishnan et al.90 characterized the hemodynamics associated with BAVs using surgically modified normal porcine AV as BAV models. The valve models as such were investigated in terms of effective orifice area, transvalvular pressure gradient, and peak flow rate. Particle image velocimetry (PIV) was extensively used to study the fluid dynamics in the vicinity of BAV in terms of vorticity, total kinetic energy (TKE), Reynolds shear stresses (RSS), and viscous shear stresses (VSS). Figure 2 shows the comparison between velocity fields of a normal tricuspid AV (TAV) and a BAV during peak systole, indicating a strong eccentric jet coming out of the BAV and the associated vortex formation. In BAVs, presence of two sinuses (instead of three in normal valves) drastically alters the vortex evolution (temporally and spatially), which directly affects the shear stress experienced by the valve leaflets. In general, BAVs were found to have higher TKE and RSS values compared to normal TAV indicating higher fluctuations in fluid flow through these BAVs as shown in Fig. 3. These fluctuations may evoke adverse mechanobiological reactions in BAVs.
FIGURE 2.
Velocity field of a normal tricuspid AV (bottom row) and a BAV (top row) model, showing the eccentric systolic jet in BAV. Red arrow indicated the direction of flow90 (RSS—Reynolds shear stress, VSS—viscous shear stress, TKE—total kinetic energy).
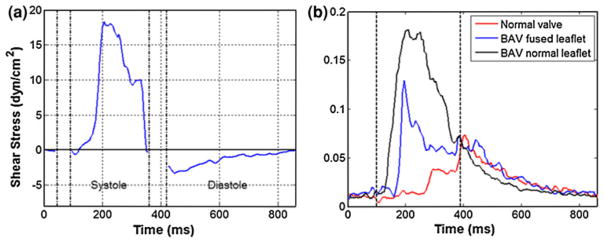
FIGURE 3.
(a) Shear stress variation on AV leaflet surface on aortic side during systole and diastole. (b) Shear stress fluctuations in comparison with normal tricuspid valve and a BAV. Note the higher fluctuations in shear stress values as reflected in the “standard deviation” values for BAV.127
MECHANICAL ENVIRONMENT AND MECHANOBIOLOGY OF AV
The structure and function of the AV is influenced by its surrounding hemodynamics and mechanical microenvironment9 (Fig. 1). Valve mechanobiology studies have focused on elucidating how ECs and ICs sense and respond to hemodynamics-induced mechanical stimuli such as stretch, shear, and transvalvular pressure. It has been well established that AV degradation is not a passive process brought about by wear and tear due to aging. It is rather an active process involving perturbation of valvular ECs and ICs by the local mechanical forces.20,21 Ex vivo studies have shown that different mechanical forces act in synergy to modulate and maintain a normal cellular phenotype. 119,122,123 Another important aspect of AV cellular mechanobiology is the interactions between ECs and ICs. There are relatively few co-culture studies performed on AV cells or tissues. Butcher and Nerem20 developed a 3D model of the AV leaflet comprising both ICs and ECs and determined the cellular responses when exposed to different luminal flow patterns and suggested that ECs are necessary to regulate the ICs phenotype and ECM synthesis. The significance of interactions between ECM and valvular cells has been investigated as well. It has been found that cellular and molecular events leading to AV disease are interdependent and entwined with extra cellular-matrix maladaptation.23,30,71 Unfortunately AV disease is detected late in the disease progression and is typically treated by AV replacement surgery. Thus, the gross pathological changes and surgical treatments of sclerotic valves have received much attention while the molecular mechanisms underlying AV disease are not well understood yet. This situation warrants a detailed understanding of complex molecular pathways that can lead to AV disease to be able to develop non-surgical treatment methods. Further, identification of the biomarkers that can be detected in early AV disease is also vital to successfully prevent and/or treat AV disease.
The following sections describe individual mechanical stimuli experienced by the native AV, under normal and pathological conditions, with an emphasis on their relevance to AV mechanobiology.
Shear Stress
Physiologically, shear stress occurs on AV leaflet surface due to friction with flowing blood. Shear stresses experienced by AV depend on flow conditions through the valve as well as cyclic dynamic leaflet motion. Shear stresses directly affect the ECs lining each valve cusp, which in turn transmit the signal to the underlying matrix and ICs. Different shear stress patterns are known to elicit different EC responses, and disturbed flow has been shown to promote cell proliferation, as well as morphology and gene expression dissimilar to laminar flow in ECs.25,65 Further, shear stresses on the valve can also change with developing pathology. Thus an accurate estimation of shear stresses experienced by the AV under both physiological and pathological hemodynamics is critical for mechanobiology studies.
Fluid mechanics of AV such as flow profiles, RSS, and turbulence characteristics have been estimated using both experimental techniques (laser Doppler velocimetry, hot film anemometry, PIV, MRI, Doppler ultrasound as well as computational modeling.54 Amongst experimental techniques, laser Doppler velocimetry, hot film anemometry are point-by-point high-resolution measurement techniques used to assess local hemodynamics, whereas PIV is comparatively a lower resolution technique, but can be used to visualize global hemodynamics such as turbulence, kinetic energy, and fluid flow characteristics. Using these techniques, several research groups have reported shear stresses upstream,33 downstream72 and near AV region in vitro under static and pulsatile flow conditions through either prosthetic or native valves. Compared to the measurements on aorta near the valve region, there are relatively few studies that reported the shear stresses on leaflet surface. Computational models have been used to predict the average wall shear stresses across the entire leaflet surface40 and also at varying degree of stenosis,99 however the geometry of the aortic root used was simplified. Recently, Yap et al. measured shear stresses on both the fibrosa (physiological and altered hemodynamics) and ventricularis (physiological hemodynamics only) of the leaflet using two-component laser Doppler velocimetry. The peak magnitudes of the shear stresses were found to be about 70 dynes/cm2 on ventricular side,128 which occurs during systole and 23 dynes/cm2 on the fibrosa side127 during diastole. On the ventricularis side, systolic shear stresses were much higher in, unidirectional with little or no reverse flow, and similar to a half sine wave. This is contrary to the findings that shear stresses experienced by the fibrosa are oscillatory in nature.24,41 Further, the same study showed that the shear stresses experienced by the fibrosa are dictated by the hemodynamics. As alterations in the hemodynamics such as high heart rate and low cardiac output have been shown to affect several stages of cardiovascular disease continuum such as endothelial dysfunction, plaque rupture, and myocardial infarction, 29,64 the shear stresses under these altered conditions can be used in mechanobiology studies to gain more insights into AV pathobiology. Conversely, ventricularis shear stress resembled half sinusoid during systole and the direction of this shear reversed during late systole with a significant magnitude, which could be attributed to the Womersley effect.
Growing body of evidence suggest that the morphology of BAV can lead to adverse hemodynamics both near the valve24,90,126 as well as in the ascending aorta,10,104 resulting in turbulence and higher leaflet surface shear stresses. In these studies, shear stresses, turbulence, and unsteadiness were found to be much higher in the normal, mildly, and severely calcified BAV models relative to the normal TAV. The flow patterns associated with fused leaflets of BAV were different in terms of wall shear stress pulsatility and magnitude compared to that of its non-fused leaflet or TAV. The systolic shear stresses had a sinusoidal variation and the diastolic shear stress resembled a decaying exponential curve. Both systolic and diastolic shear stresses were higher on nonfused leaflet than the fused leaflet. These variations have been shown in Fig. 3. Although genetic factors have been implicated in the formation of BAVs,39,73 this altered microenvironment due to the BAV morphology itself could be the reason behind accelerated progression of the disease condition.102
Shear Stress: Mechanobiology
Shear stress regulates expression of proteins in ECs that control many opposing functions such as vasodilation and vasoconstriction, thrombo-resistance and thrombogenesis, and normal cell morphology and atherosclerosis. The morphological changes of ECs under shear were induced by the assembly and re-orientation of the stress fibers, accompanied by translocation and remodeling of cell substrate adhesion complexes, and transient formation of punctate cell–cell adherent junctions, that may signal the nucleus to elicit a specific cellular response. Several studies have characterized the role of shear stresses on vascular biology and indicated that low and oscillatory shear stress is atheroprone; whereas, high shear is atheroprotective. 18,53,56 It is also speculated that the reduced shear stresses on the non-coronary leaflet of the AV due to the lack of coronary flow is responsible for the increased susceptibility to calcification of that leaflet37,77 (Table 1).
TABLE 1.
Mechanobiological effects of shear stress.
| Markers | With increase in shear stress | References |
|---|---|---|
| ECM proteins | Collagen ↑, sGAG ↓, MMP-2, 9 ↑, TIMP-2 ↑, cathepsin-L ↓ on ventricularis | 81, 121 |
| Inflammation | ICAM-1 and VCAM-1 ↑ on fibrosa | 50, 100, 102 |
| Osteogenesis | BMP-2, 4 ↑, TGF-β ↑ on fibrosa, higher in BAVs | 50, 100, 102 |
| miRNA | LPS/IL-1 mediated inhibition of RXR function | 51 |
Although AV stenosis may be akin to atherosclerosis, there exist distinct differences in the phenotypes of both the cell types, warranting further studies on AV cells and its biology. Further, compared to vasculature, there have been very few studies on the effects of shear stress on AV. Two types of shear stress bioreactors—parallel plate system62 and cone and plate viscometer,101 have been used thus far to investigate the role of shear stress in AV biology and pathobiology, both in vitro and ex vivo. The parallel plate system is typically used to apply a uniform laminar shear stress while the cone and plate device can be used to impose uni-directional laminar shear or oscillatory and much more complex shear stress variations. Recently Sun et al.103 refined the design of the original cone and plate device to be able to expose either sides of AV to different shear stresses simultaneously, that closely mimics the in vivo scenario. Earlier studies using these bioreactors mainly focused on the role of shear stress in regulating valve matrix composition, remodeling, and phenotypic changes. ECs sense the shear stresses and transmit the mechanical and biochemical signals that regulate the phenotype and proliferation of ICs as well as control the ECM protein expressions.20 Using parallel plate flow chamber, earlier studies clearly demonstrated that changes in shear stress patterns (steady or pulsatile) are capable of affecting the ECM protease and protein turnover balance in short durations. 81,119,122,123
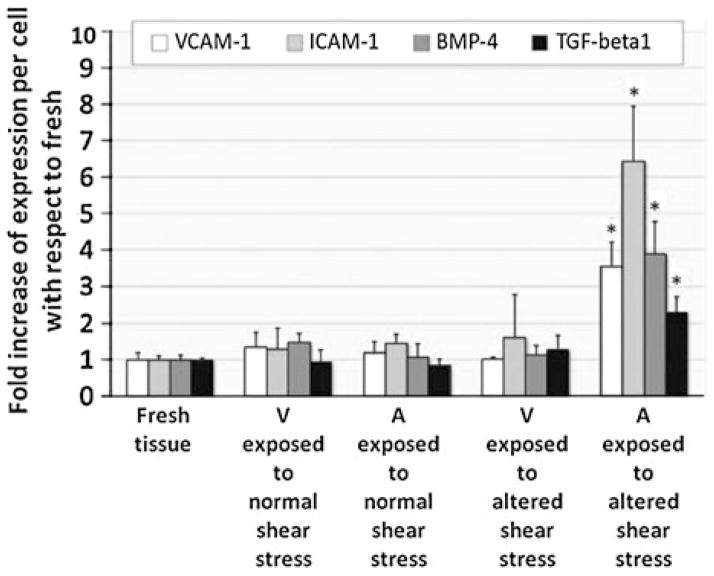
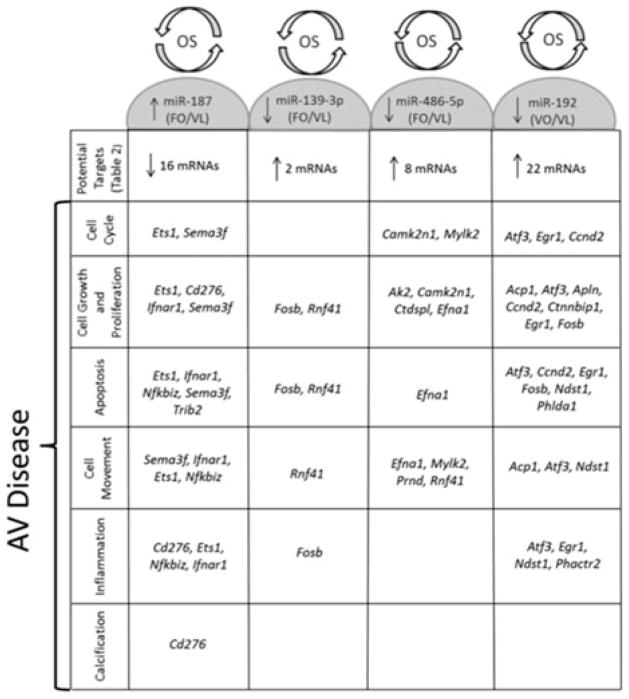
The magnitude and nature of shear stress experienced by either side of the valve differs greatly and perhaps responsible for the differential gene transcription profiles observed in healthy, porcine AVs.93 Gene profiling of porcine AV ECs showed that the expression of protective markers such as osteoprotegerin (key regulator of inflammation), parathyroid hormone (regulates Ca + 2 level and osteogenesis), c-type natriuretic peptide (cardiac and smooth muscle cell growth regulator) and chordin (BMP antagonist) that regulate the ectopic calcification were relatively low on fibrosa suggesting that fibrosa is more prone to disease initiation compared to ventricularis. This difference is more pronounced in the calcified valves, where the expression of the markers related to canonical BMP pathway such as phosphorylated SMAD-1/5/8 were found to be higher on the fibrosa endothelium corroborating the fact that fibrosa is more prone to disease initiation and progression.1,2 Side- and shear-specific expression on fibrosa side was also seen in response to altered shear stresses, where inflammation markers such as VCAM-1, ICAM-1, TGFβ-1, and BMP-2, four were significantly upregulated compared to ventricularis and were detected in the endothelial and sub-endothelial regions (Fig. 4).50,51,100 Expression of these markers on fibrosa endothelium in the presence of altered shear stresses indicates that the disease initiation could be side-specific and shear-dependent and could be potentially mediated by BMP-dependent pathway. Holliday et al.51 investigated the side-and shear-dependent miRNA and mRNA expression in human AV ECs and identified some of the mechanosensitive-miRNAs which were found to be important in remodeling, inflammation, cell proliferation, and migration elsewhere (Fig. 5). Using the Ingenuity pathway analysis, LPS/IL-1 mediated inhibition of RXR function (possibly a pro-atherogenic molecule78) and sulfur metabolism (known in myocardium protection91) were suggested as shear-regulated mechanisms that could possibly play a role in AV pathology, warranting further investigation.
FIGURE 4.
Semi-quantitative analysis of immunohistochemical staining of cell-adhesion molecules, BMP-4, and TGF-β1 after exposing porcine AV leaflets to normal and altered shear stress for 48 h in DMEM medium. A—aortic surface, V—ventricular surface, * p < 0.05 vs. fresh.100
FIGURE 5.
Predicted targets of shear responsive miRNAs in AV showing cellular functions important in aortic valve disease using Ingenuity Pathway Analysis and AmiGO. OS—oscillatory shear, LS—laminar shear, FO—fHAVECs under OS, VL—vHAVECs under LS, VO—vHAVECs under OS.51
In BAVs, surface shear stresses differ between the fused and non-fused leaflets. One of the studies that looked at the biological implications of the altered shear stresses due to BAV morphology was by Sun et al.102 Non-fused leaflet of BAV and TAV wall shear stresses maintained homeostasis whereas the shear stress of fused leaflet of BAV activated the fibrosa endothelium by up-regulating the expression of ECM proteases, inflammatory markers as well as osteogenesis related proteins in short time frame as less as 48 h. These findings demonstrate that altered shear stresses can significantly alter the valve cell phenotype, inducing inflammation, osteogenic differentiation in a side specific manner and thus eventually valve degeneration and calcification, apart from the underlying genetic factors.
Pressure
During the course of a single cardiac cycle, the pressure on the aortic cusps cyclically varies changing the stress and the strain in the leaflet tissue. In healthy individuals, the AV offers negligible resistance to the forward flow of blood (systole) and the pressure drop across the valve is under a few22 mmHg. In mild stenosis cases, the mean systolic pressure drop is around 20 mmHg during systole. In severe cases, the mean systolic pressure drop is higher than 40 mmHg.46 During diastole, a closed AV endures a transvalvular pressure gradient of approximately 80–100 mmHg acting normal to the leaflet area. With systemic hypertension, the transvalvular gradient can reach as high as 180–200 mmHg122,123 during diastole. This normal force is supported by the AV leaflets’ fibrosa layer and transmitted from the collagen fibers to the preferentially aligned ICs.112
Pressure: Mechanobiology
Hypertension plays an important role in the initial stages of aortic stenosis as it is found in one-third of patients with symptomatic aortic stenosis.3 Elevated pressures experienced during mild to severe hypertension increase the mechanical strain experienced by the leaflets and may play a key role in activation of several complex biological networks that induce endothelial dysfunction, altered remodeling, and inflammation.83 In the past, most of the studies were performed with chondrocytes, human umbilical vein cells,49 osteoblasts, vascular smooth muscle48 and AV ECs.114 Apoptosis, ECM composition and stiffness, cell proliferation signaling and adhesion are influenced by pressure and the modulation levels of these processes are mixed depending on the pressure level. However, it is unclear as to how hypertensive pressure is involved in early AV disease pathophysiology (Table 2).
TABLE 2.
Mechanobiological effects of pressure.
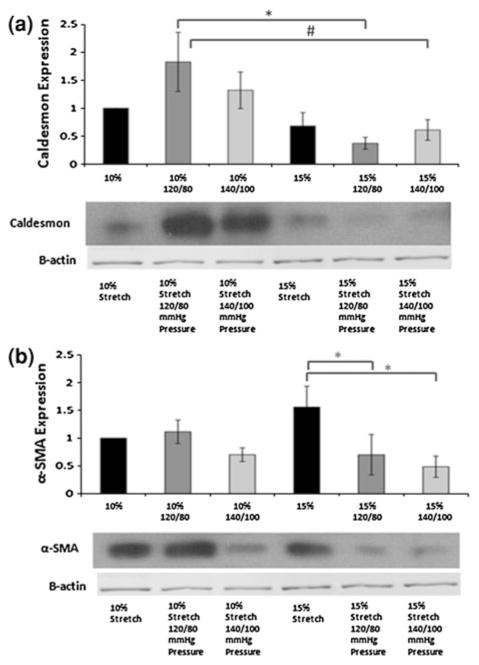
In vitro studies have progressed from static pressure experiments122 to investigating the effects of dynamic pressure on AV mechanobiology.67,95 One of the early studies that examined the effects of pressure on AV leaflets demonstrated that increase in pressure decreased the α-SMA expression.119 Isolated pathological stretch on the other hand increased the α-SMA expression.6 The opposing effects of stretch and pressure on α-SMA expression therefore suggest that the combined normal physiological hemodynamic forces may act to maintain the quiescent phenotype and prevent expression of activated contractile phenotype which was also shown using a novel ex vivo stretch and pressure bioreactor.106 Calponin and Caldesmon, associated with the actin bundling and polymerization, showed similar trends to that of α-SMA, indicating that these markers play a role in maintaining the valve IC phenotype as shown in the Fig. 6. Vimentin, a protective cytoskeletal component that provides stiffness, also decreased at combined pathological stretch and pressure, indicating that its protective function may be compromised under pathological conditions. Hypertensive pressure significantly reduced cathepsin L activity and down regulated MMP-2/9 expression moderately, indicating the pressure-dependent regulation of these proteases,81 and further, thickness of fibrosa and spongiosa increased relative to ventricularis in case of pathological stretch and pressure conditions, 106 owing to altered ECM remodeling. Warnock et al. investigated the immediate response of the elevated pressure on valve ICs, and found significant up regulation of VCAM-1 (inflammatory marker) and down regulation of osteopontin (endogenous downregulator of ectopic calcification). Further, gene array results indicated that approximately 50% of the genes including matrix metalloproteinase (MMP)-1, MMP-3, interleukin (IL)-6, and pentraxin-3 that are involved in tumor necrosis factor (TNF)-alpha network were impacted by the hypertensive conditions. In a separate study by the same group using AV ICs on collagen constructs, it was found that cyclic strain up regulated the expression of VCAM-1, suggesting that cyclic strain might be a more significant stimulus in evoking this response. These markers are known to be involved in inflammation, tissue remodeling, and calcification, and indicate that the pathological processes can be triggered due to hypertensive pressures. Thus these studies indicate that hypertensive pressures alter the inflammatory and remodeling response mediated by valvular ICs, and thus contribute to the AV disease progression.
FIGURE 6.
Immunoblot bands for (a) α-SMA and (b) Caldesmon with β-actin as loading controls. Expression of the various proteins normalized to β-actin and then the 10% stretch case (* p<0.05, # p<0.10).66
Although the above studies have investigated the mechanobiological effects of pressure in isolation, 66,67,95,117 it must be noted that pressure causes increased stretch on the leaflets through tensile-compressive and bending forces. Hence, future studies must investigate these two forces in combination, rather than in isolation.4,106
Leaflet Strain
The mechanics of valve tissue is complex with a highly non-linear stress–strain relationship. A majority of these stresses and strains are induced by a complex interplay between blood flow dynamics and vale cusp tissue. The changes in internal structure of the AV leaflet tissue with strain majorly affect the stress distribution in the leaflet. During the course of each cardiac cycle, the AV undergoes a combination of normal, bending, tensile, compressive, and shear stresses. Shear and normal stresses (induced by pressure) have been discussed in previous sections. Bending stress in AV is both tensile and compressive, with the inflow-side experiencing tensile stress while the outflow-side experiences compressive stress. Indeed, the curvature of the leaflet is integral in ensuring proper coaptation and long-term functionality and viability of the valve cusp.109–111 Ragaert et al.82 have characterized the flexural mechanical properties of porcine AV leaflets (coronary and non-coronary) using indentation and static rupture tests, and quantified the maximum extension before breaking (~3 mm), stiffness (~6 N/mm), and maximum load before rupture (~13 N). Layer specific flexural properties have been extensively studied as well.34
The AV leaflets exhibit anisotropic strain because the collagen in the circumferential direction provides greater tensile strength. Leaflet strain may be rapidly lost as the tissues become less extensible with increasing age. This is primarily because continued collagen fibrillogenesis over the lifetime of an individual increases the diameter of some of the constituent fibrils, requiring greater force to produce the same extension.108 This progressively reduces the AV strain with age progression, with drastic reduction occurring before the age of 25.26 Radial direction, which is mainly composed of elastic fibers, shows higher strain. Missirlis and Chong,69 Brewer et al.,19 Thubrikar et al.112 reported in vivo AV leaflet strains to be approximately 10 and 40% in the circumferential and radial directions, respectively. This data is comparable across different species. Yap et al.125 measured the strains on porcine AV cusps under different hypertensive pressure conditions in an ex vivo flow loop system by tracking markers on leaflet surface using stereo photogrammetry. In terms of strain profile, the diastolic strain of the leaflets followed the transvalvular pressure gradient and the systolic strain followed the flow curve. These dynamic strain characteristics were used as the reference value for various physiological and pathophysiological ex vivo studies6 from the same investigators, as explained in the next section. Efforts are underway by Kai et al.57 to measure strain in surgically stitched BAV models. Sacks and co-workers98 have characterized the biaxial mechanical behavior of fibrosa and ventricularis layers separately and found out that these layers exhibit different nonlinear anisotropic (quasi-elastic) behavior. The fibrosa behaved similar to the intact native tissue, but less compliant, under biaxial loading. They also inferred that the ventricularis dominates the mechanical response of the intact tissue in the radial direction at higher stress levels. This biomechanics study sheds light on the layer specific properties of the AV leaflets, which is very critical in developing constitutive models of the AV for numerical studies.
Numerical models are gaining prominence in recent times to characterize the stress distribution on native AV leaflets and malformed (bicuspid) leaflets.31,42,54,118 The maximum in-plane stresses in these BAVs increased by as high as 161% when the material properties were changed by ±25% as compared to normal TAV stresses. Weinberg et al.118 developed a multiscale finite element model for comparing BAV and normal TAV and they observed that cellular deformations between these two are not significantly different. It was implied that the calcification found in BAVs may be triggered by factors other than simple geometric parameters, suggesting that calcific aortic stenosis in BAVs may be caused by genetic factors.
The knowledge gained from strain quantification studies has been translated to cell level in vitro studies to investigate differentiation of ICs to osteoblasts or myofibrolasts.32 Cell level effects are investigated by isolating the cells36 and subjecting them to strain or by using imaging techniques to investigate the cell behavior while the entire AV leaflet tissue is stretched.66 Even though different strain levels have been used in various in vitro and ex vivo studies to elucidate cell level effects, it has been established that non-physiological strain leads to pathological conditions in AV leaflets in terms of inflammation, remodeling, and calcification.
Strain: Mechanobiology
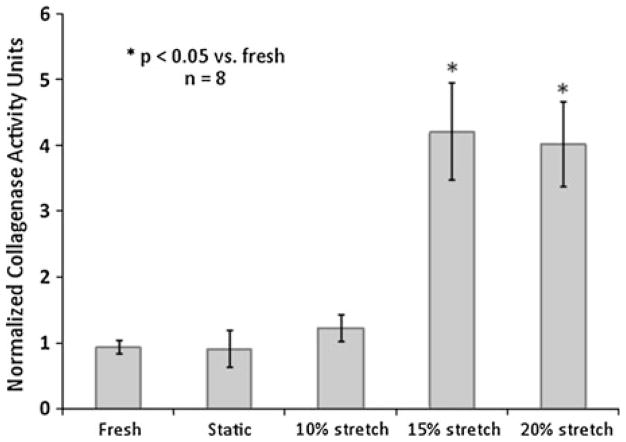
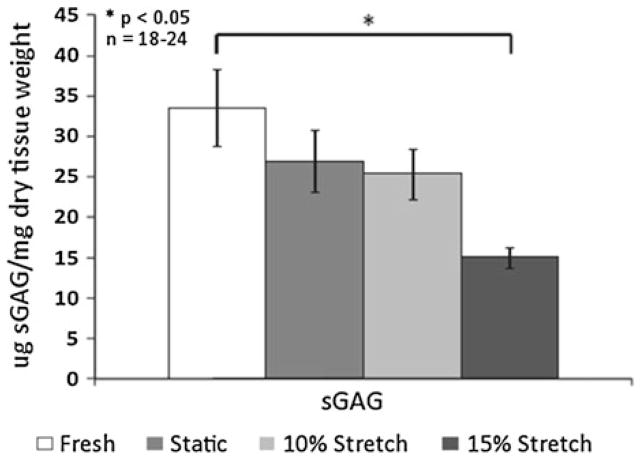
Ex vivo and in vitro stretch simulation studies on AV leaflets have been broadly of two kinds: (1) Effect of cyclic strain on native leaflets in terms of remodeling, inflammation and calcification markers and (2) cyclic strain effects on isolated AV cells loaded in scaffolds, and studying them from tissue engineering perspective. Native porcine AV leaflets when subjected to varying stretch magnitudes, responded in a biphasic manner. The responses were either biphasic in stretch magnitude in terms of metalloproteinase (MMP) activity and tissue inhibitor of metalloproteinase (TIMP) expression, or reached a plateau, with no significant difference between 15 and 20% stretch groups. The remodeling potential, quantified in terms of MMP/TIMP ratio, it was observed that it peaked at 15% stretch group in comparison with fresh and 10% stretch groups. The collagen content of the AV leaflets, stretched to pathological levels for 48 h, was increased when compared to fresh and static control leaflets (Fig. 7), while sGAG content was decreased in stretched leaflets compared to fresh leaflets (Fig. 8).7 This increase in collagen content suggests that the leaflets adapt to altered mechanical loading by either increasing synthesis, or decreasing degradation of collagen. The reduced levels in sGAG content are attributed to the lack of compressive stresses in this study (Table 3).
FIGURE 7.
Collagenase activity progressively increased in porcine AV leaflets with increasing cyclic stretch. Activity was significantly higher at 15 and 20% cyclic stretch compared to fresh controls, static controls and 10% stretch. There was no significant difference in collagenase activity between fresh, static and 10% stretch groups (n refers to number of experimental samples).7
FIGURE 8.
Changes in the content of sulfated glycosaminoglycan of porcine AV leaflets subjected to the following treatments: fresh control, static incubation, 10% cyclic stretch, 15% cyclic stretch after 48 h. sGAG significantly decreased with increase in cyclic stretch levels (n refers to number of experimental samples).6
TABLE 3.
Mechanobiological effects of strain.
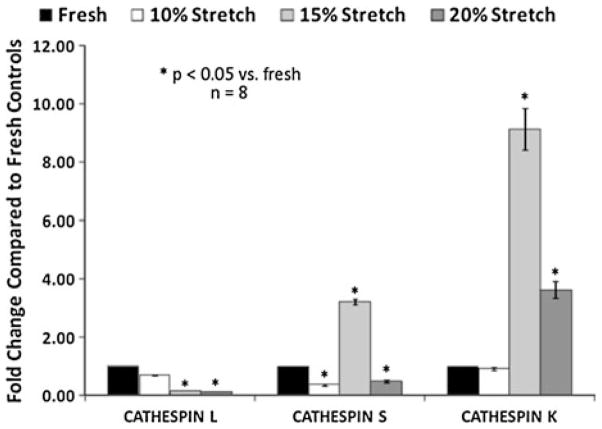
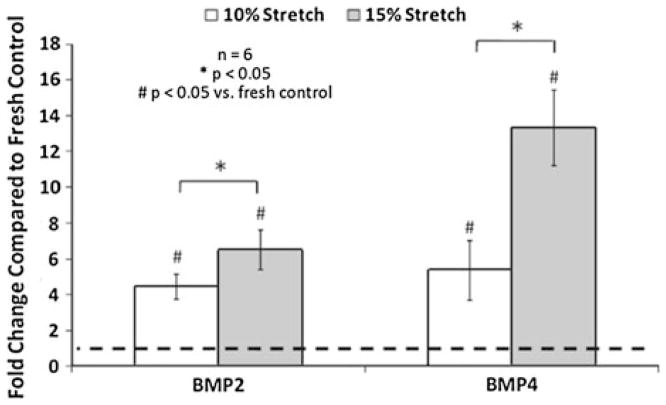
In terms of ECM remodeling enzymes, it was observed that cathepsin L expression was reduced by elevated cyclic stretch, while cathepsin S and K expression was increased (Fig. 9). In a recent study by Helske et al.47 it was revealed that cathepsin S, K, and V expression and activity were the cathepsin sub-types that were upregulated in stenotic AVs. Elevated cyclic stretch also increased the collagenase and gelatinase activity. Further, pathological level of stretch has been shown to induce AV calcification in a BMP-dependent manner (Fig. 10). BMPs have been established as markers in early calcification progression in cultured vascular and valvular cells.27,68,75,96,97 It was also observed that the BMP antagonist noggin was able to downregulate the stretch induced osteogenic and calcification events (ALP activity, Runx2 expression, and calcium levels in the leaflets). It has been indicated that an atherogenic environment results in activation of the valve ICs leading to initial expression of BMP-2 and BMP-4 leading to expression of the downstream transcription factor Runx2.8 The fibrosis effect of neurotransmitter serotonin (5-hydroxytryptamine, 5HT) on heart valves has been well documented.38,45,55 Peña-Silva et al.80 reported that elevated serotonin levels can result in increased oxidative stress in the valve cusp potentially leading to stenotic valve disease. 5-HT-induced valve stiffening may occur throughout the valve cusp resulting in reduced valve curvature and ability of the valve to coapt effectively.
FIGURE 9.
Semi-quantification of cathepsin immunohistochemical staining of porcine AV leaflets subjected to cyclic stretch. Cathepsin L appears to be dominant one in the fresh valve, while 15% stretch significantly increased expression of cathepsins S and K. Cathepsin S and K expression was significantly (p <0.05) lower at 20% stretch compared to 15% stretch (n refers to number of experimental samples).7
FIGURE 10.
BMP-2 and BMP-4 expression in porcine AV leaflets was significantly (p<0.05) increased by 15% stretch compared with 10% stretch (n refers to number of experimental samples).38
Cell level cyclic strain studies have been garnering lot of interest from tissue engineering and regenerative medicine community. These cell level models have progressed from 2D strain application61,105 to 3D cultures.43,44 Valve fibroblasts in these 3D models have shown cell differentiation and matrix synthesis. To understand the activation of fibroblasts, cell, and tissue based models have been developed.79 Different substrates (fibrin, collagen based) for tissue engineered heart valves were investigated17 and their mechanical behavior, fiber orientation, and resultant ECM of the construct in response to mechanical conditioning have been reported.28,87 Further an in vitro model was also developed to quantify the stress generation, compaction, and retraction of tissue-engineered constructs seeded with human vascular-derived cells.113 On the other hand, it was found that a synergistic combination of biological (BMP4) and mechanical forces are required to induce the same level of SMA, runx2, and OPN expressions in human valvular ICs populated scaffolds as that of isolated cells. Butcher et al. demonstrated that cyclic strain induces time dependent (48 and 96 h) valvular IC orientation and collagen alignment, which in turn influenced alpha SMA (gene ACTA2) levels. Recent gene expression studies by Warnock and co-workers95 indicated that 15% cyclic strain reduces expression of pro-inflammatory genes by ICs loaded in collagen constructs. Any other strain value induced inflammatory response by the valvular ICs as measured by inflammatory marker VCAM-1 and mechanosensitive gene OPN.95
EFFECTS OF COMBINED MECHANICAL STIMULI
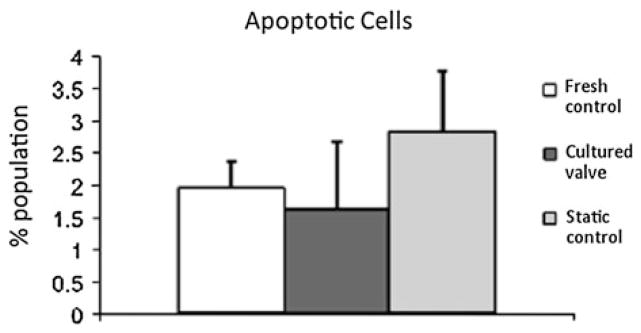
Mechanobiology studies of AV in the presence of each of the isolated mechanical stimuli (shear, stretch or pressure) while keeping the others constant aid in understanding how each mechanical stimulus plays a role in regulating AV biology and pathophysiology. However, the knowledge gained from the studies where AV is subjected to the combined mechanical stimuli, i.e., mimicking its native in vivo state is as critical and helps us understand the interplay of each stimuli in regulating the AV health. There have been recent initiatives to study the mechanobiological effects of combined stimuli.4,11,12,58,106,116 One such attempt was made by Konduri et al.58 using an ex vivo pulsatile organ culture system, subjecting a native AV along with its root to its physiological hemodynamics. ECM composition (sGAG, elastin, and collagen content), cell viability, proliferation as well as the α-SMA content was preserved in cultured leaflets. However, under static conditions, a decrease in sGAG, elastin, and α-SMA content with significant cell death (Fig. 11) compared to fresh and cultured AVs was seen indicating that mechanical stimuli are indeed required to preserve native composition and function of the AV leaflets. Warnock and co-workers95 studied the inflammatory response of AV ICs when subjected to cyclic strain and pressure together, and found the synergistic response to be different than the individual response in terms of inflammatory markers such as VCAM and OPN mechanosensitive genes. Another group developed a flexure, stretch, and flow bioreactor where each of these stimuli can be independently controlled and can be used for studying the mechanobiological responses to physiological and pathological stimuli.35 Apart from understanding the effect of complex mechanical stimuli on AV biology, bioreactors like these could potentially be used to mechanically condition the tissue-engineered AVs to develop the natural ECM92 and also assess the resultant deformations experienced by the valves using the real time non-invasive measurement techniques.59,60
FIGURE 11.
Apoptotic cell population in porcine AV leaflets showed no significant difference between the fresh and leaflets cultured in organ culture system, while there was significant increase in static controls compared to cultured leaflets.58
DISCUSSION
Calcification of the AVs was once thought to be a degenerative process and passive deposition of hydroxyapatite crystals. It has been established that AV disease progression is a very dynamic and complex process, involving interplay of altered mechanical environment and molecular mechanisms. New approaches and models have helped to characterize the mechanical environment of the AV better. This review highlighted recent progress in understanding the complex mechanical environment of AV and its mechanobiological implications that play major role in maintaining AV health. A three-pronged approach of in vitro (cell level), ex vivo (tissue level), and in vivo (animal models and patient trials) have been adapted by several groups to investigate the molecular pathways and key genes involved in AV disease at multiple scales, right from the gene level to molecular level studies. These investigations clearly indicate that mechanical stimuli, even when slightly altered, can alone trigger AV disease progression apart from the genetics and other biochemical factors. However, most of these mechanobiology studies employed simplified stimuli. Future studies should focus on investigating the mechanobiological implications of the complex physiological and realistic mechanical environment in order to gain a comprehensive understanding of the cellular and pathophysiologic processes involved in AV inflammation and calcification. This knowledge will also aid in development of more competitive tissue engineered valves as well as in devising potential therapeutics and early diagnostics against the AV disease.
Acknowledgments
Authors wish to thank the Cardiovascular Fluid Mechanics Lab, Dr. Hanjoong Jo’s lab, Dr. Robert M. Nerem’s Lab members, and all other researchers for their contributions to thework presented in this paper. Authors also would like to thank all the relevant funding sources for the Cardiovascular Fluid Mechanics lab at Georgia Tech: American Heart Association under the Post-doctoral Research Awards (0625620B, 10POST3050054) and Predoctoral Research Award (09PRE2060605), National Science Foundation through the Engineering Research Center program at the Georgia Tech under the award EEC 9731643, National Heart, Lung and Blood Institute grant number HL-07262, the Wallace H. Coulter Distinguished Faculty Chair funds, Petit Undergraduate Research Scholars Program, a gift from Tom and Shirley Gurley, and all other sources. Finally, the authors acknowledge the kindness of Mr. Holifield for donating porcine heart valves, and thank the machine shop crew at School of Chemical and Biomolecular Engineering, Georgia Tech for machining the ex vivo bioreactors for some of the studies presented in this paper.
References
- 1.Ankeny RF. The Role of Bone Morphogenic Proteins in Human Aortic Valvular Endothelial Cells. Atlanta, GA: Georgia Institute of Technology; 2010. [Google Scholar]
- 2.Ankeny RF, V, Thourani H, Weiss D, Vega JD, Taylor WR, Nerem RM, et al. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves—association with low BMP antagonists and SMAD6. PLoS ONE. 2011;6(6):e20969. doi: 10.1371/journal.pone.0020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonini-Canterin F, Huang G, Cervesato E, Faggiano P, Pavan D, Piazza R, et al. Symptomatic aortic stenosis: does systemic hypertension play an additional role? Hypertension. 2003;41(6):1268–1272. doi: 10.1161/01.HYP.0000070029.30058.59. [DOI] [PubMed] [Google Scholar]
- 4.Arjunon S, Rathan S, Yoganathan A. Design of a novel ex vivo bioreactor to investigate the effect of pressure induced stretch on aortic valve biology. QScience Proceedings: Heart Valve Biology and Tissue Engineering; Greece. 2012. p. 72. [Google Scholar]
- 5.Balachandran K, Bakay MA, Connolly JM, Zhang X, Yoganathan AP, Levy RJ. Aortic valve cyclic stretch causes increased remodeling activity and enhanced serotonin receptor responsiveness. Ann Thorac Surg. 2011;92(1):147–153. doi: 10.1016/j.athoracsur.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran K, Konduri S, Sucosky P, Jo H, Yoganathan A. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann Biomed Eng. 2006;34(11):1655–1665. doi: 10.1007/s10439-006-9167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296(3):H756–H764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PubMed] [Google Scholar]
- 8.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am J Pathol. 2010;177(1):49–57. doi: 10.2353/ajpath.2010.090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflamm. 2011;2011:263870. doi: 10.4061/2011/263870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker AJ, Markl M, Bürk J, Lorenz R, Bock J, Bauer S, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5(4):457–466. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 11.Barzilla JE, Acevedo FE, Grande-Allen KJ. Organ culture as a tool to identify early mechanisms of serotonergic valve disease. J Heart Valve Dis. 2010;19(5):626–635. [PubMed] [Google Scholar]
- 12.Barzilla JE, McKenney AS, Cowan AE, Durst CA, Grande-Allen KJ. Design and validation of a novel splashing bioreactor system for use in mitral valve organ culture. Ann Biomed Eng. 2010;38(11):3280–3294. doi: 10.1007/s10439-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellhouse B, Bellhouse F. Fluid mechanics of model normal and stenosed aortic valves. Circ Res. 1969;25(6):693–704. [PubMed] [Google Scholar]
- 14.Bellhouse BJ, Talbot L. The fluid mechanics of the aortic valve. J Fluid Mech Digit Arch. 1969;35(4):721–735. [Google Scholar]
- 15.Bischoff J, Aikawa E. Progenitor cells confer plasticity to cardiac valve endothelium. J Cardiovasc Transl Res. 2011;4(6):710–719. doi: 10.1007/s12265-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation. 2006;114(5):E84–E231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 17.Bouten CVC, Dankers PYW, Driessen-Mol A, Pedron S, Brizard AMA, Baaijens FPT. Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev. 2011;63(4–5):221–241. doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Braddock M, Schwachtgen J-L, Houston P, Dickson MC, Lee MJ, Campbell CJ. Fluid shear stress modulation of gene expression in endothelial cells. Physiology. 1998;13(5):241–246. doi: 10.1152/physiologyonline.1998.13.5.241. [DOI] [PubMed] [Google Scholar]
- 19.Brewer RJ, Mentzer RM, Jr, Deck JD, Ritter RC, Trefil JS, Nolan SP. An in vivo study of the dimensional changes of the aortic valve leaflets during the cardiac cycle. J Thorac Cardiovasc Surg. 1977;74(4):645–650. [PubMed] [Google Scholar]
- 20.Butcher JT, Nerem RM. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 2006;12(4):905–915. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 21.Butcher JT, Nerem RM. Valvular endothelial cells and the mechanoregulation of valvular pathology. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1445–1457. doi: 10.1098/rstb.2007.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol. 2004;24(8):1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 23.Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, et al. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol. 2003;41(10):1753–1758. doi: 10.1016/s0735-1097(03)00295-x. [DOI] [PubMed] [Google Scholar]
- 24.Chandra S, Rajamannan N, Sucosky P. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech Model Mechanobiol. 2012;11(7):1085–1096. doi: 10.1007/s10237-012-0375-x. [DOI] [PubMed] [Google Scholar]
- 25.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie GW, Barratt-Boyes BG. Age-dependent changes in the radial stretch of human aortic valve leaflets determined by biaxial testing. Ann Thorac Surg. 1995;60(2 Suppl):S156–S158. doi: 10.1016/0003-4975(95)00219-b. discussion S159. [DOI] [PubMed] [Google Scholar]
- 27.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, et al. Transforming growth factor-beta1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg. 2007;83(3):946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Cox MAJ, Kortsmit J, Driessen N, Bouten CVC, Baaijens FPT. Tissue-engineered heart valves develop native-like collagen fiber architecture. Tissue Eng Part A. 2010;16(5):1527–1537. doi: 10.1089/ten.TEA.2009.0263. [DOI] [PubMed] [Google Scholar]
- 29.Custodis F, Schirmer SH, Baumhakel M, Heusch G, Bohm M, Laufs U. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol. 2010;56(24):1973–1983. doi: 10.1016/j.jacc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Davies PF, Passerini AG, Simmons CA. Aortic valve: turning over a new leaf(let) in endothelial phenotypic heterogeneity. Arterioscler Thromb Vasc Biol. 2004;24(8):1331–1333. doi: 10.1161/01.ATV.0000130659.89433.c1. [DOI] [PubMed] [Google Scholar]
- 31.De Hart J, Baaijens FP, Peters GW, Schreurs PJ. A computational fluid–structure interaction analysis of a fiber-reinforced stentless aortic valve. J Biomech. 2003;36(5):699–712. doi: 10.1016/s0021-9290(02)00448-7. [DOI] [PubMed] [Google Scholar]
- 32.Du Y, Yip H. Effects of bone morphogenetic protein 2 on Id expression and neuroblastoma cell differentiation. Differentiation. 2010;79(2):84–92. doi: 10.1016/j.diff.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Einav S, Stolero D, Avidor JM, Elad D, Talbot L. Wall shear stress distribution along the cusp of a tri-leaflet prosthetic valve. J Biomed Eng. 1990;12(1):13–18. doi: 10.1016/0141-5425(90)90108-y. [DOI] [PubMed] [Google Scholar]
- 34.Engelmayr Jr, Hildebrand GCDK, Sutherland FW, Mayer JE, Jr, Sacks MS. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials. 2003;24(14):2523–2532. doi: 10.1016/s0142-9612(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 35.Engelmayr Jr, Sacks GCMS. Prediction of extracellular matrix stiffness in engineered heart valve tissues based on nonwoven scaffolds. Biomech Model Mechanobiol. 2008;7(4):309–321. doi: 10.1007/s10237-007-0102-1. [DOI] [PubMed] [Google Scholar]
- 36.Fisher CI, Chen J, Merryman WD. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol. 2013;12(1): 5–17. doi: 10.1007/s10237-012-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111(24):3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 38.Frishman WH, Huberfeld S, Okin S, Wang YH, Kumar A, Shareef B. Serotonin and serotonin antagonism in cardiovascular and non-cardiovascular disease. J Clin Pharmacol. 1995;35(6):541–572. doi: 10.1002/j.1552-4604.1995.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 39.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 40.Ge L, Leo HL, Sotiropoulos F, Yoganathan AP. Flow in a mechanical bileaflet heart valve at laminar and near-peak systole flow rates: CFD simulations and experiments. J Biomech Eng. 2005;127(5):782–797. doi: 10.1115/1.1993665. [DOI] [PubMed] [Google Scholar]
- 41.Ge L, Sotiropoulos F. Direction and magnitude of blood flow shear stresses on the leaflets of aortic valves: is there a link with valve calcification? J Biomech Eng. 2010;132(1):014505. doi: 10.1115/1.4000162. [DOI] [PubMed] [Google Scholar]
- 42.Gnyaneshwar R, Kumar RK, Balakrishnan KR. Dynamic analysis of the aortic valve using a finite element model. Ann Thorac Surg. 2002;73(4):1122–1129. doi: 10.1016/s0003-4975(01)03588-3. [DOI] [PubMed] [Google Scholar]
- 43.Gould RA, Chin K, Santisakultarm TP, Dropkin A, Richards JM, Schaffer CB, et al. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 2012;8(5):1710–1719. doi: 10.1016/j.actbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res. 2006;72(3):375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson BI, Hauso O, Drozdov I, Kidd M, Modlin IM. Carcinoid heart disease. Int J Cardiol. 2008;129(3):318–324. doi: 10.1016/j.ijcard.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 46.Haerten K, Dohn G, Dohn V, Seipel L, Loogen F. Natural history of patients with severe aortic valve disease under medical therapy (author’s transl) Z Kardiol. 1980;69(11):757–762. [PubMed] [Google Scholar]
- 47.Helske S, Syvaranta S, Lindstedt KA, Lappalainen J, Oorni K, Mayranpaa MI, et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol. 2006;26(8):1791–1798. doi: 10.1161/01.ATV.0000228824.01604.63. [DOI] [PubMed] [Google Scholar]
- 48.Hishikawa K, Nakaki T, Marumo T, Hayashi M, Suzuki H, Kato R, et al. Pressure promotes DNA synthesis in rat cultured vascular smooth muscle cells. J Clin Investig. 1994;93(5):1975–1980. doi: 10.1172/JCI117189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hishikawa K, Nakaki T, Marumo T, Suzuki H, Kato R, Saruta T. Pressure enhances endothelin-1 release from cultured human endothelial cells. Hypertension. 1995;25(3):449–452. doi: 10.1161/01.hyp.25.3.449. [DOI] [PubMed] [Google Scholar]
- 50.Hoehn D, Sun L, Sucosky P. Role of pathologic shear stress alterations in aortic valve endothelial activation. Cardiovasc Eng Technol. 2010;1(2):165–178. [Google Scholar]
- 51.Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301(3):H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hope MD, Hope TA, Meadows AK, Ordovas KG, Urbania TH, Alley MT, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255(1):53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 53.Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells: role of β1 integrins and tyrosine kinases. Circ Res. 1996;79(2):310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 54.Jermihov PN, Jia L, Sacks MS, Gorman RC, Gorman JH, 3rd, Chandran KB. Effect of geometry on the leaflet stresses in simulated models of congenital bicuspid aortic valves. Cardiovasc Eng Technol. 2011;2(1):48–56. doi: 10.1007/s13239-011-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, et al. Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am J Pathol. 2002;161(6):2111–2121. doi: 10.1016/s0002-9440(10)64489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonathan Butcher CA S, Warnock JN. Mechanobiology of the aortic heart valve. J Heart Valve Dis. 2008;17(1):62–73. [PubMed] [Google Scholar]
- 57.Kai Szeto PP, Nigam V, Lasheras J. Deformation of congenital bicuspid aortic valves in systole. DFD12 Meeting of The American Physical Society; San Diego, USA. 2012. [Google Scholar]
- 58.Konduri S, Xing Y, Warnock JN, He Z, Yoganathan AP. Normal physiological conditions maintain the biological characteristics of porcine aortic heart valves: an ex vivo organ culture study. Ann Biomed Eng. 2005;33(9):1158–1166. doi: 10.1007/s10439-005-5506-4. [DOI] [PubMed] [Google Scholar]
- 59.Kortsmit J, Driessen NJB, Rutten MCM, Baaijens FPT. Real time, non-invasive assessment of leaflet deformation in heart valve tissue engineering. Ann Biomed Eng. 2009;37(3):532–541. doi: 10.1007/s10439-008-9621-x. [DOI] [PubMed] [Google Scholar]
- 60.Kortsmit J, Driessen NJB, Rutten MCM, Baaijens FPT. Nondestructive and noninvasive assessment of mechanical properties in heart valve tissue engineering. Tissue Eng Part A. 2009;15(4):797–806. doi: 10.1089/ten.tea.2008.0197. [DOI] [PubMed] [Google Scholar]
- 61.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, et al. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. 2006;71(3):548–556. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Levesque MJ, Nerem RM. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107(4):341–347. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 63.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):E46–E215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 64.Maio R, Miceli S, Sciacqua A, Leone G, Bruni R, Naccarato P, et al. Heart rate affects endothelial function in essential hypertension. Intern Emerg Med. 2011 doi: 10.1007/s11739-011-0618-3. [DOI] [PubMed] [Google Scholar]
- 65.Mazzag B, Barakat A. The effect of noisy flow on endothelial cell mechanotransduction: a computational study. Ann Biomed Eng. 2011;39(2):911–921. doi: 10.1007/s10439-010-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Metzler SA, Digesu CS, Howard JI, Filip To SD, Warnock JN. Live en face imaging of aortic valve leaflets under mechanical stress. Biomech Model Mechanobiol. 2012;11(3–4):355–361. doi: 10.1007/s10237-011-0315-1. [DOI] [PubMed] [Google Scholar]
- 67.Metzler SA, Pregonero CA, Butcher JT, Burgess SC, Warnock JN. Cyclic strain regulates proinflammatory protein expression in porcine aortic valve endothelial cells. J Heart Valve Dis. 2008;17(5):571–577. discussion 578. [PubMed] [Google Scholar]
- 68.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, et al. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113(24):2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 69.Missirlis YF, Chong M. Aortic valve mechanics—part I: material properties of natural porcine aortic valves. J Bioeng. 1978;2(3–4):287–300. [PubMed] [Google Scholar]
- 70.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94(11):1396–1402. doi: 10.1016/j.amjcard.2004.08.013. (A6) [DOI] [PubMed] [Google Scholar]
- 71.Monzack EL, Gu X, Masters KS. Efficacy of simvastatin treatment of valvular interstitial cells varies with the extracellular environment. Arterioscler Thromb Vasc Biol. 2009;29(2):246–253. doi: 10.1161/ATVBAHA.108.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nandy S, Tarbell MJ. Flush Mounted Hot Film Anemometer Measurement of Wall Shear Stress Distal to a Tri-Leaflet Valve for Newtonian and Non-Newtonian Blood Analog Fluids. Amsterdam: PAYS-BAS, IOS Press; 1987. [DOI] [PubMed] [Google Scholar]
- 73.Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009;47(6):828–834. doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishimura RA. Cardiology patient pages. Aortic valve disease. Circulation. 2002;106(7):770–772. doi: 10.1161/01.cir.0000027621.26167.5e. [DOI] [PubMed] [Google Scholar]
- 75.Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation. 2006;114(1 Suppl):I566–I572. doi: 10.1161/CIRCULATIONAHA.105.001214. [DOI] [PubMed] [Google Scholar]
- 76.Otto CM. Calcification of bicuspid aortic valves. Heart. 2002;88(4):321–322. doi: 10.1136/heart.88.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341(3):142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 78.Padovani AMS, Molina MF, Mann KK. Inhibition of liver X receptor/retinoid X receptor-mediated transcription contributes to the proatherogenic effects of arsenic in macrophages in vitro. Arterioscler Thromb Vasc Biol. 2010;30(6):1228–1236. doi: 10.1161/ATVBAHA.110.205500. [DOI] [PubMed] [Google Scholar]
- 79.Paolo Poggio RS, Grau JB, Branchetti E, Lai E, Gorman RC, Gorman JH, III, Bavaria JE, Sacks MS, Ferrari G. Biomechanical Activation of Human Valvular Interstitial Cells from Early Stage of CAVD QScience Proceedings. 2012 (Heart Valve Biology and Tissue Engineering) [Google Scholar]
- 80.Pena-Silva RA, Miller JD, Chu Y, Heistad DD. Serotonin produces monoamine oxidase-dependent oxidative stress in human heart valves. Am J Physiol Heart Circ Physiol. 2009;297(4):H1354–H1360. doi: 10.1152/ajpheart.00570.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Platt MO, Xing Y, Jo H, Yoganathan AP. Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J Heart Valve Dis. 2006;15(5):622–629. [PubMed] [Google Scholar]
- 82.Ragaert K, De Somer F, Somers P, De Baere I, Cardon L, Degrieck J. Flexural mechanical properties of porcine aortic heart valve leaflets. J Mech Behav Biomed Mater. 2012;13:78–84. doi: 10.1016/j.jmbbm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 83.Rajamannan NM, Bonow RO, Rahimtoola SH. Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med. 2007;4(5):254–262. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 84.Reul H, Talukdar N. Heart valve mechanics. In: Hwang N, Gross D, Patel D, editors. Quantitative Cardiovascular Studies Clinical and Research Applications of Engineering Principles. Baltimore, MD: University Park Press; 1979. [Google Scholar]
- 85.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111(7):920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 86.Robicsek F, Thubrikar MJ, Cook JW, Fowler B. The congenitally bicuspid aortic valve: how does it function? Why does it fail? Ann Thorac Surg. 2004;77(1):177–185. doi: 10.1016/s0003-4975(03)01249-9. [DOI] [PubMed] [Google Scholar]
- 87.Robinson PS, Tranquillo RT. Planar biaxial behavior of fibrin-based tissue-engineered heart valve leaflets. Tissue Eng Part A. 2009;15(10):2763–2772. doi: 10.1089/ten.tea.2008.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossvoll O, Samstad S, Torp HG, Linker DT, Skjaerpe T, Angelsen BA, et al. The velocity distribution in the aortic anulus in normal subjects: a quantitative analysis of two-dimensional Doppler flow maps. J Am Soc Echocardiogr. 1991;4(4):367–378. doi: 10.1016/s0894-7317(14)80447-1. [DOI] [PubMed] [Google Scholar]
- 89.Sacks MS, David Merryman W, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42(12):1804–1824. doi: 10.1016/j.jbiomech.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saikrishnan N, Yap CH, Milligan NC, Vasilyev NV, Yoganathan AP. In vitro characterization of bicuspid aortic valve hemodynamics using particle image velocimetry. Ann Biomed Eng. 2012;40(8):1760–1775. doi: 10.1007/s10439-012-0527-2. [DOI] [PubMed] [Google Scholar]
- 91.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, et al. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G-dependent generation of hydrogen sulfide. Circulation. 2009;120(11 suppl 1):S31–S36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schenke-Layland K, Riemann I, Opitz F, König K, Halbhuber KJ, Stock UA. Comparative study of cellular and extracellular matrix composition of native and tissue engineered heart valves. Matrix Biol. 2004;23(2):113–125. doi: 10.1016/j.matbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96(7):792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simmons CA, Zilberberg J, Davies PF. A rapid, reliable method to isolate high quality endothelial RNA from small spatially-defined locations. Ann Biomed Eng. 2004;32(10):1453–1459. doi: 10.1114/b:abme.0000042360.57960.2b. [DOI] [PubMed] [Google Scholar]
- 95.Smith KE, Metzler SA, Warnock JN. Cyclic strain inhibits acute pro-inflammatory gene expression in aortic valve interstitial cells. Biomech Model Mechanobiol. 2010;9(1):117–125. doi: 10.1007/s10237-009-0165-2. [DOI] [PubMed] [Google Scholar]
- 96.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95(8):773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 97.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278(33):31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 98.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129(5):757–766. doi: 10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 99.Stevenson DM, Yoganathan AP, Williams FP. Numerical simulation of steady turbulent flow through trileaflet aortic heart valves—II. Results on five models. J Biomech. 1985;18(12):909–926. doi: 10.1016/0021-9290(85)90035-1. [DOI] [PubMed] [Google Scholar]
- 100.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-{beta}1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29(2):254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sucosky P, Padala M, Elhammali A, Balachandran K, Jo H, Yoganathan AP. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J Biomech Eng. 2008;130(3):035001. doi: 10.1115/1.2907753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun L, Chandra S, Sucosky P. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS ONE. 2012;7(10):e48843. doi: 10.1371/journal.pone.0048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun L, Rajamannan N, Sucosky P. Design and validation of a novel bioreactor to subject aortic valve leaflets to side-specific shear stress. Ann Biomed Eng. 2011;39(8):2174–2185. doi: 10.1007/s10439-011-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve. Circulation. 2009;119(6):880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 105.Tan W, Scott D, Belchenko D, Qi HJ, Xiao L. Development and evaluation of microdevices for studying anisotropic biaxial cyclic stretch on cells. Biomed Microdevices. 2008;10(6):869–882. doi: 10.1007/s10544-008-9201-8. [DOI] [PubMed] [Google Scholar]
- 106.Thayer P, Balachandran K, Rathan S, Yap C, Arjunon S, Jo H, et al. The effects of combined cyclic stretch and pressure on the aortic valve interstitial cell phenotype. Ann Biomed Eng. 2011;39(6):1654–1667. doi: 10.1007/s10439-011-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc Pathol. 2006;15(5):256–263. doi: 10.1016/j.carpath.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 108.Thubrikar M. The Aortic Valve. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- 109.Thubrikar M, Bosher LP, Nolan SP. The mechanism of opening of the aortic valve. J Thorac Cardiovasc Surg. 1979;77(6):863–870. [PubMed] [Google Scholar]
- 110.Thubrikar MJ, Heckman JL, Nolan SP. High speed cine-radiographic study of aortic valve leaflet motion. J Heart Valve Dis. 1993;2(6):653–661. [PubMed] [Google Scholar]
- 111.Thubrikar MJ, Nolan SP, Aouad J, Deck JD. Stress sharing between the sinus and leaflets of canine aortic valve. Ann Thorac Surg. 1986;42(4):434–440. doi: 10.1016/s0003-4975(10)60554-1. [DOI] [PubMed] [Google Scholar]
- 112.Thubrikar M, Nolan SP, Bosher LP, Deck JD. The cyclic changes and structure of the base of the aortic valve. Am Heart J. 1980;99(2):217–224. doi: 10.1016/0002-8703(80)90768-1. [DOI] [PubMed] [Google Scholar]
- 113.van Vlimmeren MAA, Driessen-Mol A, Oomens CWJ, Baaijens FPT. An in vitro model system to quantify stress generation, compaction, and retraction in engineered heart valve tissue. Tissue Eng Part C. 2011;17(10):983–991. doi: 10.1089/ten.TEC.2011.0070. [DOI] [PubMed] [Google Scholar]
- 114.Vouyouka AG, Salib SS, Cala S, Marsh JD, Basson MD. Chronic high pressure potentiates the antiproliferative effect and abolishes contractile phenotypic changes caused by endothelial cells in cocultured smooth muscle cells. J Surg Res. 2003;110(2):344–351. doi: 10.1016/s0022-4804(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 115.Warnock JN, Burgess SC, Shack A, Yoganathan AP. Differential immediate-early gene responses to elevated pressure in porcine aortic valve interstitial cells. J Heart Valve Dis. 2006;15(1):34–41. [PubMed] [Google Scholar]
- 116.Warnock JN, Konduri S, He Z, Yoganathan AP. Design of a sterile organ culture system for the ex vivo study of aortic heart valves. J Biomech Eng. 2005;127(5):857–861. doi: 10.1115/1.1992535. [DOI] [PubMed] [Google Scholar]
- 117.Warnock JN, Nanduri B, Pregonero Gamez CA, Tang J, Koback D, Muir WM, et al. Gene profiling of aortic valve interstitial cells under elevated pressure conditions: modulation of inflammatory gene networks. Int J Inflamm. 2011;2011:176412. doi: 10.4061/2011/176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weinberg EJ, Kaazempur Mofrad MR. A multiscale computational comparison of the bicuspid and tricuspid aortic valves in relation to calcific aortic stenosis. J Biomech. 2008;41(16):3482–3487. doi: 10.1016/j.jbiomech.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 119.Weston M, Yoganathan A. Biosynthetic activity in heart valve leaflets in response to in vitro flow environments. Ann Biomed Eng. 2001;29(9):752–763. doi: 10.1114/1.1397794. [DOI] [PubMed] [Google Scholar]
- 120.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, et al. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res. 2011;109:183–192. doi: 10.1161/CIRCRESAHA.111.245035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xing Y. Effects of Mechanical Forces on the Biological Properties of Porcine Aortic Valve Leaflets. Georgia Institute of Technology; 2004. [Google Scholar]
- 122.Xing Y, He Z, Warnock JN, Hilbert SL, Yoganathan AP. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Ann Biomed Eng. 2004;32(4):555–562. doi: 10.1023/b:abme.0000019175.12013.8f. [DOI] [PubMed] [Google Scholar]
- 123.Xing Y, Warnock JN, He Z, Hilbert SL, Yoganathan AP. Cyclic pressure affects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Ann Biomed Eng. 2004;32(11): 1461–1470. doi: 10.1114/b:abme.0000049031.07512.11. [DOI] [PubMed] [Google Scholar]
- 124.Yacoub MH, Takkenberg JJM. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–61. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 125.Yap CH, Kim HS, Balachandran K, Weiler M, Haj-Ali R, Yoganathan AP. Dynamic deformation characteristics of porcine aortic valve leaflet under normal and hypertensive conditions. Am J Physiol Heart Circ Physiol. 2010;298(2):H395–H405. doi: 10.1152/ajpheart.00040.2009. [DOI] [PubMed] [Google Scholar]
- 126.Yap CH, Saikrishnan N, Tamilselvan G, Vasilyev N, Yoganathan AP. The congenital bicuspid aortic valve can experience high-frequency unsteady shear stresses on its leaflet surface. Am J Physiol Heart Circ Physiol. 2012;303(6):H721–H731. doi: 10.1152/ajpheart.00829.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yap C, Saikrishnan N, Tamilselvan G, Yoganathan A. Experimental measurement of dynamic fluid shear stress on the aortic surface of the aortic valve leaflet. Biomech Model Mechanobiol. 2012;11:171–182. doi: 10.1007/s10237-011-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yap C, Saikrishnan N, Yoganathan A. Experimental measurement of dynamic fluid shear stress on the ventricular surface of the aortic valve leaflet. Biomech Model Mechanobiol. 2012;11:231–244. doi: 10.1007/s10237-011-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]