Abstract
Gingerol is a major dietary compound that occurs in several plants belonging to the Zingiberaceae family. In the current study, the protective effect of gingerol on STZ-induced sporadic Alzheimer’s disease (SAD) was determined. Gingerol was isolated from the seeds of Aframomum melegueta K. Schum and tested at doses of 10 and 20 mg/kgbwt for its possible effect on the SAD model in mice, using celecoxib (30 mg/kg bwt) as a reference standard. The curative effects of gingerol were assessed through measurement of β-amyloid (Aβ-42), α-, β- secretases, APH1a and COX-2 levels. In addition, improvement in the cognitive deficit in mice after treatment was confirmed using the water maze and Y-maze with intra-maze cues. Gingerol improved the cognitive and behavioral impairment and AD-like pathology in streptozotocin model mice. These beneficial effects occurred with an increase in α-secretase activity and a decrease in cerebral Aβ-42, β- secretase, APH1a activity and COX-2-linked neuro-inflammation.
Subject terms: Cognitive control, Alzheimer's disease
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by loss of cognitive function as well as pathological deposition of β-amyloid (Aβ) senile plaques and neurofibrillary tangles1. AD is classified into late-onset sporadic AD (SAD) and early onset familial AD (FAD). The majority of AD cases are SAD, which involves many etiopathogenic mechanisms including environmental, genetic and metabolic factors2.
The intracerebroventricular (ICV) injection of streptozotocin results in a well-established mouse model showing many aspects of SAD including neuroinflammation, brain insulin resistance, cholinergic deficits, accumulation of β-amyloid and tau proteins, and oxidative stress as well as memory and learning impairment3.
In AD, the amyloidogenic pathway results in the production of Aβ through the cleavage of amyloid precursor protein (APP) by β-secretase followed by the APH1a subunit of the γ-secretase complex. However, α-secretase produces the non-amyloidogenic soluble amyloid precursor protein α (sAPPα)4. Accumulating evidence has demonstrated that COX inhibitors are involved in the management of AD through their action on the downstream effects of the insulin signaling pathway inhibiting neuroinflammation and oxidative stress5. Moreover, it was previously suggested that expression of cyclooxygenases (COXs) may affect β-amyloid peptide generation through mechanisms that involve the PGE2-mediated potentiation of γ-secretase activity, further supporting a role for COX-2 in the neuropathology of AD6.
Natural products are a well-known source of most of the discovered medicines. Most of the active compounds that have shown protective effects against SAD are from plants, with little contribution from marine and microbial origins7. Most of the discovered plant-derived compounds have shown activity as acetyl cholinesterase inhibitors (AChEIs), but they only address the symptoms of AD. However, certain plant extracts and compounds with antioxidant and anti-inflammatory effects have also been reported to alleviate AD5.
6-Gingerol is a major phenolic constituent and hydroxyphenyl alkane of several plants belonging to the Zingiberaceae family such as ginger, cardamom and grain of paradise. The formerly mentioned plants are widely used in Middle Eastern and Asian cuisine as spices and every-day beverages. According to Arabian folklore, ginger has been claimed to improve memory and has been traditionally used as an ingredient for cognitive enhancement8.
6-Gingerol is reported to display several biochemical and pharmacologic activity such as anticarcinogenic, antimutagenic, anti-apoptotic9, antioxidant, anti-inflammatory10, and cardio- and hepatoprotective effects11, 12. It is also known to inhibit nitric oxide synthase and cyclo-oxygenase13 and the expression of tumor necrosis factor alpha (TNF-α)14. The antioxidant, anti-apoptotic and anti-inflammatory potentials of 6-gingerol suggest its possible effect for protection against some SAD manifestations.
The objective of this study is to investigate the effect of gingerol on some molecular biomarkers of SAD including anestimation of the level of β-amyloid, α- and β-secretases and the APH1a subunit of γ-secretase as well as the cognitive skills, suggesting that it could be a promising component of a clinical nutrition regime or used as an adjuvant therapy4–6.
Results
Purified gingerol was identified using 1H and 13C NMR analysis and comparison of the data with that of the reported literature (Supplementary data, Figs 1–3)15, 16. All experiments were designed to test the possible effect of gingerol at two dosages (10 and 20 mg/kg bwt) on the improvement of cognitive function as well as Aβ formation in the brain in STZ-induced cognitive impairment and amyloid genesis in a mouse model. As no significant difference was found during the pilot study between the two gingerol (10 mg/kg and 20 mg/kg) groups and the saline-treated group (Supplementary data, Tables 1–7), only the saline control group was represented in all of the following graphs. The possible anti-inflammatory effect of gingerol was tested in comparison with the effect of the anti-inflammatory celecoxib as a reference standard.
Morris water maze
Cognitive impairment was induced by ICV injection of STZ as manifested in the water maze test and modified Y maze test. The Morris water maze is used to investigate the spatial reference learning and memory of mice. During the training phase, all mice learned the platform location, as revealed by a decrease in the latency and the distance travelled to locate the submerged platform. However, the training was delayed for the ICV-STZ mice as they needed more time and travelled a longer distance to find the platform than the control mice, which clearly indicated a short-term memory impairment in the STZ-treated mice. The treated groups (groups A, B and C) showed a significant improvement starting from the first day as they exhibited a significantly lower escape latency than those in the STZ group (Fig. 1).
Figure 1.
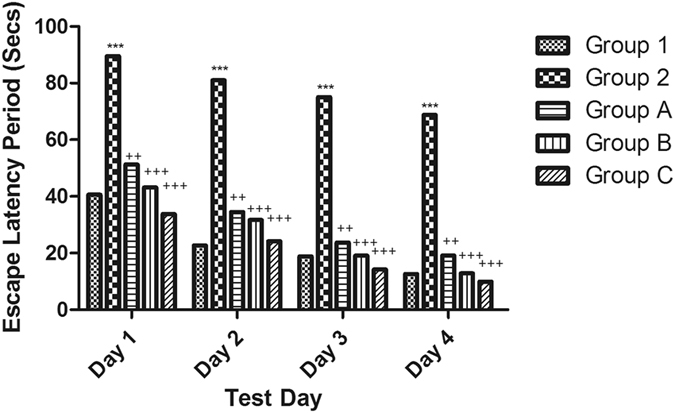
Effect of STZ, celecoxib and gingerol on the mean escape latency in the Morris water maze. Animals were divided into 5 groups. Group 1 received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B received a single injection of STZ (3 mg/kg, i.c.v.), and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to the behavioral tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by the Tukey-Kramer multiple comparison test with the aid of GraphPad Prism Software version 5. ***Significantly different from the normal group P < 0.001. ++Significantly different from the STZ group P < 0.01. +++Significantly different from the STZ group P < 0.001.
In the probe test, The STZ-treated group showed a significant decrease in the mean time spent in the target quadrant by approximately 70% compared to that of the normal group. Moreover, the treated groups A, B and C showed a significant increase in the mean time spent in the target quadrant by approximately 60% compared to that of the STZ-treated group (Fig. 2).
Figure 2.
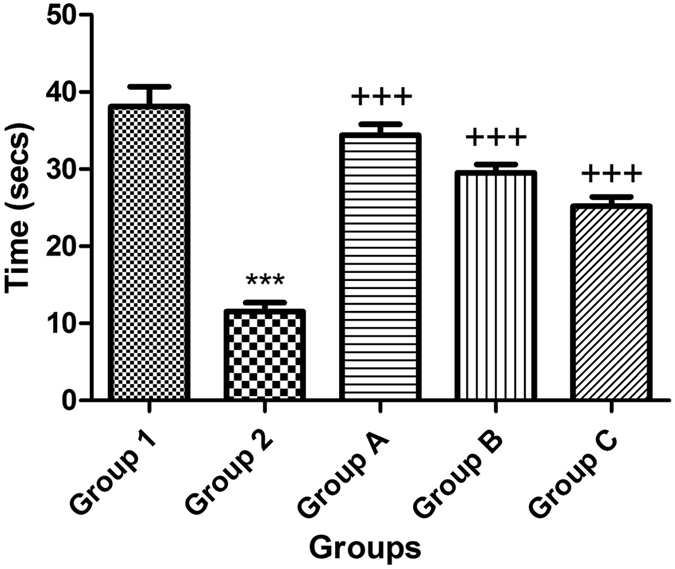
Effect of STZ, celecoxib, and gingerol (10 and 20 mg/kg) on the Morris water maze probe test. Animals were divided into 5 groups. Group 1 received a single dose of saline ICV; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B animals were injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to the behavioral tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by a Tukey-Kramer multiple comparison test with the aid of GraphPad Prism Software version 5. ***Significantly different from the normal group P < 0.001. +++Significantly different from the STZ group P < 0.001. +++Significantly different from the STZ group P < 0.001.
These results suggest an impairment to encode and remember the spatial coordinates of the platform within the environment. Retrieval of spatial memory was more specifically analyzed with the probe trials performed 24 hours after the last training trial. The ICV-STZ mice crossed the former platform site much less often than the control mice and did not show marked preference toward the target quadrant. These results indicate that the ICV-STZ mice have a decreased ability to encode the environment as a spatial map and to localize the platform, suggesting an impairment in spatial reference memory.
Intra-maze cue version of the Y-maze
The effect of STZ (3 mg/kg, ICV) was investigated on the novel arm count in the intra-maze cue version of the Y-maze and demonstrated significant impairment in novelty-seeking behavior and spatial memory compared with that of the control mice. Treatment of mice with the positive control (celecoxib, 30 mg/kg, i.p.) and gingerol (10 mg/kg and 20 mg/kg, i.p.) for 7 days resulted in significant improvement in both the novelty-seeking behavior as well as spatial memory (Fig. 3).
Figure 3.
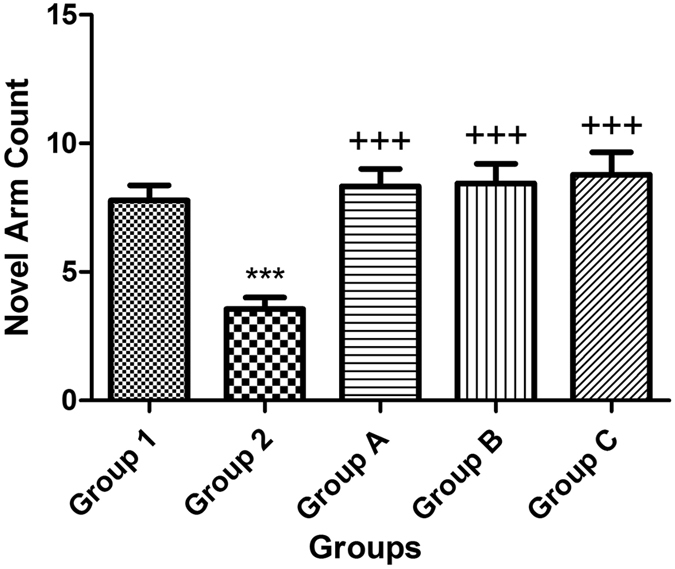
Effect of STZ, celecoxib, and gingerol (10 and 20 mg/kg) on the novel arm count in the intra-maze cue version of the Y-maze. Animals were divided into 5 groups. Group 1 received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B animals were injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to the behavioral tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by a Tukey-Kramer multiple comparison test with the aid of GraphPad Prism Software version 5. ***Significantly different from the normal group P < 0.001. +++Significantly different from the STZ group P < 0.001.
STZ-induced Aβ-42 generation in mouse brains
To further investigate the anti-amyloid genesis effects of gingerol, the inhibitory effect on the levels of Aβ-42 as well as α-, β- and γ-secretases was examined.
The mean levels of Aβ-42 in mouse brains were evaluated by a mouse Aβ-42 ELISA kit. The STZ-treated mice showed a significantly higher concentration of amyloid beta than the normal group. However, treatment with celecoxib or gingerol (10 mg/kg and 20 mg/kg) resulted in a significant reduction in the concentration of Aβ-42 when compared with that in the STZ-treated mice (Fig. 4).
Figure 4.
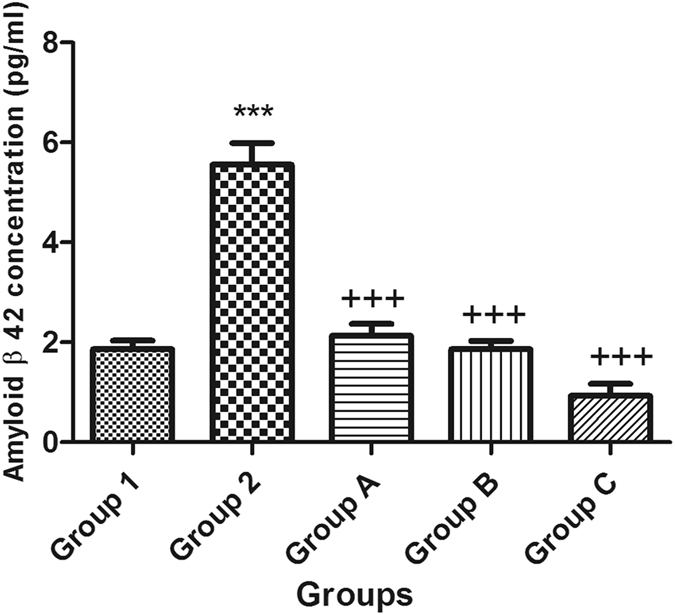
Effect of STZ, celecoxib, and gingerol (10 and 20 mg/kg) on the mean mouse amyloid beta 42 concentration using an ELISA assay. Animals were divided into 5 groups. Group 1 received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B animals were injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by Tukey-Kramer multiple comparison test with the aid of GraphPad Prism Software version 5. ***Significantly different from the normal group P < 0.001. +++Significantly different from the STZ group P < 0.001.
STZ decreased α-secretase activity but increased β-secretase activity and the APH1a concentration
Tumor necrosis factor alpha (TNF-α)-converting enzyme (TACE), also called ADAM17 or α-secretase, belongs to the ADAM (A Disintegrin and Metalloprotease) family of proteins involved in neurogenesis through the ectodomain shedding of cell surface proteins17. TACE, the protease responsible for the generation of mature TNF-α, plays an important role in acute and chronic inflammation. The inhibition of TACE by a pharmacological agent may represent an alternative approach to modulate the effect of TNF-α18. TACE is also responsible for the proteolytic cleavage of amyloid precursor proteins, L-selectin and transforming growth factor-α19.
Moreover, APH1a encodes a component of the γ-secretase complex that cleaves integral membrane proteins such as β-amyloid precursor protein (APP). Accordingly, the APH1a concentration was used in the present study as a way to represent the level of γ-secretase in the brains of mice. Following a single ICV injection of STZ, the β-secretase activity and APH1a concentration increased, whereas the activity of α-secretase was reduced in mouse brains. Intraperitoneal injection of celecoxib orgingerol (10 mg/kg and 20 mg/kg) resulted in a significant reduction in the level of β and APH1a and a significant increase in the activity of α-secretase (Figs 5, 6 and 7).
Figure 5.
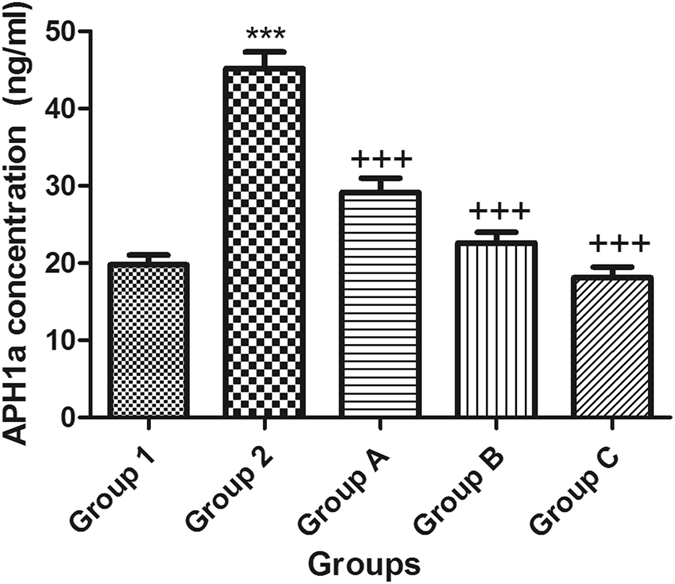
Effect of STZ, celecoxib, andgingerol (10 and 20 mg/kg) on the mean mouse APH1a concentration using an ELISA assay. Animals were divided into 5 groups. Group 1 received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.). and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B animals were injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by Tukey-Kramer multiple comparison test with the aid of GraphPad Prism Software version 5. ***Significantly different from the normal group P < 0.001. +++Significantly different from the STZ group P < 0.001.
Figure 6.
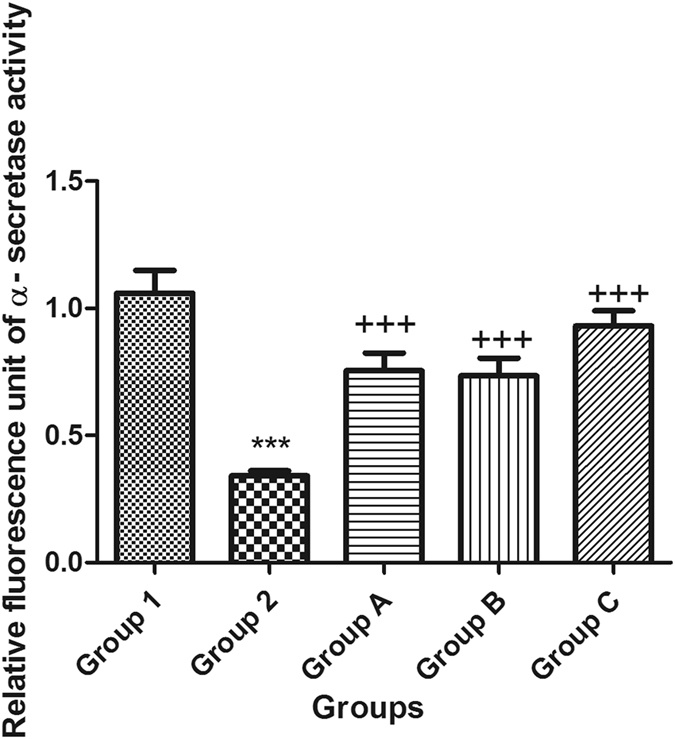
Effect of STZ, celecoxib, and gingerol (10 and 20 mg/kg) on the mean mouse α–secretase activity using an ELISA assay. Animals were divided into 5 groups. Group 1 received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B animals were injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by Tukey-Kramer multiple comparison test by the aid of Graph Pad Prism Software version 5. ***Significantly different from normal group P < 0.001. +++Significantly different from STZ group P < 0.001.
Figure 7.
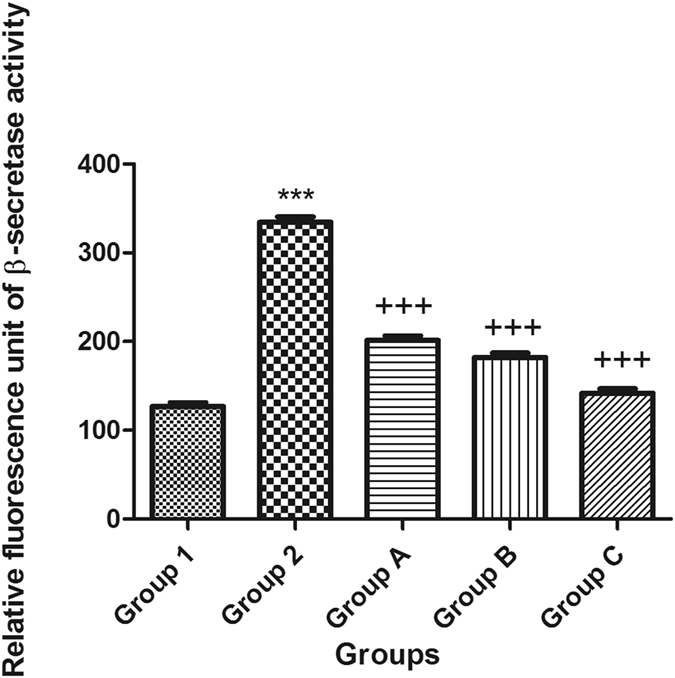
Effects of STZ, celecoxib, and gingerol (10 and 20 mg/kg) on the mean mouse β-secretase cleavage activity using an ELISA assay. Animals were divided into 5 groups. Group 1 received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. Group 2 received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. Group A was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. Group B animals were injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C was injected with STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to tests after the 3rd week of injections. Each result represents the mean value for 10 mice ± SE of the mean. Statistical analysis was carried out by one-way ANOVA followed by a Tukey-Kramer multiple comparison test with the aid of GraphPad Prism Software version 5. ***Significantly different from the normal group P < 0.001. +++Significantly different from the STZ group P < 0.001.
Immunohistochemistry: Effect of various drug treatments on the expression of COX-2 in the brain
ICV injection of STZ showed a marked increase in the expression of COX-2 in the mouse brains compared to that in normal animals. However, COX-2 expression was markedly decreased in the celecoxib and gingerol (10 mg/kg and 20 mg/kg) -treated animal groups compared to that in the STZ-treated animals (Fig. 8).
Figure 8.
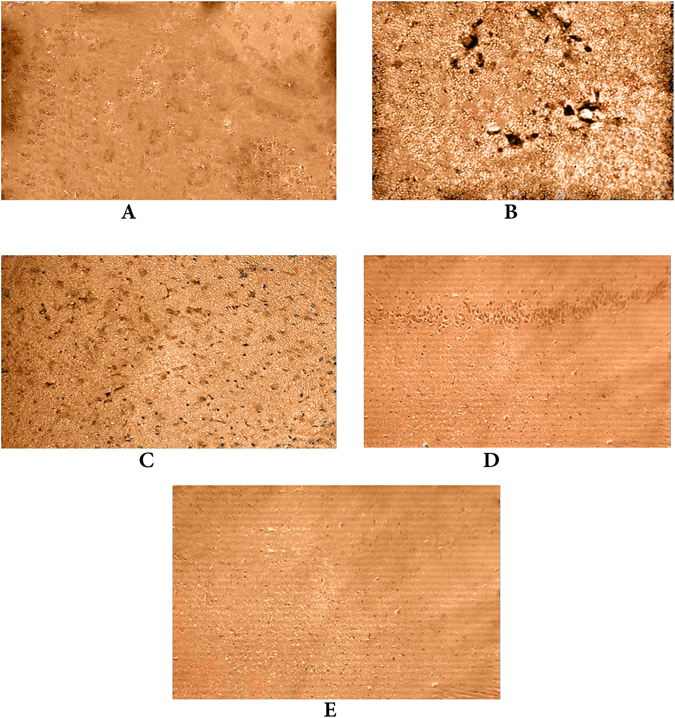
Effects of STZ, celecoxib, and gingerol (10 and 20 mg/kg) on the immunohistochemical analysis of COX-2 expression in the brain. (A) The normal group received a single ICV dose of saline; then, after 2 weeks, animals were intraperitoneally injected with saline for seven days. (B) The control group received a single injection of STZ (3 mg/kg, i.c.v.), and then, after 2 weeks, they were intraperitoneally injected with saline for 7 days. (C) The celecoxib group received STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with celecoxib (30 mg/kg, i.p.) for 7 days. (D) The gingerol (10 mg/kg) group received STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (10 mg/kg/day, i.p.) for 7 days. (E) The gingerol (20 mg/kg) group received STZ (3 mg/kg, i.c.v.) once, and after 2 weeks, they were injected with gingerol (20 mg/kg/day, i.p.) for 7 days. Scale bar, 60 µm (A–E).
Discussion
AD is an age-linked neurodegenerative disease characterized by severe dementia. Symptoms of AD occur over a long period of time, and therefore, treatments should target this silent period of the disease to retard the disease progression towards dementia20. Centrally administered STZ is known to cause SAD-like memory deterioration, and STZ-treated mice are therefore one of the non-transgenic mice models used to represent AD21, 22.
ICV-STZ-treated rats showed an impaired learning and memory performance. Cognitive impairment after ICV-STZ administration is induced by the degradation of phospholipids and the resulting increases in the free fatty acid, arachidonic acid. COX is a rate-limiting enzyme in the metabolism of arachidonic acid to prostanoids, particularly prostaglandins (PGs), which significantly contribute to neuro-inflammation with oxidative stress. PGs such as PGE1 and PGE2 have also been reported to cause neurotoxicity and stimulate the release of glutamate from astrocytes. In a recent study, the use of COX inhibitors, such as indomethacin, has been shown to cause some improvement in the symptoms of AD, and the mechanism has been hypothesized to involve a reduction in inflammation and oxidative stress. In addition, celecoxib (a selective COX-2 inhibitor) aids in slowing the progression of the disease through its anti-inflammatory property resulting in reduced neuronal cell death23. Accordingly, it was used as a positive control in this study.
In this study, the neuroprotective effects of gingerol on the molecular biomarkers of SAD and cognitive skills were investigated. Its protective effect was assessed through measuring the levels of Aβ, α-and β-secretases, APH1a (a component of γ-secretase) and COX-2. In addition, the improvement in the cognitive skills ofmice after treatment was confirmed using the Y and water maze.
In the present study, fourteen days following a single ICV injection of STZ, the level of Aβ-42 as well as the activity of β-secretase and the APH1a concentration in the whole brain increased, whereas that of α-secretase decreased. Treatment with celecoxib and gingerol resulted in a significant reduction in the levels of Aβ-42, APH1a and β-secretase activity as well as a significant increase in the activity of α-secretase.
Aβ precursor protein (APP) is generally known to be a limiting reagent in the cell, and it has been postulated that α- and β-secretases compete for APP proteolysis24. Reduced β-secretase activity could therefore be responsible for the alleviation of the cerebral Aβ pathology in gingerol-treated mice. In this regard, it was previously demonstrated that another polyphenol, epigallocatechin gallate (EGCG), was also able to promote non-amyloidogenic APP metabolism in the Tg2576 cerebral amyloidosis mouse model. Interestingly, the mechanism for the beneficial effects of EGCG on non-amyloidogenic APP processing relied on promoting the activity of the candidate α-secretase, a disintegrin and metalloprotease 1025. Recently, nutraceuticals, including the green tea polyphenol and citrus bioflavonoid EGCG26, luteolin27, grape-derived polyphenols28, and caffeine29 have been found to exhibit anti-amyloidogenic properties. Moreover, a plant-derived polyphenol, tannic acid (TA), has been shown to oppose the behavioral impairment and AD-like pathology in transgenic mice. These beneficial effects occur with a reduction in cerebral Aβ pathology, cleaved β-CTF, sAPP-β, BACE1 (beta-site amyloid precursor protein cleaving enzyme 1) protein expression and activity, and neuro-inflammation30.
Neuro-inflammation plays a crucial role in the pathogenesis of SAD. In the current study, the expression levels of COX-2 were observed to be significantly up-regulated in STZ-induced AD mice and down-regulated strongly by gingerol compared with that in control mice. Similarly, it was previously demonstrated that the STZ-induced learning and memory impairments in mice are due to stimulation of the neuro-inflammatory response31.
The protective effect of gingerol was further confirmed through immunohistochemistry, in which we studied the effects of these compounds on expression of COX-2 in the brain. The protective effect of gingerol on Aβ-42, β-secretase and APH1a levels was accompanied by a marked decrease in the expression of COX-2 in the brain tissue. Recently, the effects of shogaol, a structurally related compound to gingerol and isolated from Zingiberofficinale, on microglia activation induced by lipopolysaccharide (LPS) were studied both in primary cortical neuron-glia cultures and in an in vivo neuro-inflammatory model. Shogaol exerted an anti-inflammatory effect by inhibiting the production of PGE2 and by down-regulating COX-2 expression better than gingerol32. In addition, a recent study done by Ilic and his colleagues on the ethanolic extract of grains of paradise (A. melegueta), the source of gingerol in the current study, showed that the major phenolics from the extract,6-paradol, 6-gingerol, and 6-shogaol, reduced inflammation, which is in part due to the inhibition of COX-2 enzyme activity and the expression of pro-inflammatory genes33.
In addition, the improvement in the cognitive skills of mice after treatment was established using the Y and water maze. In the present study, the MWM was used to observe the effects of STZ and gingerol on cognitive dysfunction in a mouse model. Our results indicated that STZ can significantly increase the escape latency and decrease the percentage of time spent in the 4th quadrant, which is in agreement with previous studies that concluded that ICV-STZ-treated mice had deficits in spatial learning and memory indicated by impaired acquisition and retention in the MWM and passive avoidance tasks34. Moreover, during the probe trial done on last day with the platform removed, mice failed to remember the precise location of the platform, spending significantly less time in the target quadrant than the normal groups; this result was proven earlier35. Similarly, STZ resulted in severe significant impairment in novelty-seeking behavior and spatial memory in the modified Y-maze test. This finding coincides with a recent study that proved that the deficits in Y-maze performance following chronic stress reflect neurochemical and/or neurobiological changes underlying spatial memory ability36. In addition, the relationship between higher expression of inflammatory cytokines and STZ-caused cognitive impairment has been widely acknowledged. Previous studies have shown that cognitive function will be improved if the excessive activation of the inflammatory response has been corrected37. This indicates that the STZ model is beneficial as a mouse model of cognitive impairment. In this light, recent focus has been given to a group of phenolics such as flavonoids, which have been found to be potentially anti-amyloidogenic38. The beneficial behavioral neuroprotective effects of the studied phenolics and gingerol coincide with a recent finding that pretreatment with hesperidin improved the cognitive impairment induced by ICV injection of STZ in the MWM test. This was concluded to be mediated by inhibiting the overexpression of inflammatory markers such as COX-239, and their efficacies were found to be at the level of celecoxib, used as a positive control in this study.
Finally, it could be concluded from the present work that gingerol prevented memory impairments in a SAD mouse model, which could be attributable to the amelioration of STZ- induced neuroinflammation and amyloidogenesis. This study gives a perspective on the wide variety of interactions between the inflammatory COX-2 and APP secretases and the beneficial effect of gingerol. On the one hand, overexpression of COX-2by STZ is able to increase the activity of β-secretase and γ-secretase and to decrease that of α-secretase. On the other hand, gingerol and celecoxib are able to modulate the activity of the secretases by regulating their levels in the case of BACE1 and APH1a or by directly shifting the cleavage site of γ-secretase. This study is the first, to the best of our knowledge, to report the protective effects of gingerol against STZ-induced biochemical changes and behavioral impairments, rendering gingerol a promising compound to treat or prevent AD.
Methods
General experimental procedures
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Unity INOVA 850 instrument at 850 MHz for 1H- and 213 MHz for 13C-NMR (Bruker BioSpin, Billerica, MA, USA) using CDCl3 as the solvent. ESIMS was recorded on an Agilent 1200 system connected to an Agilent 6320 Ion Trap HPLC-ESI-MS (Agilent Technology, Waldbronn, Germany). TLC was carried out on a pre-coated silica gel 60 F254 (0.25 mm, Merck) and RP-18 F254S (0.25 mm, Merck Co., Darmstadt, Germany). Column chromatography (CC) was carried out on a silica gel 60 for column chromatography (Fluka, 70-230 mesh, Germany). The plant material and the isolation and purification methods of gingerol can be seen in the Supplementary Information.
Biological Study
Experimental Design
Adult male Swiss albino mice weighing between 20–25 g were utilized. Mice were obtained from the animal state of the National Institute of Research (NRC) (Giza, Egypt). Mice were housed in a temperature-controlled room (23–24 °C) and presented with 12-hour dim/light cycles. Free access to food and water was permitted. All procedures were done following the guide for the treatment and care of laboratory animals published by US National Institute of Health and approved by the Ethics Committee at the German University in Cairo. All actions were made to minimize mouse suffering.
A pilot study was performed whereby two doses of gingerol (10 and 20 mg/kg) were employed in addition to the saline control group. The behavioral and biochemical tests were performed on the three control groups. The findings of this study revealed that there were no statistically significant differences noted in the results among the three control groups. Therefore, the normal saline control group was the only group used in the experimental design and the description of the results.
Mice were then randomly isolated into five groups, and every group comprised ten mice. These groups were defined as follows: Group 1: Mice received a solitary ICV infusion of saline; then, following 2 weeks, they received intraperitoneal injections with the vehicle (saline) for seven days. Group 2: Mice were infused with STZ (3 mg/kg, i.c.v.) once, and then received intraperitoneal injections of saline after 2 weeks for seven days. Group A: Mice were infused with STZ (3 mg/kg, i.c.v.) once, and following 2 weeks, they were treated with celecoxib (30 mg/kg, i.p.) for 7 days. Group B: Mice were injected with STZ (3 mg/kg, i.c.v.) once and following 2 weeks, they were treated with gingerol (10 mg/kg/day, i.p.) for 7 days. Group C: Animals were injected with STZ (3 mg/kg, i.c.v.) once, and following 2 weeks, they were treated with gingerol (20 mg/kg/day, i.p.) for 7 days. Animals from groups A, B, and C were subjected to the behavioral and biochemical tests after the third week of infusions.
All mice were subjected to the Morris water maze and Ymi behavioral tests toward the end of the already specified medications. After that, they were euthanized, and their brains were harvested. Two whole brains from each group were placed in 4% formaldehyde to be utilized for immunohistochemical examination of COX-2 expression in the brain, and the remaining brains were separated into 2 hemispheres and solidified at −80 °C to be later homogenized and utilized for ELISA investigation for Aβ content and α-, β- and γ-secretase activity.
STZ-induced SAD
SAD was induced in mice by intracerebroventricular (ICV) injection of STZ (3 mg/kg) dissolved in saline40. The detailed procedures can be reviewed in the Supplementary Information.
Morris water maze
The Morris water maze (MWM) test was performed to assess spatial reference learning and memory as previously described41. The detailed procedures can be reviewed in the Supplementary Information.
Ymitest
The Y-maze included remarkable intra-maze cues (Ymi). In this prompted version, one vast wooded, painted object (unique in relation to those utilized as a part of the object recognition test and sufficiently heavy to keep the mice from moving it) was set toward the end of every arm. The detailed procedures can be reviewed in the Supplementary Information.
ELISA measurement of amyloid β-42
The mean of the Aβ-42 concentration present in mouse brains was calculated by using a mouse Aβ-42 ELISA kit (Anaspec, Germany)42. The detailed procedures can be reviewed in the Supplementary Information.
Estimation of gamma-secretase in the brain homogenate
Quantitative determination of the mouse gamma secretase subunit APH-1a concentration in the brain homogenate was done using a mouse gamma-secretase subunit APH-1a ELISA kit (Catalog No: E0406m, EIAab, China).The detailed procedures can be reviewed in the Supplementary Information.
Estimation of alpha-secretase in the brain homogenate
The total activity of α-secretase or tumor necrosis factor alpha-converting enzyme (TACE) present in mouse brain homogenates was determined using a commercially available Sensolyte520 TACE (α-secretase) activity kit (Anaspec, CA, USA), according to the provided instructions. The detailed procedures can be reviewed in the Supplementary Information.
Estimation of beta-secretase in the brain homogenate
In this assay, a beta-secretase-specific peptide was used and conjugated to two reporter molecules, EDANS and DABCYL. Then, the fluorescent emissions from EDANS were quenched by the physical proximity of the DABCYL moiety in the un-cleaved form. The detailed procedures can be reviewed in the Supplementary Information.
Immunohistochemistry for COX-2 deposition in the brain
The immunohistochemistry procedures for COX-2 deposition in the brain were performed according to Ramos-Vara et al.43. The detailed procedures can be reviewed in the Supplementary Information.
Statistical analysis
Statistical analysis was performed using instant automated software (GraphPad Prism Software version 5.01, Inc., California, USA). The results were expressed as the mean ± standard error of mean (SEM). One-tailed Student’s t-tests and one-way ANOVAs followed by Tukey-Kramer multiple comparison tests were used. Immunohistochemistry was only performed to ensure the deposition of COX-2; consequently, it was only subjected to visual examination. A P value less than 0.05 was considered significant.
Electronic supplementary material
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. (250/166/1434). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Author Contributions
A.M.E., H.M.A. and R.S.E. all performed the isolation and identification of gingerol and shared in designing the experiments and editing the manuscript. N.S.E. also helped to design the experiments, performed the pharmacological study, carried out the data analysis and graphical presentation, and shared in editing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-023-36652-w"
Change history
6/9/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-023-36652-w
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02961-0
References
- 1.Querfurth HW, LaFerla FM. Mechanisms of disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal K, Grundke-Iqbal I. Alzheimer’s disease, a multifactorial disorder seeking multitherapies. Alzheimers.Dement. 2010;6:420–424. doi: 10.1016/j.jalz.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salkovic-Petrisic M, Osmanovic J, Grünblatt E, Riederer P, Hoyer S. Modeling sporadic Alzheimer’s disease: The insulin resistant brain state generates multiple long-term morphobiological abnormalities including hyperphosphorylated tau protein and amyloid-β. J. Alzheimer’s.Dis. 2009;18:729–750. doi: 10.3233/JAD-2009-1184. [DOI] [PubMed] [Google Scholar]
- 4.Chasseigneaux S, Allinquant B. Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J. Neurochem. 2012;120:99–108. doi: 10.1111/j.1471-4159.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- 5.Dhull DK, et al. Neuroprotective effect of cyclooxygenase inhibitors in ICV-STZ induced sporadic Alzheimer’s disease in rats. J. Mol. Neurosci. 2012;46:223–235. doi: 10.1007/s12031-011-9583-6. [DOI] [PubMed] [Google Scholar]
- 6.Qin W, et al. Cyclooxygenase (COX)-2 and COX-1 potentiate β-amyloid peptide generation through mechanisms that involve γ-secretase activity. Journal.of.Biological.Chemistry. 2003;278:50970–50977. doi: 10.1074/jbc.M307699200. [DOI] [PubMed] [Google Scholar]
- 7.Houghton P, Howes M-J. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals. 2005;14:6–22. doi: 10.1159/000085382. [DOI] [PubMed] [Google Scholar]
- 8.Saenghong, N. et al. Zingiber officinale improves cognitive function of the middle-aged healthy women. Evid. BasedComplement. Alternat. Med. 2012 (2011). [DOI] [PMC free article] [PubMed]
- 9.Seo HB, Kwon TD, Song YJ. The effect of ginger extract ingestion and swimming exercise on insulin resistance and skeletal muscle antioxidant capacity and apoptosis in hyperglycemic rats fed a high-fructose diet. J. Exerc. Nutrition. Biochem. 2011;15:41–48. doi: 10.5717/jenb.2011.15.1.41. [DOI] [Google Scholar]
- 10.Surh Y-J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res.-Fundam. Mol. Mech. Mutag. 1999;428:305–327. doi: 10.1016/S1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 11.El-Halawany, A. M., El Dine, R. S., El Sayed, N. S. & Hattori, M. Protective effect of Aframomum.melegueta phenolics against CCl4-induced rat hepatocytes damage; role of Apoptosis and pro-inflammatory cytokines inhibition. Sci. Rep. 4 (2014). [DOI] [PMC free article] [PubMed]
- 12.El-Bakly WM, Louka ML, El-Halawany AM, Schaalan MF. 6-gingerol ameliorated doxorubicin-induced cardiotoxicity: role of nuclear factor kappa B and protein glycation. Cancer.Chemother. Pharmacol. 2012;70:833–841. doi: 10.1007/s00280-012-1975-y. [DOI] [PubMed] [Google Scholar]
- 13.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 14.Oyagbemi AA, Saba AB, Azeez OI. Molecular targets of [6]‐gingerol: Its potential roles in cancer chemoprevention. BioFactors. 2010;36:169–178. doi: 10.1002/biof.78. [DOI] [PubMed] [Google Scholar]
- 15.Tackie A, et al. Hydroxyphenylalkanones from Amomum.melegueta. Phytochemistry. 1975;14:853–854. doi: 10.1016/0031-9422(75)83070-6. [DOI] [Google Scholar]
- 16.Shih H-C, et al. Synthesis of analogues of gingerol and shogaol, the active pungent principles from the rhizomes of Zingiber officinale and evaluation of their anti-platelet aggregation effects. Int. J. Mol. Sci. 2014;15:3926–3951. doi: 10.3390/ijms15033926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 18.Siegel SA, et al. The mouse/human chimeric monoclonal antibody cA2 neutralizes TNF in vitro and protects transgenic mice from cachexia and TNF lethality in vivo. Cytokine. 1995;7:15–25. doi: 10.1006/cyto.1995.1003. [DOI] [PubMed] [Google Scholar]
- 19.Levin J, et al. Acetylenic TACE inhibitors. Part 1. SAR of the acyclic sulfonamide hydroxamates. Biorg. Med. Chem. Lett. 2003;13:2799–2803. doi: 10.1016/S0960-894X(03)00514-6. [DOI] [PubMed] [Google Scholar]
- 20.Aso E, Ferrer I. Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Front. Pharmacol. 2014;5:1–11. doi: 10.3389/fphar.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, et al. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse) Mol. Neurobiol. 2013;47:711–725. doi: 10.1007/s12035-012-8375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraska A, et al. In vivo cross-sectional characterization of cerebral alterations induced by intracerebroventricular administration of streptozotocin. PloS.one. 2012;7:e46196. doi: 10.1371/journal.pone.0046196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Sayed NS, Kassem LA, Heikal OA. Promising therapy for Alzheimer’s disease targeting angiotensinconverting enzyme and the cyclooxygense-2 isoform. Drug.Discov. Ther. 2009;3:307–315. [PubMed] [Google Scholar]
- 24.Gandhi S, Refolo LM, Sambamurti K. Amyloid precursor protein compartmentalization restricts β-amyloid production. J. Mol. Neurosci. 2004;24:137–143. doi: 10.1385/JMN:24:1:137. [DOI] [PubMed] [Google Scholar]
- 25.Rezai-Zadeh K, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obregon DF, et al. ADAM10 activation is required for green tea (−)-epigallocatechin-3-gallate-induced α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 2006;281:16419–16427. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- 27.Rezai‐Zadeh K, et al. Flavonoid‐mediated presenilin‐1 phosphorylation reduces Alzheimer’s disease β‐amyloid production. J. Cell. Mol. Med. 2009;13:574–588. doi: 10.1111/j.1582-4934.2008.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono K, et al. Potent anti‐amyloidogenic and fibril‐destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 29.Arendash GW, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-β levels in aged Alzheimer’s disease mice. J. Alzheimer’s.Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 30.Mori T, et al. Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice. J. Biol. Chem. 2012;287:6912–6927. doi: 10.1074/jbc.M111.294025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu S, et al. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014;19:317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Ha SK, et al. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology. 2012;63:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Ilic NM, et al. Anti-inflammatory activity of Grains of paradise (Aframomum.melegueta.Schum) extract. J. Agric. Food. Chem. 2014;62:10452–10457. doi: 10.1021/jf5026086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehan S, et al. Effect of mitogen activated protein kinase inhibitor in animal model of Alzheimer’s diseases. Int. J. Pharm. Prof. Res. 2011;2:212–223. [Google Scholar]
- 35.Singh B, Sharma B, Jaggi AS, Singh N. Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer’s disease type: possible involvement of PPAR-γ agonistic property. J. Renin.Angiotensin.Aldosterone.Syst. 2013;14:124–136. doi: 10.1177/1470320312459977. [DOI] [PubMed] [Google Scholar]
- 36.Wright RL, Conrad CD. Short Communication Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress:.The.International.Journal.on.the.Biology.of.Stress. 2005;8:151–154. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dik M, et al. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- 38.Yang F, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 39.Javed H, et al. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipids alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. Journal.of.the.neurological.sciences. 2014;348:51–59. doi: 10.1016/j.jns.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 40.Mehla J, Pahuja M, Gupta YK. Streptozotocin-induced sporadic Alzheimer’s disease: selection of appropriate dose. J. Alzheimer’s.Dis. 2013;33:17–21. doi: 10.3233/JAD-2012-120958. [DOI] [PubMed] [Google Scholar]
- 41.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 42.Mehta PD, et al. Plasma and cerebrospinal fluid levels of amyloid β proteins 1-40 and 1-42 in Alzheimer disease. Arch. Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 43.Ramos-Vara JA, et al. Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J. Vet. Diag. Invest. 2008;20:393–413. doi: 10.1177/104063870802000401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


