Abstract
Biofilms in chronic wounds are known to contain a persister subpopulation that exhibits enhanced multidrug tolerance and can quickly rebound after therapeutic treatment. The presence of these “persister cells” is partly responsible for the failure of antibiotic therapies and incomplete elimination of biofilms. Electrochemical methods combined with antibiotics have been suggested as an effective alternative for biofilm and persister cell elimination, yet the mechanism of action for improved antibiotic efficacy remains unclear. In this work, an electrochemical scaffold (e-scaffold) that electrochemically generates a constant concentration of H2O2 was investigated as a means of enhancing tobramycin susceptibility in pre-grown Pseudomonas aeruginosa PAO1 biofilms and attacking persister cells. Results showed that the e-scaffold enhanced tobramycin susceptibility in P. aeruginosa PAO1 biofilms, which reached a maximum susceptibility at 40 µg/ml tobramycin, with complete elimination (7.8-log reduction vs control biofilm cells, P ≤ 0.001). Moreover, the e-scaffold eradicated persister cells in biofilms, leaving no viable cells (5-log reduction vs control persister cells, P ≤ 0.001). It was observed that the e-scaffold induced the intracellular formation of hydroxyl free radicals and improved membrane permeability in e-scaffold treated biofilm cells, which possibly enhanced antibiotic susceptibility and eradicated persister cells. These results demonstrate a promising advantage of the e-scaffold in the treatment of persistent biofilm infections.
Antibiotics: Enhanced effect with an electrical scaffold
Using an electrically conductive fabric to generate hydrogen peroxide could eradicate persistent biofilms in chronically infected wounds. Electrochemical scaffolds (e-scaffolds) are thin networks of conductive material such as carbon fiber used to generate chemical responses in media they are in contact with. Haluk Beyenal and colleagues at Washington State University, USA, investigated the effect of a carbon fabric e-scaffold on cultured biofilms of the bacterium Pseudomonas aeruginosa. The procedure enhanced the susceptibility of this troublesome multidrug-resistant bacterium to the antibiotic tobramycin. Crucially, it eradicated so-called persister cells that can evade antibiotic treatment to reform biofilms in chronic wounds. The research suggests that the effect involves the production of hydroxyl free radicals from hydrogen peroxide and increased permeability of the bacterial cell membranes. The potential of e-scaffolds for treating infected wounds warrants further exploration.
Introduction
Chronic and recalcitrant biofilm infections on wounds are often caused by the presence of a bacterial subpopulation of “persister cells” that are particularly tolerant to bacteriostatic antibiotics.1 Biofilms protect bacterial communities in part because the extracellular polymeric substances (EPS) that form the biofilm matrix serve as a diffusion barrier.2 This barrier limits antibiotic penetration into biofilms3 and immobilizes antibiotics.4 In addition, the diffusive barrier results in nutrient gradients, causing decreased growth and metabolic inactivity in parts of the biofilm community, which allows persister cells to arise.1 In particular, increased persister cell formation is observed in Gram-negative bacterial biofilms because the bacterial cell membranes are composed of lipopolysaccharides that further limit antibiotic penetration into the cells5.
In lieu of classical antibiotics, a number of alternative antimicrobial treatments are being explored either individually (e.g., silver,6 mannitol7) or in combination with conventional antibiotics.8–10 Unfortunately, high concentrations of these antimicrobials/antibiotics have toxic side effects11,12 while at low concentrations they often decompose before completely eliminating biofilm communities.13 Moreover, any persister cells can regrow and form biofilms with potentially enhanced tolerance to antibiotics.14 Interestingly, the application of an antibiotic in combination with a direct current (DC) (ranging from µA to mA/cm2) can be effective against several Gram-negative bacteria,15,16 including against putative persister cells.16 The mechanism underlying this effect was unclear until a recent study demonstrated the presence of electrochemically generated H2O2.17 In that study, an electrochemical scaffold (e-scaffold) made of conductive carbon fabric generated ~25 µM H2O2 near the surface of the e-scaffold and this was sufficient to reduce an A. baumannii biofilm (~3-log) that was established on a dermal explant. The constant but relatively low production of H2O2 did not appear to be cytotoxic to the mammalian tissue.17
A similar study demonstrated that electrochemically generated H2O2 is sufficient to prevent or delay Pseudomonas aeruginosa PAO1 biofilm growth in vitro 18 despite the production of two important catalase enzymes (katA and katB) that can protect P. aeruginosa from H2O2.19 Nevertheless, complete elimination of mature P. aeruginosa PAO1 biofilms by e-scaffold treatment can be difficult. Adjunct antibiotic treatment can be helpful,16 but the mechanism underlying this combined effect is not understood.15 For instance, Nodzo et al. reported an enhanced efficacy of vancomycin when it was combined with a cathodic potential of −1.8 VAg/AgCl against biofilms formed on Ti implants in a rodent model, but did not report any mechanism.20 Niepa et al. reported that a stainless steel (SS304) electrode released metal cations that enhanced antibiotic efficacy against P. aeruginosa PAO1 persister cells in an electrochemical system applying ~70 µA/cm2 DC.16 Under this condition, it is likely that SS304 corroded and released iron ions.21 A similar increased efficacy of antibiotic was reported when an inert carbon electrode under the same applied DC was used against P. aeruginosa PAO1 persister cells in the same system.22 An inert carbon electrode does not release metal cations as SS304 does; thus, the release of metal cations is unlikely to be the mechanism for the efficacy of a combination of DC and antibiotic treatment.22 The authors speculated that electrochemically generated reactive oxygen species (ROS) (e.g., H2O2 and OH•) were responsible for this effect, but they did not confirm this experimentally.22
An e-scaffold generates H2O2, which enters the bacterial periplasm through porins,23,24 where it can induce intracellular production of highly reactive hydroxyl free radicals (OH•)25,26 that degrade membrane lipids, proteins, and DNA.26,27 Recent research also found that H2O2 eliminates some of the persister cells in biofilms, facilitates the disruption of biofilm architecture and mediates the generation of metabolically active dispersal cells in a range of Gram-negative bacterial biofilms.28,29 Such metabolic activity in surviving dispersal cells and OH• production have been reported to induce bacterial sensitivity to antibiotic treatment.30–32 Therefore, e-scaffold generated H2O2 possibly promotes intracellular OH• production that in turn improves antibiotic sensitivity in biofilms and attacks persister cells.
In this work, we used P. aeruginosa PAO1 with an aminoglycoside antibiotic (tobramycin). P. aeruginosa PAO1 can resist tobramycin by producing periplasmic glucans, mutations of ribosome-binding sites or increased efflux pump action inhibiting cellular uptake.33 Furthermore, P. aeruginosa PAO1 biofilm persister cells are reportedly less sensitive to tobramycin.9 We isolated persister cells from P. aeruginosa PAO1 biofilms after treating them with ciprofloxacin following published protocols.16,34 We hypothesized that the bacterial subpopulation that survived e-scaffold generated H2O2 would be more sensitive to tobramycin than these ciprofloxacin-tolerant persister cells. The objectives of this study were (1) to evaluate the tobramycin susceptibility of P. aeruginosa PAO1 biofilms treated with an, e-scaffold and compare it with the tobramycin susceptibility of persister cells, (2) to evaluate the efficacy of the e-scaffold against persister cells and (3) to determine whether e-scaffold treatment would increase intracellular production of OH• radicals and increase membrane permeability in the bacterial cells, making them more susceptible to antibiotics. In addition, change in bacterial cell morphology after e-scaffold treatment was observed using scanning electron microscopy (SEM). Finally, based on these observations, a possible mechanism of e-scaffold enhanced antibiotic susceptibility is proposed and a future mechanistic study is suggested.
Results
Electrochemical scaffold enhances tobramycin susceptibility in biofilm cells
When biofilm treatments were combined with different concentrations of tobramycin, the surviving cells responded differently. The tobramycin susceptibility of P. aeruginosa PAO1 biofilms regrown from fresh culture, untreated biofilm cells, and persister cells isolated from biofilms appeared to follow a dose response at tested concentrations between 0 and 40 µg/ml (Fig. 1). The biofilms regrown from persister cells showed tolerance to tobramycin, with only a (1.2 ± 0.16)-log reduction in viable cells for 10 µg/ml tobramycin and no further significant decrease at higher concentrations. These persister cells had the same minimum inhibitory concentration (MIC) as the fresh culture (Supplementary information); however, consistent with the characteristic behavior of “persister cells”, they survived antibiotic treatment, regrew and developed tolerance to tobramycin identical to their regular population.1,35 Interestingly, tobramycin tolerance was observed in biofilms regrown from untreated biofilm cells and persister cells isolated from biofilms. In contrast, no tolerance to tobramycin was identified for biofilms regrown from e-scaffold treated cells when different concentrations of tobramycin were combined with e-scaffold treatment (Fig. 1). This confirms the prevention of persistence by the e-scaffold. A linear dose response was observed for the log reduction of e-scaffold treated biofilm cells which received tobramycin treatment, leading to a complete eradication at 40 µg/ml tobramycin (Fig. 1). This concentration (20×MIC) is still considerably lower than what is typically required for P. aeruginosa PAO1 biofilm treatment with tobramycin (>500× of MIC) as reported in the literature.36 Overall, a significant increase in tobramycin susceptibility was attained for e-scaffold treated biofilms compared to biofilms that were not treated with an e-scaffold (P < 0.05, paired t-tests) (Fig. 1). Among the tested tobramycin concentrations, we observed a maximum tobramycin susceptibility at 40 µg/ml in e-scaffold treated biofilms compared to that for persister cells.
Fig. 1.
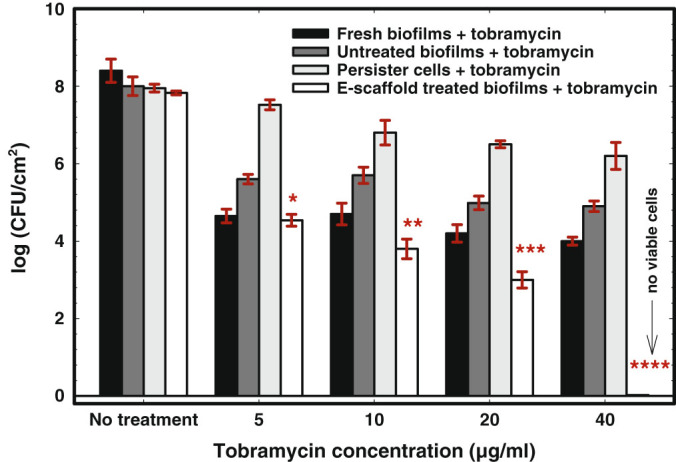
E-scaffold enhances tobramycin susceptibility in P. aeruginosa PAO1 biofilms. Bars represent means of logarithms of colony-forming units of viable biofilm cells. Error bars represent the standard error of the means from three biological replicates. The symbols *, **, ***, and **** represent significant differences in tobramycin susceptibility between e-scaffold treated biofilms + tobramycin and untreated biofilms + tobramycin (n = 3, *, P = 0.002; **, ***, **** P ≤ 0.001; paired t-test)
Electrochemical scaffold eradicates persister cells
The effect of e-scaffold generated H2O2 on P. aeruginosa PAO1 persister cells isolated from ciprofloxacin-treated biofilms was explored. As Fig. 2 shows, ~0.31 % of the total biofilm cells belonged to the persister population, which is in the range reported in the literature.37 Within 6 h of these persister cells being treated with an e-scaffold, no viable persister cells were apparent. This corresponds to a 5-log reduction in persistence compared to the control initial persister cells (P ≤ 0.001, one-way ANOVA followed by Bonferroni test). No growth was observed even after the treated cells were exposed to fresh medium alone for 24 h upon removal of the e-scaffold. This confirmed complete eradication of persister cells by e-scaffold treatment. In contrast, the final population of persister cells did not change within the additional 6 h of ciprofloxacin treatment. The e-scaffold was also found to be effective against total biofilm cells, with (4.2 ± 0.23) and (4.95 ± 0.20)-log reductions in viable cells within 6 and 24 h of treatment, respectively, compared to the control final biofilm cells. Together these results suggest that the e-scaffold is effective against both regular biofilm cells in active states and persister cells in inactive metabolic states. They also confirm that persister cells are inherently more sensitive to e-scaffold generated H2O2 exposure.
Fig. 2.
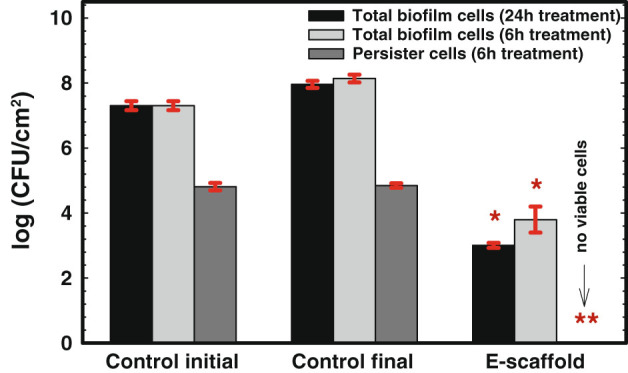
Exposure to e-scaffold eradicates the persister cells in P. aeruginosa PAO1 biofilms. Bars represent means of logarithms of colony-forming units of viable biofilm cells. Error bars represent the standard errors of the means from three biological replicates. The symbol * denotes a significant difference compared to total biofilm cells (n = 3, P < 0.05, one-way ANOVA), and ** denotes a significant difference compared to control initial persister cells (n = 3, P < 0.001, one-way ANOVA)
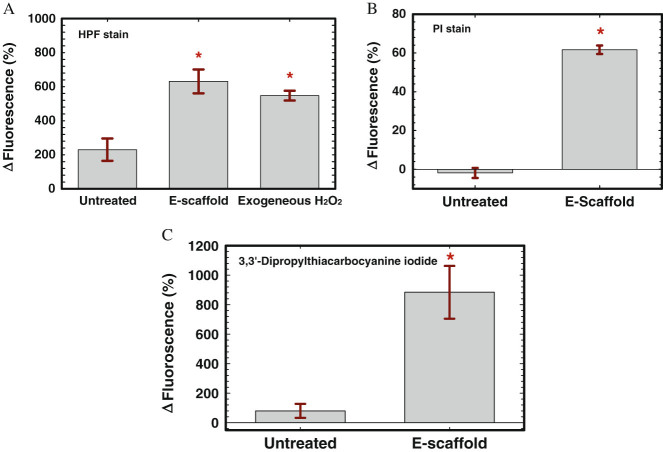
E-scaffold induces OH• generation and increases membrane permeability
We observed increased fluorescence for both e-scaffold treated and exogenous H2O2 treated biofilm cells, indicating enhanced OH• formation after e-scaffold generated H2O2 treatment (Fig. 3a). Furthermore, an increase in the propidium iodide (PI) fluorescence of e-scaffold treated cells was observed compared to untreated biofilm cells (Fig. 3b). PI is a membrane-impermeable dye and can only enter a bacterial cell if the outer membrane is damaged. It can permeate the cytoplasm and bind to the DNA, showing an increased fluorescence intensity, only upon disruption of the cell membranes by the e-scaffold.22,38 Thus, an increase in PI intensity corresponds to an increased membrane permeability. Figure 3b shows that PI permeability increased in e-scaffold treated cells, indicating possible damage to the outer cell membrane by the e-scaffold. Increased membrane permeability was additionally verified using 3,3′-dipropylthiacarbocyanine iodide, a membrane potential sensitive dye. An increase in the fluorescence intensity of 3,3′-dipropylthiacarbocyanine iodide was observed in e-scaffold treated cells compared to untreated biofilm cells (Fig. 3c), indicating membrane depolarization after exposure to e-scaffold. The dissipation of the membrane potential may be caused by the perturbation of the membrane lipid bilayers.39 Membrane potential sensitive dye 3,3′-dipropylthiacarbocyanine iodide enters only depolarized cells, where it binds reversibly to lipid-rich intracellular components.40 The increased fluorescence intensity of this dye in e-scaffold treated cells (Fig. 3c) indicates that the e-scaffold disrupted the cytoplasmic membrane and that this induced depolarization of the plasma membrane potential. Thus Fig. 3 suggests that the e-scaffold increased membrane permeability, possibly upon the membrane integrity of the cells being compromised by OH•.
Fig. 3.
E-scaffold increases OH• formation and membrane permeability. a Increase in fluorescence of HPF-stained e-scaffold treated and exogenous H2O2 treated P. aeruginosa PAO1 biofilm cells indicates increased OH• formation compared to untreated biofilms. b Increase in fluorescence of propidium iodide (PI) indicates increased membrane permeability of P. aeruginosa PAO1 cells after exposure to e-scaffold. c Increase in fluorescence of 3, 3′-dipropylthiacarbocyanine iodide, a membrane potential sensitive dye, in e-scaffold treated P. aeruginosa PAO1 cells verifies bacterial membrane depolarization by e-scaffold resulting in increased membrane permeability. Error bars represent standard errors of means for at least three biological replicates. The symbol * indicates a significant difference from the untreated biofilm cells (n = 3, P < 0.001; paired t-test)
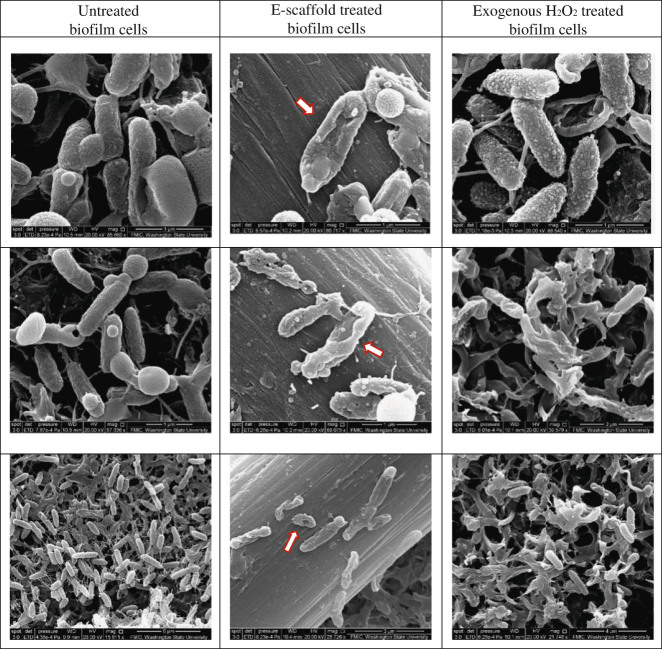
Additionally, membrane-compromised cells similar to those in exogenous H2O2 treated samples were observed in e-scaffold treated samples in SEM images (Fig. 4). Similar deformation was observed previously by Istanbullu et al. in cells treated with electrochemically generated H2O2 18 and by DeQueiroz et al. and Diao et al. in cells treated with exogenous H2O2.41 Untreated biofilm cells showed intact cell walls and membranes (Fig. 4). Thus, Fig. 4 indicates e-scaffold generated H2O2 induced morphological changes in cells and structural damage of the outer membrane that might increase membrane permeability.42,43
Fig. 4.
Scanning electron microscopy (SEM) images showing untreated, e-scaffold treated and exogenous H2O2 treated P. aeruginosa PAO1 biofilm cells. Three representative images are shown for each treatment. Red arrows indicate cells showing a stressed membrane
Discussion
Electrochemical method combined with antibiotic has been suggested to be effective against biofilms in several previous studies, yet the mechanism behind this increased antibiotic efficacy remains unclear.15,44 In this work, an e-scaffold that electrochemically generates a constant concentration H2O2 was investigated as a mean of enhancing antibiotic efficacy against biofilm cells and its efficacy against ciprofloxacin-tolerant persister cells was evaluated. Overall, our results indicate that the e-scaffold induced intracellular formation of OH• and improved membrane permeability. These mechanisms enhanced tobramycin efficacy, including against persister cells.
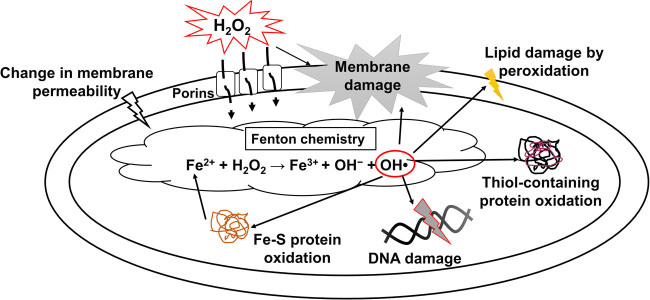
The biofilm removal mechanism of e-scaffolds is electrochemical generation of H2O2, which is a potential biocide and oxidizing agent.17 H2O2 mediates dispersal in biofilms, disrupts various bacterial processes and cellular networks, and disrupts the cell envelope through intracellular production of ROS such as OH• in Gram-negative bacteria, as shown in Fig. 5.26,28,45 Our observations suggest that e-scaffold generated H2O2 increases intracellular OH• formation in Gram-negative P. aeruginosa PAO1 biofilm cells. Furthermore, in membrane permeability assays and SEM image analysis, we observed increased permeability with moderate membrane damage in cells after e-scaffold treatment. Thus, we propose that when e-scaffold generated H2O2 enters a bacterial cell, it induces intracellular ROS production such as OH•, which can increase the permeability of bacterial membranes.26,27 Increased permeability can facilitate better antibiotic penetration. These effects can potentiate the tobramycin susceptibility of P. aeruginosa PAO1 biofilms and eradicate persister cells in various metabolic states.31,46
Fig. 5.
Proposed mechanism for e-scaffold generated H2O2 enhancing antibiotic susceptibility. H2O2 generated extracellularly by e-scaffold diffuses through the bacterial cell envelope and reacts with intracellular Fe2+, forming radicals that oxidize lipids, proteins and DNA, which prompts cell death. Through membrane damage or changing membrane permeability, antibiotic penetration into the bacterial cell is increased, which in turn enhances the antibiotic susceptibility of the cells
That the e-scaffold increases the permeability of the outer membrane of Gram-negative bacteria opens up the possibility of enhancing susceptibility to a range of antibiotics. As noted above, recent papers questioned the role of electric current in electrochemically generating H2O2 that enhanced antibiotic susceptibility in biofilms and associated persister cells.15,22,47 It has already been confirmed that an e-scaffold at a constant applied potential (−600 mVAg/AgCl) electrochemically generates H2O2, which is the mechanism of action for biofilm removal.17 Here, we explained that e-scaffold generated H2O2 can lead to intracellular OH• production in bacteria that possibly enhances the efficacy of tobramycin against P. aeruginosa PAO1 biofilms and thus eradicates biofilms and associated persister cells.
The observed effect of increased intracellular OH• formation as a possible mechanism for enhanced antibiotic susceptibility in bacteria needs to be further confirmed by more mechanistic studies. For instance, decreasing OH• through the addition of thiourea (150 mM), an OH• scavenger,48 inhibited bacterial cell death due to e-scaffold and tobramycin (Fig. S2, Supplementary information), indicating that OH• production may be critical for the observed bactericidal activity. Additionally, overexpression of cellular ROS scavengers such as superoxide dismutase (SOD) can be used to inhibit e-scaffold induced OH• formation and bacterial cell death. Thus, it can be verified whether OH• formation is a critical factor for the observed bacterial killing by e-scaffold and tobramycin. As indicated in Fig. 5, OH• formation occurs through the Fenton reaction and Fe2+ plays a vital role in this reaction. Testing the efficacy of e-scaffold and tobramycin against mutant strains with impaired iron regulation would be another way to confirm OH• formation as the fundamental mechanism for e-scaffold enhanced antibiotic susceptibility.38
Finally, this research proposes future use of the e-scaffold as an effective individual or adjuvant therapy against persistent biofilm infections. Notably, the application of AC and DC electric fields combined with antibiotics involves a different mechanism49 than that of the electrochemical biofilm control method proposed in this work.
Materials and methods
Growth medium, chemicals, and antibiotics
For all experiments, 20 g/L (1×) Luria broth (LB) medium (Sigma-Aldrich, catalog #L3522) was used to grow overnight P. aeruginosa PAO1 cultures and 1 g/L (0.05×) LB was used as the growth medium for biofilms. Tobramycin (Sigma Aldrich, catalog #T4014) and ciprofloxacin (Sigma Aldrich, catalog #17850) solutions were diluted in 1 g/L (0.05×) LB for antibiotic susceptibility experiments and persister cell isolation, respectively. Minimum inhibitory concentrations (MICs) were determined for both antibiotics following the Clinical and Laboratory Standards Institute (CLSI) protocol as detailed in Supplementary information.50 Other key compounds included 3′-p-hydroxyphenyl fluorescein dye (Invitrogen, catalog #H36004), propidium iodide (Invitrogen, catalog #L-7012), 3, 3′-dipropylthiacarbocyanine iodide (Sigma Aldrich, catalog #318434), thiourea (AK Scientific, Inc., catalog #S726) and H2O2 (VWR, catalog #RC3819-16).
Culture and biofilm preparation
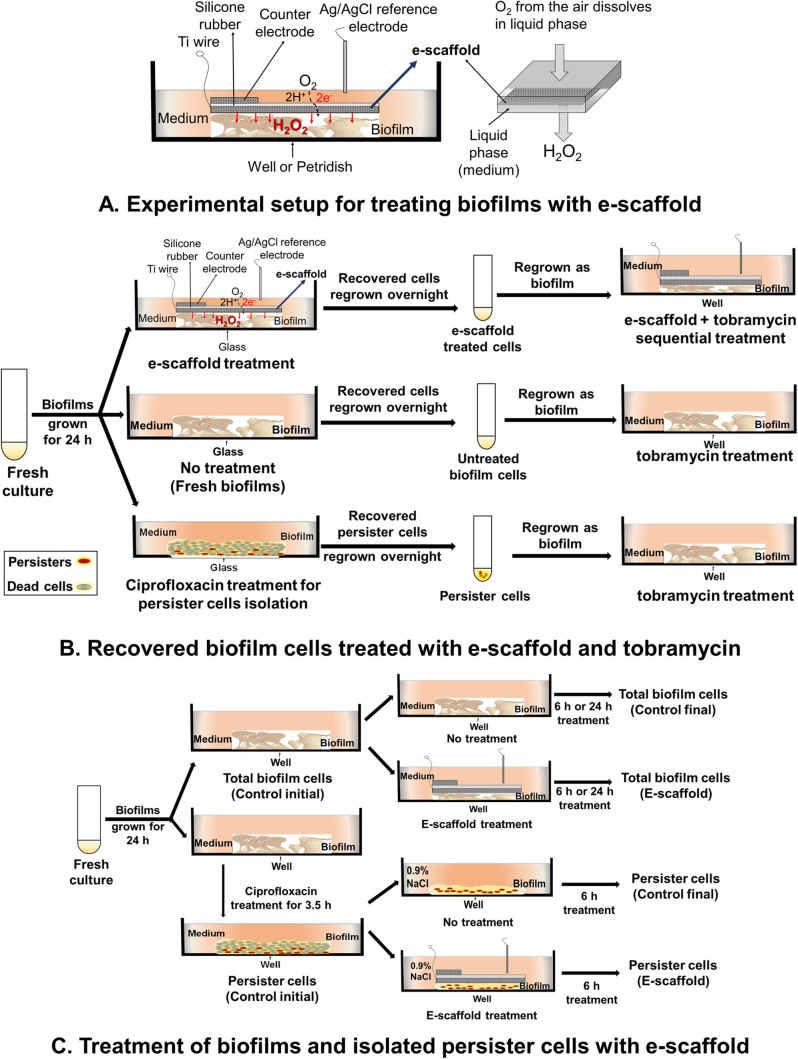
Frozen stocks of P. aeruginosa PAO1 were cultured overnight in LB at 37 °C on a rotating table (70 rpm). For biofilm experiments, overnight culture was adjusted to OD600 ≈ 0.5 in LB and used as inoculum.17 Briefly, 2 ml of culture was used to inoculate sterile glass bottom petri dishes (MatTek Corporation, catalog #P35G-1.5-20-C) and allowed to form biofilms for 24 h. These one-day-old biofilms were treated with e-scaffolds for 24 h, as described previously.17 Briefly, untreated and e-scaffold treated biofilm cells were washed twice to remove loosely attached cells and then remaining cells were recovered for antibiotic susceptibility testing. To identify and isolate P. aeruginosa PAO1 persister cells, planktonic culture was grown to the stationary phase and the dose-dependent killing curve for ciprofloxacin (0–200 μg/mL) was investigated (Supplementary information, Fig. S1).34 A plateau of the surviving subpopulation was observed for ciprofloxacin concentrations above 50 μg/mL (200× of MIC), and these were identified as persister cells.34,51 We assessed the antibiotic treatment on biofilms from three different populations: cells recovered after no treatment (“untreated biofilms”), cells recovered after e-scaffold treatment (“e-scaffold treated biofilm”) and “persister cells” that were isolated from biofilms treated with 200 μg/mL ciprofloxacin for 3.5 h according to a published protocol.34 For experiments with persister cells, the ciprofloxacin-treated biofilms were washed and refreshed with 0.9 % NaCl for subsequent experiments. This method for culture, biofilm preparation and treatments is illustrated in Fig. 6.
Fig. 6.
a Schematic of experimental setup for the treatment of biofilm exposed to an e-scaffold with an illustration of electrochemical H2O2 production. The electrodes are connected to a potentiostat (not shown in figure). Scientific Reports, 2015. 5, 14908. DOI: 10.1038/srep14908. © Creative Commons Attribution 4.0 International License. b Recovered biofilm cells treated with e-scaffold and tobramycin. c Treatment of biofilms and persister cells with e-scaffold
Biofilm treatment with e-scaffold
P. aeruginosa PAO1 biofilms were exposed to e-scaffold treatment for 24 h, as described previously.17 Briefly, a custom-built e-scaffold was fabricated using carbon fabric as detailed in Supplementary information.17 Biofilms were carefully washed (2×) with LB (0.05×) and an e-scaffold was overlaid onto the biofilms followed by 4 mL of fresh medium. A standard Ag/AgCl (saturated KCl) reference electrode was introduced to apply a constant potential (-600 mVAg/AgCl) to an e-scaffold using a Gamry Series G 300 potentiostat (Gamry Instruments, Warminster, PA, USA) to reduce oxygen, generate a low concentration of H2O2 and deliver it continuously to the biofilms (Fig. 6a). After treatment, the viable cell counts were determined using a modified drop-plate cell counting method.17,52 Loosely attached cells were removed by carefully washing the biofilms with 0.9 % NaCl before they were resuspended in 5 mL of 0.9 % NaCl and vortexed for 30 s. These suspensions were centrifuged (4180 × g for 10 min), and the resulting cell pellet was resuspended in 1 mL of 0.9 % NaCl. Aliquots (250 µL) were then serially diluted, and 10 µL of each dilution was plated onto LB agar. Plates were incubated for 24 h (37 °C), and colony-forming units (CFU) were enumerated.
Tobramycin susceptibility of e-scaffold treated cells
To determine whether e-scaffold treatment altered susceptibility to antibiotics,35 biofilm treatments were combined with tobramycin treatments. For this experiment, the recovered biofilm cells were harvested (Fig. 6b) and adjusted to OD600 ≈ 0.5 in LB medium (0.05×). A 1-mL cell suspension was used to inoculate a 24-well plate, where biofilms were allowed to form. Biofilms were treated with an e-scaffold for 2 h. Each well was then washed (2×) and challenged with 1 mL of one of the test concentrations (5, 10, 20 and 40 µg/mL) of tobramycin for 6 h. After treatment, the cells were washed (2×), resuspended in 0.9 % NaCl and processed to enumerate CFU.52 Cell counts were compared for e-scaffold and other treated and untreated biofilms. The treatments are summarized in Fig. 6b.
Effect of e-scaffold on persister cells
Biofilms were grown for 24 h in 6-well plates and treated with 200 μg/mL ciprofloxacin for 3.5 h to isolate persister cells from ciprofloxacin-treated biofilms.16 The total number of viable cells was determined for ciprofloxacin-treated and untreated biofilms using the modified drop-plate cell counting method.52 Remaining persister cells were then exposed to either 200 μg/ml ciprofloxacin or e-scaffold treatment for 6 h (in the 0.9 % NaCl solution). Final CFU numbers were then determined following the procedure described above. Figure 6c summarizes the treatment methodology we used.
Hydroxyl free radical detection assay
Intracellular hydroxyl free radical (OH•) formation was detected using 5 µM of a fluorescent reporter dye, 3′-(p-hydroxyphenyl fluorescein) (HPF) (Invitrogen, catalog #H36004) following a published protocol.38 Briefly, e-scaffold treated, exogenous H2O2-treated and untreated biofilm cells were vortexed in 500 µL of LB in a microcentrifuge tube for 30 s. These samples were centrifuged (10,000 × g for 10 min), and then the medium was replaced with a final concentration of 5 µM HPF prepared in 500 µL of 0.1 M PBS. After staining in the dark at room temperature for 15 min, samples were centrifuged at 10,000 × g for 10 min. Supernatant was removed, and cells were rinsed and resuspended with PBS. An aliquot (100 µL) was added to each well in a 96-well plate, and fluorescence intensity was quantified using a microplate reader (Bio-Tek Cytation 5) with excitation at 490 and emission at 515 nm. For the OH• formation assays, fluorescence was estimated as follows: ((Fluorescence with dye−Fluorescence without dye)/(Fluorescence without dye))×(100).
Membrane permeability
Change in bacterial membrane permeability was evaluated by analyzing the influx of a membrane-impermeable dye, propidium iodide (PI)22,38 according to the manufacturer’s instructions (Invitrogen, catalog #L-7012). E-scaffold treated and untreated biofilm cells were centrifuged (6000 rpm, 10 mins) to cell pellets and supernatant was poured off. The cell pellets were stained with PI for 15 min in the dark, then washed twice with 0.9 % NaCl to remove any unbound dye. Cells were then resuspended in 0.9 % NaCl, and 100 µL of each suspension was transferred in triplicate into wells of a 96-well plate. Fluorescence intensity was quantified (excitation 535 nm, emission 517 nm). Fluorescence was determined as a percentage change compared to untreated sample using the following formula: ((Fluorescence with dye−Fluorescence without dye)/(Fluorescence without dye))×(100).
Additionally, the effect of e-scaffold treatment on bacterial membrane permeability was detected using a membrane potential sensitive dye, 3, 3′-dipropylthiacarbocyanine iodide. The fluorescence intensity of 3, 3′-dipropylthiacarbocyanine iodide changes in response to changes in plasma membrane potential upon structural damage.42,53 Untreated biofilm cells were washed and then resuspended in buffer A (20 mM glucose, 5 mM HEPES, pH = 7.2) with 0.1 M KCl. To achieve a stable signal, 3,3′-dipropylthiacarbocyanine iodide was added to untreated and e-scaffold treated biofilm cell suspensions and incubated for 10 min. Changes in the fluorescence intensity induced by changes in membrane potential after exposure to e-scaffold were measured at an excitation wavelength of 622 nm and an emission wavelength of 670 nm.42 The measurements were performed in triplicate for three biological replicates in wells of a 96-well plate. Fluorescence was determined as a percentage change compared to untreated sample using the following formula: ((Fluorescence with dye−Fluorescence without dye)/(Fluorescence without dye))×(100).
Scanning electron microscopy
For scanning electron microscopy (SEM) imaging, biofilms were grown for 24 h on UV-sterilized, 0.22-µm type GV membrane filters (Millipore, catalog ID #551200401) placed in sterile 6-well plates. Exogenous H2O2 was added continuously at an average 0.008 mmol/h for 24 h to mimic the e-scaffold generated H2O2 treatment described previously.17 After treatment for 24 h, both e-scaffolds and membrane filters with biofilms were aseptically collected from the untreated, e-scaffold treated and exogenous H2O2 treated wells. The membrane filters and e-scaffolds were fixed overnight with 2.5 % glutaraldehyde and 2 % paraformaldehyde in 0.1 M phosphate buffer, followed by rinsing with 0.1 M phosphate buffer (3 × 10 min each). The membranes and e-scaffolds were then dehydrated gradually by being washed sequentially with 10, 30, 50, 70, and 95 % alcohol (10 min each) and 100 % alcohol (3 × 10 min each). Hexamethyldisilizane (HMDS) was used for overnight drying. Samples were then sputter-coated with gold prior to field emission in-lens scanning electron microscopy (FEISEM) (FEI 200F) imaging.
Statistical analysis
All experiments were conducted for at least three biologically independent replicates. Technical replicates were averaged to produce replicate means that were subsequently used for analysis. Mean values were compared within and between groups using one-way ANOVA followed by Bonferroni’s post hoc test for individual comparisons. Differences were considered statistically significant if P < 0.05 (Sigma plot, version 13, Systat Software, Inc., San Jose, CA).
Conclusions
In this work, we found that P. aeruginosa PAO1 biofilms treated with e-scaffolds do not develop tolerance to tobramycin; thus, e-scaffolds reduce persistence. Our results indicate that the e-scaffold enhances tobramycin susceptibility in P. aeruginosa PAO1 biofilms and eradicates isolated persister cells. This appears to be a promising advantage of the e-scaffold for controlling persister cells. We further showed that the e-scaffold induces intracellular OH• formation and causes an increase in membrane permeability and moderate morphological changes in the bacterial membrane. We propose these effects as the mechanism by which it enhances tobramycin efficacy. These results demonstrate the potential of the e-scaffold as an alternative to conventional antibiotic treatment or as an adjuvant therapy to enhance antibiotic susceptibility in complex and persistent biofilm infections.
Electronic supplementary material
Acknowledgements
The authors would like to thank Phuc Ha for SEM imaging. The authors are also grateful to the Franceschi Microscopy and Imaging Center of Washington State University for the use of their facilities and staff assistance. The authors would like to thank Mia Mae Kiamco for helping in e-scaffold preparation. This research was supported by NSF-CAREER award #0954186.
Author contributions
STS developed the hypothesis, designed the experiments, performed all the experiments and contributed to manuscript preparation. DRC contributed to the research plan and manuscript preparation. HB conceived the idea and contributed to the experimental design and manuscript preparation.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies the paper on the npj Biofilms and Microbiomes website (doi:10.1038/s41522-016-0003-0).
References
- 1.Lewis, K. in Annual review of microbiology Vol. 64 (eds Gottesman, S. & Harwood, C. S.), 357–372 (Annual Reviews, 2010)
- 2.Flemming H-C, Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 3.Stewart PS, Grab L, Diemer JA. Analysis of biocide transport limitation in an artificial biofilm system. J Appl. Microbiol. 1998;85:495–500. doi: 10.1046/j.1365-2672.1998.853529.x. [DOI] [PubMed] [Google Scholar]
- 4.Davenport EK, Call DR, Beyenal H. Differential protection from tobramycin by extracellular polymeric substances from Acinetobacter baumannii and Staphylococcus aureus Biofilms. Antimicrob. Agents Chemother. 2014;58:4755–4761. doi: 10.1128/AAC.03071-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole K. Stress responses as determinants of antimicrobial resistance in Gram-negative bacteria. Trends Microbiol. 2012;20:227–234. doi: 10.1016/j.tim.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Marx DE, Barillo DJ. Silver in medicine: the basic science. Burns. 2014;40:S9–S18. doi: 10.1016/j.burns.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Daviskas E, Anderson SD, Eberl S, Chan HK, Bautovich G. Inhalation of dry powder mannitol improves clearance of mucus in patients with bronchiectasis. Am. J. of Respir. Crit. Care Med. 1999;159:1843–1848. doi: 10.1164/ajrccm.159.6.9809074. [DOI] [PubMed] [Google Scholar]
- 8.Gurunathan S. Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J. Ind Eng. Chem. 2015;29:217–226. doi: 10.1016/j.jiec.2015.04.005. [DOI] [Google Scholar]
- 9.Barraud, N., Buson, A., Jarolimek, W. & Rice, S. A. Mannitol enhances antibiotic sensitivity of persister bacteria in pseudomonas aeruginosa biofilms. PLoS ONE8, e84220, doi:10.1371/journal.pone.0084220 (2013). [DOI] [PMC free article] [PubMed]
- 10.Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005;57:1539–1550. doi: 10.1016/j.addr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Trop M, et al. Silver coated dressing Acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J. Trauma Inj. Infect. Crit. Care. 2006;60:648–652. doi: 10.1097/01.ta.0000208126.22089.b6. [DOI] [PubMed] [Google Scholar]
- 12.Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Tkachenko O, Karas JA. Standardizing an in vitro procedure for the evaluation of the antimicrobial activity of wound dressings and the assessment of three wound dressings. J. Antimicrob. Chemother. 2012;67:1697–1700. doi: 10.1093/jac/dks110. [DOI] [PubMed] [Google Scholar]
- 14.Dawson CC, Intapa C, Jabra-Rizk MA. “Persisters”: survival at the cellular level. PLoS Pathog. 2011;7:e1002121. doi: 10.1371/journal.ppat.1002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Pozo JL, Rouse MS, Patel R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs. 2008;31:786–795. doi: 10.1177/039139880803100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niepa THR, Gilbert JL, Ren D. Controlling Pseudomonas aeruginosa persister cells by weak electrochemical currents and synergistic effects with tobramycin. Biomaterials. 2012;33:7356–7365. doi: 10.1016/j.biomaterials.2012.06.092. [DOI] [PubMed] [Google Scholar]
- 17.Sultana ST, et al. Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Sci. Rep. 2015;5:14908. doi: 10.1038/srep14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Istanbullu O, Babauta J, Hung Duc N, Beyenal H. Electrochemical biofilm control: mechanism of action. Biofouling. 2012;28:769–778. doi: 10.1080/08927014.2012.707651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart PS, et al. Effect of catalase on hydrogen peroxide penetration into Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2000;66:836–838. doi: 10.1128/AEM.66.2.836-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nodzo, S., et al. Cathodic voltage-controlled electrical stimulation plus prolonged vancomycin reduce bacterial burden of a titanium implant-associated infection in a rodent model. Clin. Orthp. Relat. Res.474, 1668–1675 (2016). [DOI] [PMC free article] [PubMed]
- 21.Lewandowski, Z. & Beyenal, H. Mechanisms of microbially influenced corrosion. 1–30 (Springer, Berlin, 2008).
- 22.Niepa THR, et al. Sensitizing Pseudomonas aeruginosa to antibiotics by electrochemical disruption of membrane functions. Biomaterials. 2016;74:267–279. doi: 10.1016/j.biomaterials.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochimica Et Biophysica Acta-Biomembranes. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol Microbiol. 2011;80:580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haber, F. & Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. A147, 332–351, doi:10.1098/rspa.1934.0221 (1934).
- 26.Imlay JA. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 27.Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mai-Prochnow A, et al. Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several gram-negative bacteria. J. Bacteriol. 2008;190:5493–5501. doi: 10.1128/JB.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart PS, Wattanakaroon W, Goodrum L, Fortun SM, McLeod BR. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 1999;43:292–296. doi: 10.1128/aac.43.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dwyer DJ, Kohanski MA, Collins JJ. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 2009;12:482–489. doi: 10.1016/j.mib.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl. Acad. Sci. USA. 2012;109:12147–12152. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart PS, Rayner J, Roe F, Rees WM. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl Microbiol. 2001;91:525–532. doi: 10.1046/j.1365-2672.2001.01413.x. [DOI] [PubMed] [Google Scholar]
- 33.Poole K, Russell AD, Lambert PA. Mechanisms of antimicrobial resistance: opportunities for new targeted therapies. Adv. Drug Deliv. Rev. 2005;57:1443–1445. doi: 10.1016/j.addr.2005.05.001. [DOI] [Google Scholar]
- 34.Möker N, Dean CR, Tao J. Pseudomonas aeruginosa increases formation of multidrug-tolerant persister cells in response to quorum-sensing signaling molecules. J. Bacteriol. 2010;192:1946–1955. doi: 10.1128/JB.01231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulcahy LR, Burns JL, Lory S, Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010;192:6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenfeld M, et al. Serum and lower respiratory tract drug concentrations after tobramycin inhalation in young children with cystic fibrosis. J. Pediatr. 2001;139:572–577. doi: 10.1067/mpd.2001.117785. [DOI] [PubMed] [Google Scholar]
- 37.Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001;183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013;5:11. doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu MH, Maier E, Benz R, Hancock REW. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M, et al. Cytochrome P-450 metabolites but not NO, PGI2, and H2O2 contribute to ACh-induced hyperpolarization of pressurized canine coronary microvessels. Am. J. Physiol.- Heart Circ. Physiol. 2003;285:H1939–H1948. doi: 10.1152/ajpheart.00190.2003. [DOI] [PubMed] [Google Scholar]
- 41.Diao HF, Li XY, Gu JD, Shi HC, Xie ZM. Electron microscopic investigation of the bactericidal action of electrochemical disinfection in comparison with chlorination, ozonation and Fenton reaction. Process Biochem. 2004;39:1421–1426. doi: 10.1016/S0032-9592(03)00274-7. [DOI] [Google Scholar]
- 42.Park S-C, et al. Amphipathic α-helical peptide, HP (2–20), and its analogues derived from Helicobacter pylori: pore formation mechanism in various lipid compositions. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2008;1778:229–241. doi: 10.1016/j.bbamem.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann M, et al. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010;54:3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sultana ST, Babauta JT, Beyenal H. Electrochemical biofilm control: a review. Biofouling. 2015;31:745–758. doi: 10.1080/08927014.2015.1105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 46.Albesa I, Becerra MC, Battan PC, Paez PL. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys. Res. Commun. 2004;317:605–609. doi: 10.1016/j.bbrc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Neoh KG, Hu X, Kang E-T. Mechanistic insights into response of Staphylococcus aureus to bioelectric effect on polypyrrole/chitosan film. Biomaterials. 2014;35:7690–7698. doi: 10.1016/j.biomaterials.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 48.Novogrodsky A, Ravid A, Rubin AL, Stenzel KH. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc. Natl Acad. Sci. USA. 1982;79:1171–1174. doi: 10.1073/pnas.79.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YW, et al. Effect of electrical energy on the efficacy of biofilm treatment using the bioelectric effect. NPJ Biofilms Microbiomes. 2015;1:15016. doi: 10.1038/npjbiofilms.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones RN, et al. Educational antimicrobial susceptibility testing as a critical component of microbiology laboratory proficiency programs: American Proficiency Institute results for 2007–2011. Diagn. Microbiol. Infect. Dis. 2013;75:357–360. doi: 10.1016/j.diagmicrobio.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 51.Cañas-Duarte SJ, Restrepo S, Pedraza JM. Novel protocol for persister cells isolation. PLoS One. 2014;9:e88660. doi: 10.1371/journal.pone.0088660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CY, Nace GW, Irwin PL. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods. 2003;55:475–479. doi: 10.1016/S0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 53.Cho J, et al. The antifungal activity and membrane-disruptive action of dioscin extracted from Dioscorea nipponica. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2013;1828:1153–1158. doi: 10.1016/j.bbamem.2012.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.