Abstract
Metastases claim more than 90% of cancer-related patient deaths and are usually seeded by a subset of circulating tumor cells shed off from the primary tumor. In circulation, circulating tumor cells are found both as single cells and as clusters of cells. The clusters of circulating tumor cells, although many fewer in number, possess much higher metastatic potential as compared to that of individual circulating tumor cells. In this review, we highlight recent insights into molecular mechanisms that can enable the formation of these clusters—(a) hybrid epithelial/mesenchymal phenotype of cells that couples their ability to migrate and adhere, and (b) intercellular communication that can spatially coordinate the cluster formation and provide survival signals to cancer cells. Building upon these molecular mechanisms, we also offer a possible mechanistic understanding of why clusters are endowed with a higher metastatic potential. Finally, we discuss the highly aggressive Inflammatory Breast Cancer as an example of a carcinoma that can metastasize via clusters and corroborates the proposed molecular mechanisms.
Introduction
Despite decades of advances in cancer biology, metastasis remains the primary reason for cancer-related deaths.1 Cancer metastasis is a multistep cascade in which cancer cells escape the primary organ, enter and typically travel through the lymph and/or blood vasculature, and then exit at distant organs, eventually colonizing and proliferating at these sites leading to largely incurable stage IV disease. The metastatic cascade is highly challenging for those breakaway cells, with extremely high rates of attrition—only an estimated 0.2% of disseminated tumor cells being able to successfully seed secondary tumors or metastases.2 Thus, the ability to initiate metastases is a key bottleneck during cancer progression and presents an ideal window for therapeutic targeting.3
The most well-studied mechanism proposed to facilitate metastasis is single-cell dissemination enabled by an Epithelial-to-Mesenchymal Transition (EMT). EMT is a process through which epithelial cells lose their traits of apico-basal polarity and cell-cell adhesion and gain migratory and invasive traits typical of mesenchymal cells that enable the blood-borne dissemination of carcinoma cells.4 Conversely, after reaching a distant organ, these cells have been proposed to undergo an MET (Mesenchymal to Epithelial Transition)—a reverse of EMT—to regain their traits of cell-cell adhesion and polarity to establish metastases.5 However, an indispensable role of EMT and MET has been called into question recently.6–8
Besides single-cell dissemination enabled by EMT, an alternative mechanism for metastasis that has emerged from recent studies is collective migration by clusters of Circulating Tumor Cells (CTCs). Although rare as compared to individually migrating CTCs, clusters of CTCs can individually form up to 50-times more metastases.9 In vivo experiments and clinical data across multiple cancer types demonstrate that these clusters typically contain fewer than 10 cells,10, 11 clearly suggesting that clustered migration provides emergent, i.e., or ‘whole is greater than sum of its parts’, advantage for metastasis. The prognostic value of CTC clusters can be gauged by clinical observations, where patients with CTC clusters circulating in their bloodstream have significantly worse overall and progression-free survival than those in whom only individually migrating single CTCs are found.9
Therefore, identifying the molecular mechanisms that can form and maintain these clusters is of paramount importance in tackling metastasis. In this review, we highlight recent work that offers novel insights into mechanisms that can contribute to cluster formation and ascribe heightened metastatic potential to them. We then focus on a highly aggressive disease—Inflammatory Breast Cancer (IBC)—that forms clustered lymphatic emboli as a major means of metastasis and note several lines of evidence suggesting distant metastases also occur via clusters. IBC thus can serve as a model system to emphasize the critical role of the described molecular mechanisms in forming and stabilizing circulating CTC clusters—the primary villains of metastasis.
Clusters of CTCs: their formation and entry into the circulation
The ability of tumor cell clusters to traverse the lung12 and their higher efficiency at forming metastases when injected intravenously in mice has been known for over four decades.13 New insights into how these clusters are formed have emerged from recent lineage tracing techniques that showed that CTC clusters are not usually formed by random collisions during circulation; rather they are launched as clusters into the bloodstream from the primary tumors9, 10 (Fig. 1a). Aceto et al. established two differently-colored tumors on the left and right mammary fat pad of immunodeficient mice and observed that 96% of CTC clusters in the bloodstream and 92% of the lung metastatic foci were singly-colored. In contrast, when differently colored tumor cells were co-injected in the same mammary fat pad, it gave rise to multicolor clusters and metastases. Along the same lines, Cheung et al. injected different colored cancer cells intravenously at different times, yet rarely observed multicolored metastases, arguing for the absence of intravascular aggregation events. Consequently, CTC clusters can seed polyclonal metastases,9, 10, 14 thereby contributing to intra-tumor heterogeneity at the metastatic site—a prognostic marker of poor survival across many diverse cancer types, independent of other clinical, pathologic, and molecular factors.15
Fig. 1.
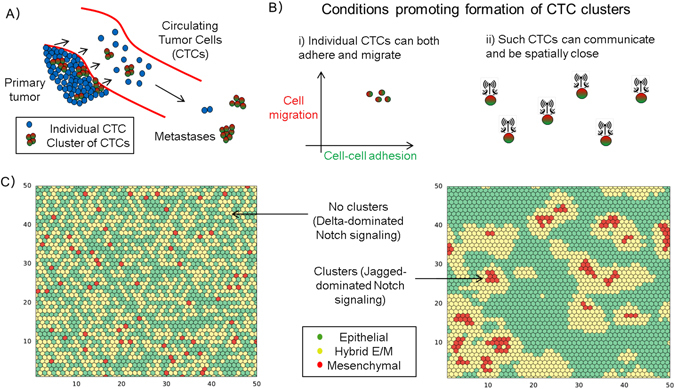
Clusters of Circulating Tumor Cells (CTCs) a Schematic illustrating that both individual CTCs and CTC clusters can be launched from the primary tumor into the bloodstream, and that clusters can form much more metastases. b Conditions that can foster formation of CTC clusters—(i) state of individual cells to be able to both migrate and adhere (i.e., hybrid E/M phenotype), and (ii) cell-cell communication among these cells to spatially rearrange to form a cluster. c Simulation of a layer of 50 × 50 cells that interact with one another via Notch signaling (left) predominantly via Delta ligands, and (right) predominantly via Jagged ligands. The color indicates the EMT status of each cell, as mentioned in the box (Adapted from Boareto et al. J R Soc Interface 2016 Fig. 4c, d 19)
Despite their significance, CTC clusters long have been believed to be incapable of traversing capillary-sized vessels. However, recent experiments using microfluidic devices illustrate that CTC clusters up to 20 cells can traverse constrictions with similar size to human capillary constrictions (5 to 10-μm) by rapidly reversibly reorganizing into single-file chain geometries.16 Similar reorganization of CTC clusters obtained from patients when transplanted in zebrafish further suggest that multicellular CTC clusters can travel as a unit from the primary tumor through the circulation to distant organs to seed metastases.16
These striking observations are reminiscent of a study on 3-D reconstruction of serial tissue sections. Bronsert et al.17 reconstructed the stromal border of various tumor types including invasive breast cancer and found little evidence of single cell migration. They concluded that cancer cell migration relies mostly, if not completely, on collective cell invasion, where invading cells retain at least partially physical cell-cell contacts. These cells that bud off from the primary tumor displayed some traits of EMT such as a morphological shift towards a spindle-like phenotype, decreased levels and membrane localization of E-cadherin, and increased nuclear levels of ZEB1, but their cell-cell adhesions were not completely lost. Therefore, these tumor buds were proposed to exhibit a ‘partial EMT’ or a hybrid epithelial/mesenchymal (E/M) phenotype instead of a completely mesenchymal phenotype.17, 18
Clusters of CTCs and a hybrid E/M phenotype: molecular similarities
In the context of metastasis, EMT has been defined as single-cell migration and/or invasion, along with loss of cell-cell adhesion.20 Overexpression of transcription factors inducing EMT such as TWIST has been shown to facilitate metastasis,21 but the role of EMT in metastasis remains controversial because a genetic knockdown of two transcription factors inducing EMT—TWIST and SNAIL-in genetically engineered mouse models was recently shown to be dispensable for metastasis.6 Understanding of the role of EMT in metastasis has been confounded by two interrelated issues—(a) tacit assumption that EMT is an ‘all-or-none’ process,22 and (b) lack of appreciation for the concept that the set of changes in cell behavior and/or lineage that have been labeled as EMT can be context dependent. For instance, EMT during embryonic development, but not during tumor progression, refers to a lineage switch from an epithelium to a mesenchyme.23, 24 Therefore, the concept of a partial EMT or a hybrid E/M phenotype has been invoked recently to highlight that cellular plasticity during collective invasion and metastasis can be extremely fine-tuned and therefore any attempts to bin CTC clusters in a binary manner of epithelial and mesenchymal traits can be counterproductive.24–26
Cells in a hybrid E/M phenotype retain at least some levels of E-cadherin—the loss of which is considered a hallmark of EMT—and co-express epithelial and mesenchymal markers and display an amalgamation of adhesion and migration to migrate collectively24 (Fig. 1b). Such co-expressing cells have been observed in primary tumors, metastatic tumors, cell lines, mouse models and CTCs belonging to multiple cancer types.24, 27–29 For example, H1975 cells maintain a stable hybrid E/M phenotype and migrate collectively forming finger-like projections in vitro29 and adhere closely upon capture by a CTC-chip.30 Collective migration of H1975 cells was disrupted on knockdown of GRHL2 or its downstream target OVOL2.29 Similar roles for GRHL2 and OVOL2 have been indicated in developmental EMT;31, 32 their inhibition abrogates collective cell migration during lung morphogenesis and mammary development, respectively. Thus, GRHL2 and OVOL2 can be considered as potential targets to break the CTC clusters.
Further analysis of the molecular signatures of CTC clusters and hybrid E/M phenotypes enable drawing a closer parallel between them. Cheung et al. demonstrated that JAG1 was one of the top differentially expressed genes in cells leading collective invasion.10 On the other hand, Boareto et al. suggested that high JAG can contribute to formation of clusters of CTCs by mediating intercellular communication between cells in a hybrid E/M phenotype19 (Fig. 1b, c). We also compared the levels of JAG and canonical epithelial (E) and mesenchymal (M) markers in individual CTCs and CTC clusters of breast cancer patients (Aceto et al.;9 GSE 51827). We observed that while both individual cells and CTC clusters tend to express both E and M markers, the expression of JAG was restricted to clusters (Fig. 2a). Further, co-expression of E and M markers is enriched in clusters as compared to single CTCs, and the cells expressing higher levels of JAG expressed both E and M markers (Fig. 2b), thereby bolstering that targeting JAG1 can interfere with cell-cell communication among cancer and/or stromal cells33 and hence break CTC clusters.
Fig. 2.
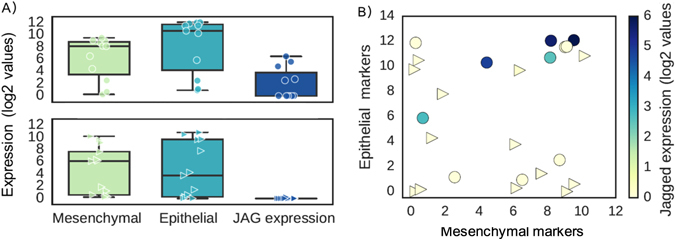
Expression pattern of 13 single CTCs and 11 CTC-clusters at single-cell level. a Expression level of mesenchymal (ZEB1, ZEB2, SNAI1, SNAI2, TWIST1, VIM, CD44) and epithelial markers (CDH1, EPCAM (Epithelial cell adhesion molecule), CD24) and Jagged (JAG1, JAG2) in individual CTCs (bottom) and CTC clusters (top). b Single cells are represented based on their levels of mesenchymal markers (x-axis) and epithelial markers (y-axis). Color code represents the expression values of the ligand Jagged. Gene counts table was directly downloaded from GSE repository (Aceto et al.;9 GSE 51827), and cells with less than 0.1 million reads were excluded from the analysis. Data was analyzed via Python and Jupyter Notebook web application (source code freely available at https://github.com/mboareto/CTC_RNAseq)
Other molecular markers expressed by cancer cells and leading to collective invasion in both mouse models and human breast tumors also have been reported to induce or maintain a hybrid E/M phenotype. For instance, these invasive cells express basal differentiation markers such as P-cadherin (CDH3) and p63,34 and knockdown of p63 is sufficient to block collective invasion. P-cadherin is a proposed marker of hybrid E/M phenotype.35 Overexpression of the transcription factor ΔNp63α in breast cancer cells can drive collective cell migration and invasion and can induce a hybrid E/M phenotype in basal-like breast cancer (BLBC) cells by both activating miR-205 that inhibits ZEB1/2 and elevating the levels of SLUG, an activator of ZEB36–38 (Fig. 3a, b). This coupling patterns is indicative of ΔNp63α acting as a ‘phenotypic stability factor’ (PSF) that can prevent the cells ‘that have gained partial plasticity’31 from undergoing a full EMT29 (i.e., single-cell migration and/or invasion) Additionally, ΔNp63α can trigger the secretion of matrix metalloproteinases (MMPs) to drive the invasive program.39 P-cadherin is a downstream target of ΔNp63α,35 and p63 gene can be activated by GRHL2,40 another PSF.29
Fig. 3.
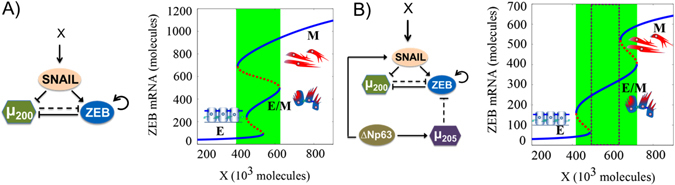
Dynamical system characteristics for miR-200/ZEB/SNAIL and miR-200/ZEB/SNAIL/ΔNp63α/miR-205 circuits. Bifurcation levels of ZEB1 mRNA in response to an external signal X driving a miR-200/ZEB/SNAIL, and b miR-200/ZEB/SNAIL/ΔNp63α/miR-205, representing the response of different circuits to varying levels of an EMT-inducing signal (shown as X). Solid blue curves denote stable states (phenotypes), while red dotted curves denote unstable states. Lower ZEB mRNA levels (<150 molecules) represent an epithelial (E) state, intermediate ZEB mRNA levels (~ 200–400 molecules) correspond to a hybrid E/M state, and higher ZEB mRNA levels (>500 molecules) denote a mesenchymal (M) state. Cartoons have been added alongside for the corresponding phenotypes. For low levels of X, cells can attain only an E state. With increasing levels of X, cells can undergo partial EMT to attain a hybrid E/M state. Further increase in X drives a complete EMT to a M state. The region marked in green represents the range of levels of X for which the hybrid E/M phenotype can exist as one of the multiple possible phenotypes; and that marked by dotted rectangle denotes the levels of X for which the hybrid E/M phenotype can exist alone. Note that the introduction of a ‘phenotypic stability factor (PSF)’ such as ΔNp63α has dramatically broadened the allowable range for a hybrid E/M phenotype. More importantly, it enabled the existence of a region where most cells can maintain a hybrid E/M state stably, i.e., a flow cytometry analysis of the region shown in dotted rectangle will identify that most cells co-express epithelial and mesenchymal markers, thereby displaying a hybrid E/M phenotype. Details of the mathematical models for both these circuits used to obtain these bifurcation diagrams are given in the Supplementary Information
Furthermore, the expression of P-cadherin (CDH3) correlates with two PSFs—GRHL2 and its target OVOL2, and the overexpression of one or more of them can predict poor overall survival, and progression-free survival across multiple cancer types.29 These observations are consistent with the prognostic power of a combined set of epithelial markers (cytokeratins 8 and 18) and mesenchymal markers (vimentin, fibronectin). Such co-expression, instead of the expression of mesenchymal markers solely, in invasive cancers like BLBC correlates with enhanced metastatic potential and poor survival,24 high histologic grade, lymphovascular invasion, and can be an independent prognostic factor.41 Cells co-expressing epithelial and mesenchymal markers are most enriched in highly aggressive cancers such as triple-negative breast cancer.24, 42 Similarly, P-cadherin is aberrantly overexpressed in local advanced IBC (see below) and other highly metastatic breast cancer cells such as 4T1.43 Put together, these results strongly suggest that a hybrid E/M phenotype can be the hallmark of collective invasion of tumors and lead to tumor aggressiveness.
It should be noted that a hybrid E/M phenotype can also be observed at a population level, i.e., in biphasic carcinomas such as carcinosarcomas that are comprised of distinct carcinomatous and sarcomatous elements components that are clonally connected.44 An epithelial morphology of the emboli and metastases of carcinosarcomas further reinforce the idea that a partial retention of epithelial traits is critical for cells to exhibit metastatic potential.24, 45–49 However, as discussed earlier, some cancers may metastasize largely via an overt single-cell EMT-MET route, for instance, metaplastic carcinomas of pure sarcomatoid subtype may also display dismal prognosis.50
Molecular mechanisms underlying higher metastatic potential of CTC clusters
Multiple factors can contribute to higher metastatic potential of CTC clusters, many of which tend to correlate with EMT, such as the ability to respond more effectively to mechanical signals and chemical gradients in primary tumor microenvironment as compared to individually migrating cells,51–53 protection against apoptosis in the bloodstream upon detachment from the ECM and/or other cells,10, 54 evasion from immune attacks due to the presence of immune cells in CTC clusters and/or altered surface markers of cancer cells,11, 55 potential cooperation among the heterogeneous cell types in clusters42 during or before entering into circulation. Moreover, CTCs, including those in clusters, can produce numerous enzymes such as MMPs56 that destroy basement membrane components,39, 57 providing access to the distant tissue once lodged at different sites, therefore potentially obviating the need to extravasate actively. However, all these advantages pertain to the steps of metastatic cascade before colonization—the limiting step in metastasis formation. How do CTC clusters overcome this last and most critical bottleneck?
A key property that can explain the colonizing potential of clusters is the high tumor-initiation potential associated with a hybrid E/M phenotype, as highlighted by multiple recent studies. Grosse-Wilde et al.48 segregated E, M, and hybrid E/M subpopulations of HMLER cells in vitro and observed that hybrid E/M cells can form up to 10-times more mammospheres than either E or M cells. Ruscetti et al.58 isolated hybrid E/M cells in vivo and demonstrated that their tumor-initiation potential was comparable to or even higher than that of the mesenchymal cells. They further illustrated that upon culturing E, hybrid E/M and M cells separately, while a majority of E and M cells retain their phenotype, more than 70% of hybrid E/M cells transition into either E or M in 24 h, suggesting their high plasticity. Similar observations have been made in silico45 and in primary ovarian cultures and tumors.59 These results strongly bolster the emerging notion that the ‘stemness window’ or ‘tumor-initiating window’ is mostly positioned midway on the ‘EMT axis’ with E and M phenotypes as its two ends.46, 47
On a molecular level, hybrid E/M cells have been shown to co-express CD24 and CD44 (CD24hi CD44hi signature).48 CD24hiCD44hi cells are present in multiple breast cancer cell lines, and their population is enriched significantly on exposure to acute chemotherapy assault, suggesting that these cells represent a drug-tolerant subpopulation capable of repopulating an entire tumor.60 These cells have upregulated JAG1 levels but lower DLL4 levels, implicating JAG1—already described as a key target to possible break CTC clusters—in mediating chemoresistance.19 Furthermore, we observed that HDAC inhibitors that can induce de-differentiation of cancer cells into tumor-initiating cells and increase the mammosphere formation efficiency and ALDH activity in metaplastic SUM159 cells61 also expands the CD24hi CD44hi subpopulation (Fig. 4). Put together, these studies exemplify an overlap of molecular mechanisms contributing to a hybrid E/M phenotype or CTC clusters and those mediating chemoresistance and/or metastasis-initiation.
Fig. 4.
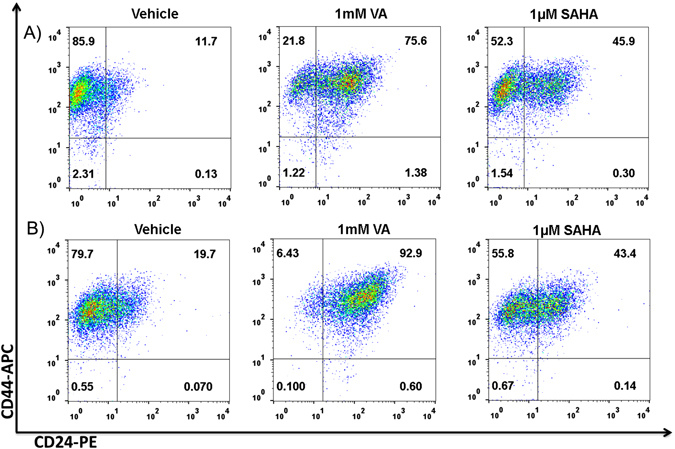
HDAC inhibitors expand the CD44+/CD24+ subpopulation. Flow cytometry analysis of expression of CD24 and CD44 in a ALDH−and b ALDH+ SUM159 cells showing a shift from the CD44+/CD24− in the vehicle-treated to a CD44+/CD24+ population in 1 mM VA or 1 µM SAHA treated cells. Representative flow cytometry data is shown. Gating was set to unstained control cells.61 For analyzing the CD44/CD24 subpopulation, cells were harvested with trypsin, centrifuged and re-suspended in phosphate buffered saline (PBS) (105 cells/ml). Cells were incubated with APC-conjugated CD44 and PE-conjugated CD24 antibodies (BD Biosciences, San Diego, CA, USA) for 30 min in ice at concentrations recommended by the manufacturer. Cells incubated in PBS and cells single-stained with APC-conjugated or PE-conjugated antibodies served as controls. Cell analysis for the expression of CD44 and CD24 was performed using a Beckman Coulter machine and the data files were analyzed using FlowJo software (Treestar, Ashland, OR)
IBC: a model for clustered dissemination
IBC is a highly aggressive locally advanced breast cancer with poor prognosis. In the USA, although it constitutes only 2–4% of breast cancer cases, IBC patients account for 10% of breast cancer related mortality annually. IBC patients typically have swelling and redness in skin and skin edema, instead of a mass detected by mammography.62 At the time of diagnosis, most IBC patients already show signs of lymph node metastasis, and 30% have distant metastases, as compared to 5% of patients in non-IBC breast cancers.63 Many molecular and behavioral aspects of IBC suggest it to be an ideal model system that manifests the traits of a hybrid E/M phenotype, collective cell invasion, and consequent aggressiveness.
A key difference in IBC and non-IBC is the ubiquitous presence of E-cadherin in primary tumors, tumor emboli or clusters in the lymphatic system, and metastases,64, 65 a presence which might appear paradoxical given the established role of E-cadherin as a metastasis suppressor in a variety of cancers.66 On the contrary, E-cadherin appears to augment the invasion of SUM 149 cells, an IBC cell line, by increasing the levels of matrix metalloprotease enzymes such as MMP-1 and MMP-9,66 suggesting that E-cadherin can promote IBC progression. Recent characterization of metastatic breast cancer cells show that they retain some levels of epithelial adhesion genes such as E-cadherin10, 67 and that E-cadherin is essential for leveraging the advantages of the osteogenic niche and consequent colonization of bone by breast cancer cells, thereby providing potential mechanistic insights into the role of E-cadherin in IBC.68
Another hallmark of IBC is the presence of numerous cohesive clusters in the lymphatics and their resistance to multiple therapies.66 As compared to non-IBC patients, IBC patients can have larger and a higher frequency of clusters of CTCs and these clusters have a stronger association with survival,69 potentially due to their resistance to severe therapeutic assaults.70 These clusters or emboli have accumulation of E-cadherin due to its altered trafficking,71 accumulation which may aid the passive dissemination of these emboli.72 Passive dissemination can facilitate metastasis in various contexts,73 and investigating active vs. passive dissemination mechanisms during intravasation can help reconcile the controversy on the role of EMT in metastasis.74
Transfection of dominant negative E-cadherin in MARY-X, a mouse model for IBC that exhibits tight aggregates of individual tumor cells held by E-cadherin in suspension, reduces the formation of these emboli,75 indicating a role for E-cadherin in maintaining the clustered phenotype. These results are reminiscent of implications of E-cadherin in collective chemotaxis in vivo,76 and that knockdown of GRHL2 or OVOL2—top activators of E-cadherin77—disrupt collective finger-like motion in vitro in lung cancer cells.29 Another potential mechanism through which E-cadherin can drive aggressive behavior is the survival of clusters in the bloodstream by ‘synoikis’, i.e., activation of survival signals through junctional adhesions between neighboring cells.78
Besides maintaining E-cadherin levels, IBC cells often also express mesenchymal proteins such as vimentin, thereby adding to the interpretation of IBC as a manifestation of a hybrid E/M phenotype79—(a) FC-IBC-02 cells express vimentin alongside E-cadherin and other markers of epithelial phenotype such as EpCAM, (b) compared to MDA-MB-231, multiple IBC cell lines—MDA-IBC-3, SUM 190, FC-IBC-02, and SUM149—have intermediate levels of ZEB1, a proposed marker for hybrid E/M phenotype80 (c) FC-IBC-02 cells both in mammospheres and adherent conditions express SLUG (SNAI2), a key mediator of a partial EMT state during mammary morphogenesis,81 and JAG1 that can contribute in maintaining a cluster of cells in a hybrid E/M phenotype.19 Furthermore, FACS analysis of cell line and mouse models of IBC—SUM 149, Mary-X, FC-IBC-01, and FC-IBC-02—indicates that a large percentage of cells are CD24hi CD44hi,82 the proposed signature for a hybrid E/M phenotype.24, 48 In contrast, mesenchymal cells such as MDA-MB-231 predominantly express CD44 (a mesenchymal stem cell marker) but lack CD24 (an epithelial marker).82
The predominance of CD24hi CD44hi cells can be an important underlying reason for resistance of IBC against chemotherapy and radiotherapy. CD24hi CD44hi cells represent the adaptive drug tolerant population that is enriched upon treatment of multiple breast cancer cell lines with docetaxel.60 These cells indicate a higher proclivity for Notch-Jagged signaling instead of Notch-Delta signaling.19 These observations are consistent with those showing that drug-resistant small cell lung cancer H69-AR cells have higher levels of JAG1 but lower levels of DLL4 as compared to the parental H69 population.83 Significantly high levels of IL-6 in serum of IBC patients as compared to non-IBC patients, overexpression of IL-6 in IBC carcinoma tissues, and secretion of IL-6 by IBC cell lines SUM149 and SUM19084, 85 and supporting stromal cells86 can augment Notch-Jagged signaling, thereby contributing to radiation resistance of these cell lines87 and IBC progression. Notch-Jagged signaling can also mediate high tumor-initiating potential in IBC. Lymphovascular emboli of MARY-X shows an addiction for Notch 3, and its knockdown can induce apoptosis and inhibit the levels of a stem cell marker CD133. The emboli of human IBC exhibits immunoreactivity for both cleaved Notch 3 intracellular domain and stem cell markers such as ALDH1.88
Multiple lines of evidence indicate that an induced transition from a hybrid E/M profile of IBC to a fully mesenchymal phenotype can reduce IBC aggressiveness. For instance, overexpression of ZEB1 or knockdown of E-cadherin, either of which can induce and often maintain a complete EMT phenotype,80, 89 reduced the in vivo growth of SUM 149 primary and metastatic tumors.90 E-cadherin knockdown also reduced the in vivo growth of 4T1 and MARY-X cells.90 Similarly, invasion of IBC cells is disrupted by exposure to TGFβ,91 a potent inducer of EMT that can induce a complete EMT89 and consequently single-cell migration phenotype.92 Consistently, a common feature of multiple pre-clinical models of IBC, independent of subtype, is the expression of SMAD6, a repressor of TGFβ signaling by its ability to inhibit SMAD4.82 Furthermore, high incidence of brain metastasis as reported in IBC patients63 can be reduced by knockdown of miR-141—a miR-200 family member that prevents EMT induction suggesting that a complete mesenchymal phenotype lacks the potential to colonize the brain.93
Conclusion
Overall, observations in IBC mouse and cell line models and in vivo experiments on CTC clusters challenge the hypothesis that a total loss of E-cadherin is necessary for metastasis. Such loss of epithelial markers has often been considered synonymous of an EMT, therefore these results question the indispensability of at least a complete abrogation of epithelial traits, and rather strongly suggest a potentially crucial role of partial retention of epithelial traits (hybrid E/M phenotype) in establishing metastasis, at least in IBC. For instance, limited E-cadherin levels at the adherens junctions of cells in a CTC cluster can orchestrate synoikis and therefore prevent CTC clusters from death in circulation.
Electronic supplementary material
Acknowledgements
This work was supported by National Science Foundation (NSF) Center for Theoretical Biological Physics (NSF PHY-1427654) and NSF DMS-1361411 (HL), the National Institutes of Health R01CA138239-01 and R01CA180061-01 (WAW), R21 CA188672-01 (BGD), National Institutes of Health Grant P01 CA098912 (MCFC), The State of Texas Grant for Rare and Aggressive Breast Cancer Research Program and The MD Anderson Morgan Welch IBC Clinic and Research Program. H.L. is also supported by CPRIT (Cancer Prevention and Research Institute of Texas) Scholar in Cancer Research of the State of Texas at Rice University. Research in Aceto lab (NA) is supported by the European Research Council (ERC), the Swiss National Science Foundation (SNSF), the Swiss Cancer League, the Basel Cancer League, the L. & Th. La Roche Foundation, the two Cantons of Basel through the ETH Zürich, and the University of Basel. MKJ is also supported by a training fellowship from the Gulf Coast Consortia on Computational Cancer Biology Training Program (CPRIT Grant No. RP170593).
Author contributions
M.K.J., H.L., M.C.F.C. developed the initial concept for this article; M.K.J., M.B., B.G.D. performed research and prepared figures; M.K.J. wrote the first draft; all authors contributed significantly to subsequent drafts.
Competing interests
The authors declare that they have no competing financial interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies the paper on the npj Breast Cancer website (doi:10.1038/s41523-017-0023-9).
Contributor Information
Mohit Kumar Jolly, Email: mkjolly.15@gmail.com.
Herbert Levine, Email: herbert.levine@rice.edu.
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Luzzi KJ, et al. Multistep nature of metastatic inefficiency. Am. J. Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celià-Terrassa T, Kang Y. Distinctive properties of metastasis- initiating cells. Genes Dev. 2016;30:892–908. doi: 10.1101/gad.277681.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer KR, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somarelli JA, et al. Distinct routes to metastasis: plasticity-dependent and plasticity-independent pathways. Oncogene. 2016;35:4302–4311. doi: 10.1038/onc.2015.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung KJ, et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. 2016;113:E854–E863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarioglu AF, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat. Methods. 2015;12:685–691. doi: 10.1038/nmeth.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeidman I, Buss JM. Transpulmonary passage of tumor cell emboli. Cancer Res. 1952;12:731–733. [PubMed] [Google Scholar]
- 13.Liotta LA, Klelnerman J, Saldel GM. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 14.Maddipati R, Stanger BZ. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 2015;5:1086–1097. doi: 10.1158/2159-8290.CD-15-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris LGT, et al. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7:10051–10063. doi: 10.18632/oncotarget.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Au SH, et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. 2016;113:4947–4952. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronsert P, et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer-host interface. J. Pathol. 2014;234:410–422. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- 18.Grigore A, Jolly MK, Jia D, Farach-Carson M, Levine H. Tumor budding: the name is EMT. Partial EMT. J. Clin. Med. 2016;5:51. doi: 10.3390/jcm5050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boareto M, et al. Notch-Jagged signaling can give rise to clusters of cells exhibiting a hybrid epithelial/mesenchymal phenotype. J. R. Soc. Interface. 2016;13:20151106. doi: 10.1098/rsif.2015.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung KJ, Ewald AJ. Illuminating breast cancer invasion: diverse roles for cell-cell interactions. Curr. Opin. Cell. Biol. 2014;30:99–111. doi: 10.1016/j.ceb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Kang Y. Probing the fifty shades of EMT in metastasis. Trends Cancer. 2016;2:65–66. doi: 10.1016/j.trecan.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung KJ, Ewald AJ. Illuminating breast cancer invasion: diverse roles for cell-cell interactions. Curr. Opin. Cell. Biol. 2014;30:99–111. doi: 10.1016/j.ceb.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolly MK, et al. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front. Oncol. 2015;5:155. doi: 10.3389/fonc.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diepenbruck M, Christofori G. Epithelial – mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr. Opin. Cell. Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Jeevan DS, Cooper JB, Braun A, Murali RAJ, Jhanwar-Uniyal M. Molecular pathways mediating metastases to the brain via epithelial-to-mesenchymal transition: genes, proteins, and functional analysis. Anticancer Res. 2016;36:523–532. [PubMed] [Google Scholar]
- 28.Andriani F, et al. Conversion to stem-cell state in response to microenvironmental cues is regulated by balance between epithelial and mesenchymal features in lung cancer cells. Mol. Oncol. 2016;10:253–271. doi: 10.1016/j.molonc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolly MK, et al. Stability of the hybrid epithelial/mesenchymal phentoype. Oncotarget. 2016;7:27067–27084. doi: 10.18632/oncotarget.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, et al. Expansion of CTCs from early stage lung cancer patients using a microfluidic co-culture model. Oncotarget. 2014;5:12383–12397. doi: 10.18632/oncotarget.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe K, et al. Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by Ovol2 transcriptional repressor. Dev. Cell. 2014;29:59–74. doi: 10.1016/j.devcel.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varma S, et al. The transcription factors grainyhead-like 2 and NK2-homeobox 1 form a regulatory loop that coordinates lung epithelial cell morphogenesis and differentiation. J. Biol. Chem. 2012;287:37282–37295. doi: 10.1074/jbc.M112.408401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Front. Oncol. 2014;4:254. doi: 10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung KJ, Ewald AJ. A collective route to metastasis: Seeding by tumor cell clusters. Science. 2016;352:167–169. doi: 10.1126/science.aaf6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro AS, Paredes J. P-Cadherin linking breast cancer stem cells and invasion: a promising marker to identify an ‘intermediate/metastable’ EMT state. Front. Oncol. 2015;4:371. doi: 10.3389/fonc.2014.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dang TT, Esparza MA, Maine EA, Westcott JM, Pearson GW. ∆Np63α promotes breast cancer cell motility through the selective activation of components of the epithelial-to-mesenchymal transition program. Cancer Res. 2015;75:3925–3935. doi: 10.1158/0008-5472.CAN-14-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarrió D, et al. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer. 2009;9:74. doi: 10.1186/1471-2407-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plutoni C, et al. P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J. Cell. Biol. 2016;212:199–217. doi: 10.1083/jcb.201505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodillinsky C, et al. p63/MT1-MMP axis is required for in situ to invasive transition in basal-like breast cancer. Oncogene. 2016;35:344–357. doi: 10.1038/onc.2015.87. [DOI] [PubMed] [Google Scholar]
- 40.Mehrazarin S, et al. p63 gene is regulated by grainyhead-Like 2 (GRHL2) through reciprocal feedback and determines epithelial phenotype in human keratinocytes. J. Biol. Chem. 2015;290:19999–20008. doi: 10.1074/jbc.M115.659144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae YK, Choi JE, Kang SH, Lee SJ. Epithelial-mesenchymal transition phenotype is associated with clinicopathological factors that indicate aggressive biological behavior and poor clinical outcomes in invasive breast cancer. J. Breast Cancer. 2015;18:256–263. doi: 10.4048/jbc.2015.18.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu M, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paredes J, et al. Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. J. Clin. Pathol. 2008;61:856–862. doi: 10.1136/jcp.2007.052704. [DOI] [PubMed] [Google Scholar]
- 44.Somarelli JA, Boss M-K, Epstein JI, Armstrong AJ, Garcia-Blanco MA. Carcinosarcomas: tumors in transition? Histol. Histopathol. 2015;30:673–687. doi: 10.14670/HH-30.673. [DOI] [PubMed] [Google Scholar]
- 45.Jolly MK, et al. Towards elucidating the connection between epithelial–mesenchymal transitions and stemness. J. R. Soc. Interface. 2014;11:20140962. doi: 10.1098/rsif.2014.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ombrato L, Malanchi I. The EMT universe: space between cancer cell dissemination and metastasis initiation. Crit. Rev. Oncog. 2014;19:349–361. doi: 10.1615/CritRevOncog.2014011802. [DOI] [PubMed] [Google Scholar]
- 47.Jolly MK, et al. Coupling the modules of EMT and stemness: a tunable ‘stemness window’ model. Oncotarget. 2015;6:25161–25174. doi: 10.18632/oncotarget.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grosse-Wilde A, et al. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS ONE. 2015;10:e0126522. doi: 10.1371/journal.pone.0126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bierie, B. et al. Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc. Natl Acad. Sci. USA114, E2337–E2346 (2017) [DOI] [PMC free article] [PubMed]
- 50.Lester TR, et al. Metaplastic sarcomatoid carcinoma of the breast appears more aggressive than other triple receptor-negative breast cancers. Breast Cancer Res. Treat. 2012;131:41–48. doi: 10.1007/s10549-011-1393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett. 2013;341:9–15. doi: 10.1016/j.canlet.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 52.Camley BA, Zimmermann J, Levine H, Rappel W-J. Emergent collective chemotaxis without single-cell gradient sensing. Phys. Rev. Lett. 2016;116:98101. doi: 10.1103/PhysRevLett.116.098101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camley BA, Zimmermann J, Levine H, Rappel WJ. Collective signal processing in cluster chemotaxis: roles of adaptation, amplification, and co-attraction in collective guidance. PLoS Comput. Biol. 2016;12:1–28. doi: 10.1371/journal.pcbi.1005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang RY-J, et al. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530) Cell Death Dis. 2013;4:e915. doi: 10.1038/cddis.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tripathi SC, et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc. Natl. Acad. Sci. 2016;113:E1555–E1564. doi: 10.1073/pnas.1521812113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray NP, Reyes E, Tapia P, Badínez L, Orellana N. Differential expression of matrix metalloproteinase-2 expression in disseminated tumor cells and micrometastasis in bone marrow of patients with nonmetastatic and metastatic prostate cancer: theoretical considerations and clinical implications-an immunocy. Bone Marrow Res. 2012;2012:259351. doi: 10.1155/2012/259351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grindel BJ, et al. Matrilysin / matrix metalloproteinase-7 (MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 2014;7:64–76. doi: 10.1016/j.matbio.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruscetti M, Quach B, Dadashian EL, Mulholland DJ, Hong W. Tracking and functional characterization of epithelial-mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Cancer Res. 2015;75:2749–2759. doi: 10.1158/0008-5472.CAN-14-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strauss R, et al. Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res. 2009;69:5115–5125. doi: 10.1158/0008-5472.CAN-09-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldman A, et al. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat. Commun. 2015;6:6139. doi: 10.1038/ncomms7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Debeb BG, et al. Histone deacetylase inhibitors stimulate dedifferentiation of human breast cancer cells through WNT/??-catenin signaling. Stem Cells. 2012;30:2366–2377. doi: 10.1002/stem.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodward WA. Inflammatory breast cancer: unique biological and therapeutic considerations. Lancet Oncol. 2015;16:e568–e576. doi: 10.1016/S1470-2045(15)00146-1. [DOI] [PubMed] [Google Scholar]
- 63.Warren LE, et al. Inflammatory breast cancer and development of brain metastases: risk factors and outcomes. Breast Cancer Res. Treat. 2015;151:225–232. doi: 10.1007/s10549-015-3381-8. [DOI] [PubMed] [Google Scholar]
- 64.Kleer CG, van Golen KL, Braun T, Merajver SD. Persistent E-cadherin expression in inflammatory breast cancer. Mod. Pathol. 2001;14:458–464. doi: 10.1038/modpathol.3880334. [DOI] [PubMed] [Google Scholar]
- 65.Colpaert CG, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br. J. Cancer. 2003;88:718–725. doi: 10.1038/sj.bjc.6600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez FJ, Lewis-Tuffin LJ, Anastasiadis PZ. E-cadherin’s dark side: possible role in tumor progression. Biochim. Biophys. Acta. 2012;1826:23–31. doi: 10.1016/j.bbcan.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27:193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mu Z, et al. Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res. Treat. 2015;154:563–571. doi: 10.1007/s10549-015-3636-4. [DOI] [PubMed] [Google Scholar]
- 70.Wang, C. et al. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res. Treat. (2016). doi:10.1007/s10549-016-4026-2. [DOI] [PubMed]
- 71.Ye Y, et al. E-cadherin accumulation within the lymphovascular embolus of inflammatory breast cancer is due to altered trafficking. Anticancer Res. 2010;30:3903–3910. [PubMed] [Google Scholar]
- 72.Alpaugh ML, Tomlinson JS, Kasraeian S, Barsky SH. Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene. 2002;21:3631–3643. doi: 10.1038/sj.onc.1205389. [DOI] [PubMed] [Google Scholar]
- 73.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444–448. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang JH, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62:452–465. doi: 10.1002/hep.27760. [DOI] [PubMed] [Google Scholar]
- 75.Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin / α, β -catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61:5231–5241. [PubMed] [Google Scholar]
- 76.Cai D, et al. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimamura T, et al. A novel network profiling analysis reveals system changes in epithelial-mesenchymal transition. PLoS ONE. 2011;6:e20804. doi: 10.1371/journal.pone.0020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am. J. Pathol. 2004;165:1315–1329. doi: 10.1016/S0002-9440(10)63390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez SV, et al. Inflammatory breast cancer (IBC): clues for targeted therapies. Breast Cancer Res. Treat. 2013;140:23–33. doi: 10.1007/s10549-013-2600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu M, Jolly MK, Levine H, Onuchic JN, Ben-Jacob E. MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc. Natl. Acad. Sci. USA. 2013;110:18144–18149. doi: 10.1073/pnas.1318192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leroy P, Mostov KE. Slug is required for cell survival during partial epithelial-mesenchymal transition of HGF-induced tubulogenesis. J. Cell. Sci. 2007;18:1943–1952. doi: 10.1091/mbc.E06-09-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robertson, F. M. et al. Genomic profiling of pre-clinical models of inflammatory breast cancer identifies a signature of epithelial plasticity and suppression of TGFβ. J. Clin. Exp. Pathol. 2, 119 (2012).
- 83.Guo L, et al. Gene expression profiling of drug-resistant small cell lung cancer cells by combining microRNA and cDNA expression analysis. Eur. J. Cancer. 2010;46:1692–1702. doi: 10.1016/j.ejca.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 84.Mohamed MM, Al-Raawi D, Sabet SF, El-Shinawi M. Inflammatory breast cancer: New factors contribute to disease etiology: a review. J. Adv. Res. 2014;5:525–536. doi: 10.1016/j.jare.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Debeb BG, et al. Pre-clinical studies of notch signaling inhibitor RO4929097 in inflammatory breast cancer cells. Breast Cancer Res. 2015;134:495–510. doi: 10.1007/s10549-012-2075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolfe AR, et al. Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models. Oncotarget. 2016 doi: 10.18632/oncotarget.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woodward WA, Debeb BG, Xu W, Buchholz TA. Overcoming radiation resistance in inflammatory breast cancer. Cancer. 2010;116:2840–2845. doi: 10.1002/cncr.25173. [DOI] [PubMed] [Google Scholar]
- 88.Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Oncogene. 2011;30:287–300. doi: 10.1038/onc.2010.405. [DOI] [PubMed] [Google Scholar]
- 89.Gregory PA, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol. Biol. Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu K, Boley KM, Moraes R, Barsky SH, Robertson F. The paradox of E-cadherin: role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism. Oncotarget. 2013;4:446–462. doi: 10.18632/oncotarget.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lehman HL, et al. Modeling and characterization of inflammatory breast cancer emboli grown in vitro. Int. J. Cancer. 2013;132:2283–2294. doi: 10.1002/ijc.27928. [DOI] [PubMed] [Google Scholar]
- 92.Giamperi S, et al. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat. Cell. Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Debeb BG, et al. miR-141-mediated regulation of brain metastasis from breast cancer. J. Natl. Cancer. Inst. 2016;108:djw026. doi: 10.1093/jnci/djw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


