Abstract

A method for regio- and stereoselective anti-addition of the perfluoroalkyl and the triflate group of phenyl(perfluoroalkyl)iodonium triflates to alkynes is presented. The radical reaction uses cheap CuCl as a smart initiator and can be conducted in gram scale. The perfluoroalkyltriflated products are readily further functionalized, rendering this transformation valuable.
Fluorinated building blocks have been widely used for preparation of pharmaceuticals, agrochemicals, and functional materials.1 This is due to the unique properties exerted by the F-substituents such as increased lipophilicity, higher metabolic stability, and better bioavailability.2 Therefore, the development of methods to introduce perfluoroalkyl groups (Rf) into organic components is important.3
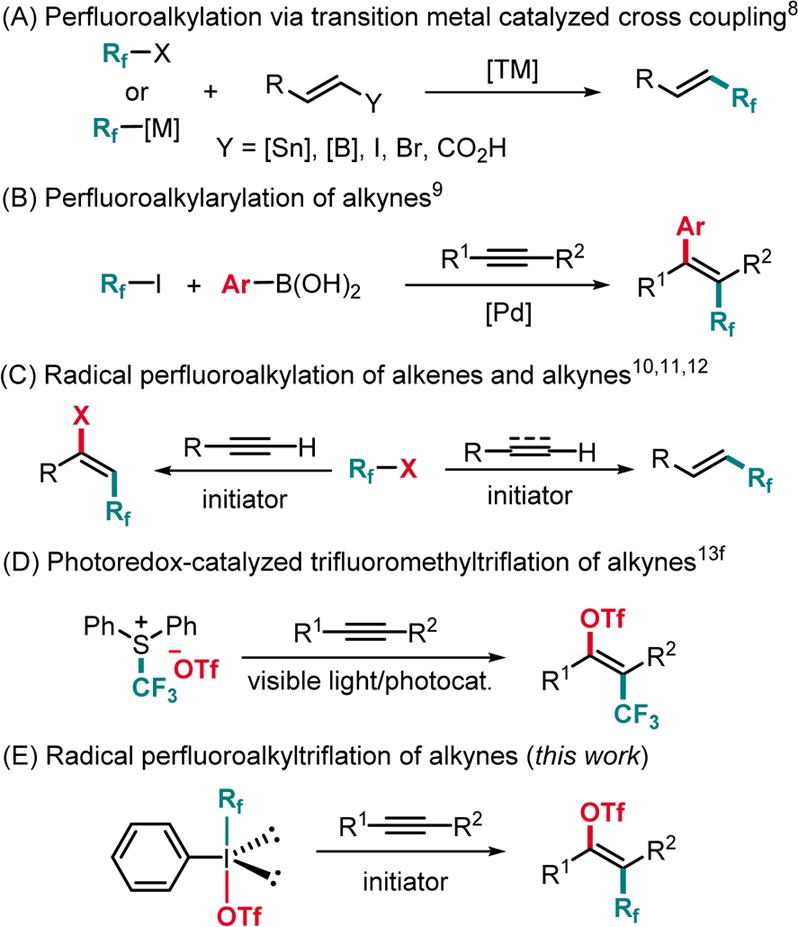
Recently, remarkable developments to attach Rf groups to (hetero)arenes,4 benzylic positions,5a allylic positions,5b the α-position of carbonyl groups,5c,5d and heteroatoms6 have been achieved. Enantioselective perfluoroalkylation was established,7 and the synthesis of vinyl perfluoroalkanes is also in high demand. Common strategies to access them involve transition metal catalyzed or mediated cross-coupling of halides or carboxylic acids with organometallic reagents (Scheme 1A).8 Nevado disclosed a Pd-catalyzed three-component arylperfluoroalkylation of terminal alkynes (Scheme 1B),9a and the Chaładaj group reported arylperfluoroalkylation of internal alkynes using Pd-catalysis.9b Other straightforward approaches to vinyl perfluoroalkanes include direct radical C–H perfluoroalkylation of alkenes,10 hydroperfluoroalkylation of alkynes,11 and alkyne perfluoroalkyliodination (Scheme 1C).12 Notably, trans-selective trifluoromethyltriflation of internal alkynes via photoredox catalysis has been developed by Akita and Koike (Scheme 1D).13f Although many valuable methods for the synthesis of vinyl perfluoroalkanes have been developed, some problems still remain to be solved. Most of the reported perfluoroalkylations suffer at least from one of the following limitations: harsh reaction conditions, narrow substrate scope, low E/Z selectivity, and moderate yield. Therefore, novel approaches to address these drawbacks are desirable.
Scheme 1. Synthesis of Vinyl Perfluoroalkanes.
Alkenyl triflates have been intensively applied as vinyl-organometallic precursors in cross-coupling reactions. The traditional route for their preparation proceeds via trapping of a deprotonated ketone with a triflating reagent (usually Tf2O or PhNTf2). Such triflates can also be accessed using HOTf13a,13b or AgOTf13c as triflate sources. Recently, electrophile induced triflation of alkynes with electrophilic reagents bearing –OTf as the counteranion has been developed (aryl and vinyl triflation by Gaunt13d and cyanotriflation by our group13e).
Hypervalent iodine(III) reagents14 of the benziodoxole type or the iodonium salt type are generally used as electrophiles where only one I(III)-ligand gets transferred to the substrate. Sequential transfer of two ligands from an iodine(III) compound to a carbon–carbon unsaturated bond would certainly improve atom economy, but such transformations are rare.13d,13e,15a−15e For example, the Waser group described an elegant strategy for oxyalkynylation of diazo compounds with alkynylbenziodoxoles.15f We herein report highly regio- and stereoselective perfluoroalkyltriflation of various alkynes with phenyl(perfluoroalkyl)iodonium triflates16 using radical chemistry (Scheme 1D). The method shows remarkable substrate scope and proceeds under mild conditions. The potential of the sequence is documented by various follow-up transformations and by the successful preparation of a fluorinated drug.
Initial screening was performed with ethyl 4-(pent-1-yn-1-yl)benzoate 1a and 1.0 equiv of iodine(III) reagent 2a (PhI(C3F7)OTf) in various solvents at room temperature for 4 h. Pleasingly, we found that in dichloroethane (DCE) the perfluoroalkylated triflate 3a was obtained in 49% yield with complete regio- and E-selectivity (Table 1, entry 6). The relative configuration of 3e, 3r, and 3v (see below) was assigned by NOE experiments, and all other compounds were assigned in analogy. Reaction did not work in MeOH or Et2O, and lower yields were achieved in MeCN, dichloromethane (DCM), and CHCl3 (Table 1, entries 1–5). Prolonging the reaction time to 15 h slightly improved the yield (Table 1, entry 7), and increasing the amount of 2a from 1.0 to 1.7 equiv let to a better result (Table 1, entry 8). The yield was further improved upon adding 1.7 equiv of a base such as pyridine or K2CO3 providing the target product 3a in 86% and 78% yield, respectively (Table 1, entries 9–10). A similar effect was achieved with BF3·Et2O as an additive (Table 1, entry 11), whereas perfluoroalkyltriflation in the presence of TfOH was lower yielding (Table 1, entry 12). Both tetrabutylammonium iodide (TBAI) and CuCl as additives (10 mol %), which are often used for SET reduction of iodine(III) reagents,17 enhanced reaction efficiency (Table 1, entries 13–14). The highest yield was obtained at 50 °C using 10 mol % of CuCl to afford 3a in 94% isolated yield (Table 1, entry 15).
Table 1. Reaction Optimization.
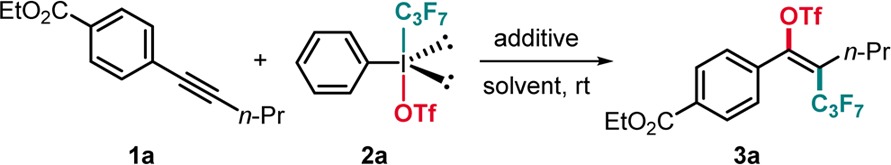
| entrya | additive | 2a (equiv) | solvent | time (h) | yield (%)b |
|---|---|---|---|---|---|
| 1 | none | 1.0 | MeOH | 4 | 0 |
| 2 | none | 1.0 | Et2O | 4 | 0 |
| 3 | none | 1.0 | MeCN | 4 | 11 |
| 4 | none | 1.0 | DCM | 4 | 40 |
| 5 | none | 1.0 | CHCl3 | 4 | 25 |
| 6 | none | 1.0 | DCE | 4 | 49 |
| 7 | none | 1.0 | DCE | 15 | 53 |
| 8 | none | 1.7 | DCE | 15 | 69 |
| 9 | pyridine (1.7 equiv) | 1.7 | DCE | 15 | 86 |
| 10 | K2CO3 (1.7 equiv) | 1.7 | DCE | 15 | 78 |
| 11 | BF3·Et2O (1.7 equiv) | 1.7 | DCE | 15 | 75 |
| 12 | TfOH (1.7 equiv) | 1.7 | DCE | 15 | 53 |
| 13 | TBAI (10 mol %) | 1.7 | DCE | 15 | 86 |
| 14 | CuCl (10 mol %) | 1.7 | DCE | 15 | 91 |
| 15d | CuCl (10 mol %) | 1.7 | DCE | 15 | 96 (94)c |
Reaction conditions: 1a (0.10 mmol, 1.0 equiv), reagent 2a, additive, solvent (1 mL), room temperature.
Yield determined by 19F NMR analysis using PhCF3 as an internal standard; isomer ratio determined by 19F NMR and GC-MS analysis on the crude product, E/Z > 20:1.
Isolated yield.
Conducted at 50 °C.
Having established the optimized conditions, we first explored the scope with respect to the alkyne component and investigated internal alkynes (Scheme 2). Various 1-aryl-1-pentynes bearing substituents at the para-, ortho-, and meta-position of the aryl group were successfully converted in good yields with excellent regio- and stereoselectivity (3a–n). Heteroaryl substituents such as pyridyl and thienyl are tolerated, and 3o and 3p were obtained in moderate to high yields. Symmetrical diaryl alkynes are suitable substrates as documented by the preparation of 3q and 3r. Complete regioselectivity and E-selectivity were also observed with methyl (3s) and cyclopropyl (3t) aryl alkynes.
Scheme 2. Alkyne and I(III)-Reagent Scope,,
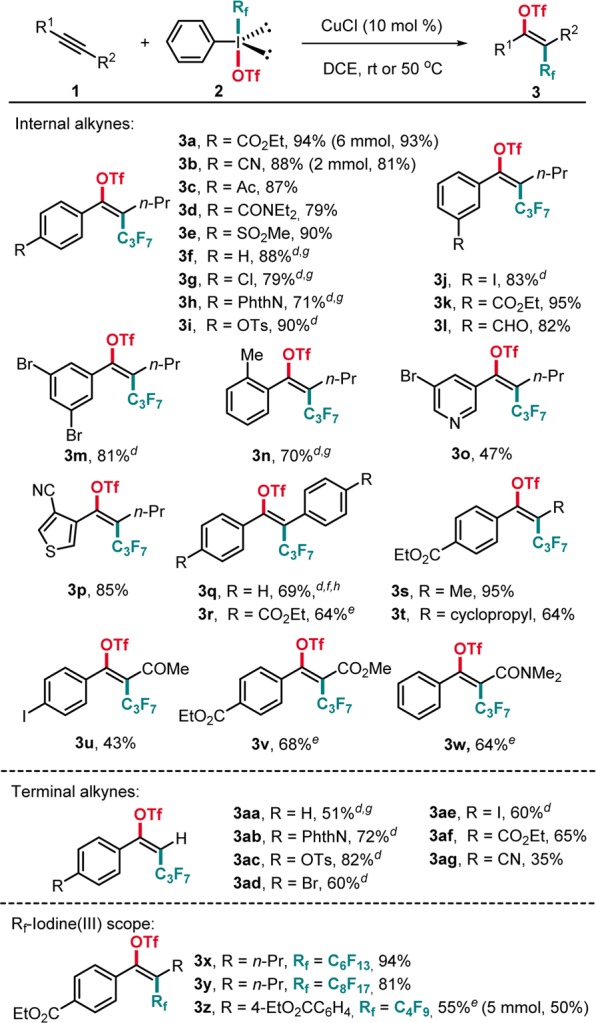
Reaction conditions: 1 (0.10 mmol, 1.0 equiv), 2 (0.17 mmol, 1.7 equiv), CuCl (0.01 mmol, 10 mol %), DCM (1 mL), 45 °C, 15 h.
Isolated yield.
E/Z isomer ratio determined by 19F NMR analysis and GC-MS analysis on the crude product. Unless noted otherwise, E/Z > 20:1.
1 (0.10 mmol, 1.0 equiv), 2 (0.15 mmol, 1.5 equiv), CuCl (0.01 mmol, 10 mol %), and DCM (1 mL), room temperature, 24 h.
After 15 h, renewed CuCl (0.01 mmol, 10 mol %), 2 (0.17 mmol, 1.7 equiv), and DCM (1 mL) addition and continued stirring at 45 °C for another 15 h.
After 24 h, renewed CuCl (0.01 mmol, 10 mol %), 2 (0.15 mmol, 1.5 equiv), and DCM (1 mL) addition and continued stirring at room temperature for another 24 h.
Yield determined by 19F NMR analysis using PhCF3 as an internal standard.
E:Z = 17:1.
Although functionalization of electron-deficient alkynes with electrophilic reagents is challenging our method proved to be applicable to such alkynes as documented by the successful transformation of an ynone (3u), a propiolic ester (3v), and a propiolic amide (3w). We next attempted to expand the newly established method to the conversion of terminal alkynes and could show that arylacetylenes bearing either electron-withdrawing or -donating groups reacted efficiently to afford the corresponding perfluoroalkyltriflation products with complete E-selectivity in moderate to good yields (3aa–3ag). Unfortunately, reactions with dialkyl alkynes failed and starting materials remained unreacted. Notably, our reaction can be conducted on gram scale without compromising the yield (3a, 3b, 3z). We found that the perfluoroalkyl group in the I(III) reagent can be readily varied as shown for the perfluorobutyl, perfluorohexyl, and perfluorooctyl congeners in the reaction with the alkyne 1a. The corresponding products 3x–z were obtained in good yields and excellent selectivities.
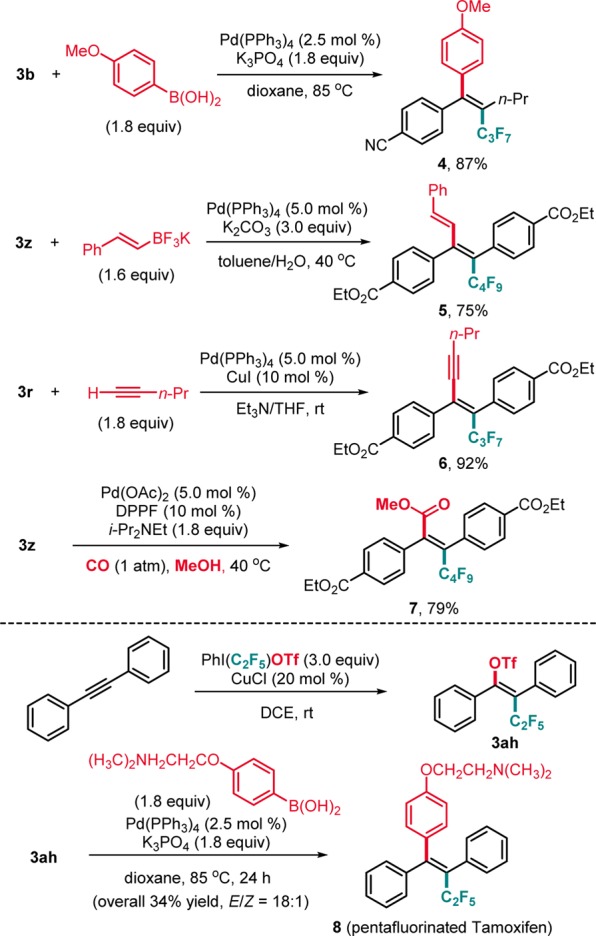
To demonstrate the synthetic value of the method, we investigated follow-up chemistry and could show that compounds of type 3 engage in various stereospecific Pd-catalyzed cross-coupling reactions, including alkenyl-aryl and alkenyl–alkenyl Suzuki couplings (4, 5), the Sonogashira reaction (6), and methoxycarbonylation (7) (Scheme 3). Moreover, pentafluorinated Tamoxifen 8 was prepared in acceptable overall yield and high E/Z selectivity via a pentafluoroethyltriflation/Suzuki coupling sequence.18
Scheme 3. Further Functionalization of Perfluoroalkyltriflated Products.
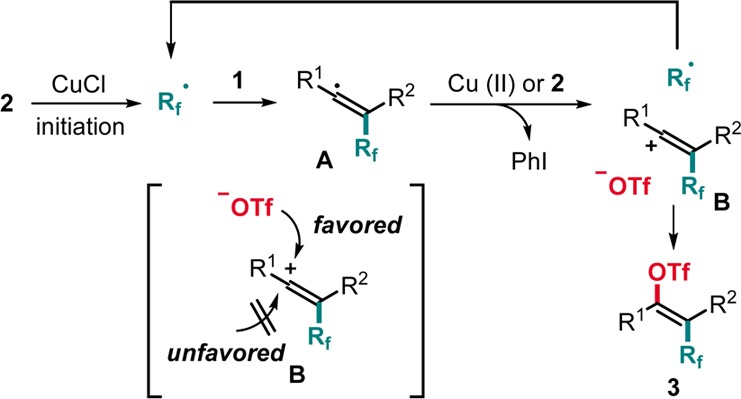
The suggested mechanism is depicted in Scheme 4. Initiation likely occurs by SET reduction of 2 with CuCl to give the Rf-radical along with Cu(II)ClOTf.16,17 Rf-radical addition to the alkyne leads to the vinyl radical A which further reacts via SET oxidation by the I(III)-reagent 2 to form the vinylic cation B along with PhI and the Rf-radical sustaining the chain. The observation that without a Cu-salt reaction proceeds in acceptable yields clearly shows that oxidation of A with reagent 2 is rather efficient, and hence the sequence belongs to an electron-catalyzed process19 where the Cu-salt acts as a smart initiator.19b Thus, regeneration of the initiating Cu(I)-complex is occurring via occasional reaction of A with the Cu(II)-salt. Trapping of B with the triflate anion eventually affords 3. The high E-selectivity can be explained by the larger steric hindrance of the Rf group as compared to the R2 substituent and by the electrostatic repulsion of the Rf-substituent and the incoming triflate during trapping.13f,20
Scheme 4. Suggested Mechanism.
In summary, we have demonstrated a general and practical protocol for direct vicinal alkyne perfluoroalkyltriflation. Prominent features of this process include mild reaction conditions, compatibility with both internal and terminal alkynes, excellent E-selectivity and complete regioselectivity, generally high yields, and easy scale up. Various follow-up reactions document that the tetrasubstituted vinyl triflates obtained as products are valuable building blocks in synthesis.
Acknowledgments
This work was supported by the Alexander von Humboldt Foundation (postdoctoral fellowship to X.W.) and the European Research Council (ERC Advanced Grant Agreement No. 692640).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b01215.
Experimental procedures and characterization data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Uneyama K.Organofluorine Chemistry; Blackwell: Oxford, U.K., 2006. [Google Scholar]; b Müller K.; Faeh C.; Diederich F. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; c Bégué J. P.; Bonnet-Delpon D.. Bioorganic and Medicinal Chemistry of Fluorine; Wiley-Interscience: Hoboken, NJ, 2008. [Google Scholar]; d Ojima I.Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009. [Google Scholar]
- a Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; b Roy S.; Gregg B. T.; Gribble G. W.; Le V.; Roy S. Tetrahedron 2011, 67, 2161–2195. 10.1016/j.tet.2011.01.002. [DOI] [Google Scholar]; c Tomashenko O.; Grushin V. V. Chem. Rev. 2011, 111, 4475–4521. 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Umemoto T. Chem. Rev. 1996, 96, 1757–1777. 10.1021/cr941149u. [DOI] [PubMed] [Google Scholar]; b Prakash G. K. S.; Yudin A. K. Chem. Rev. 1997, 97, 757–786. 10.1021/cr9408991. [DOI] [PubMed] [Google Scholar]; c Liang T.; Neumann C. N.; Ritter T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- a Kobayashi Y.; Kumadaki I. Tetrahedron Lett. 1969, 10, 4095–4096. 10.1016/S0040-4039(01)88624-X. [DOI] [Google Scholar]; b McLaughlin V. C. R.; Thrower J. Tetrahedron 1969, 25, 5921–5940. 10.1016/S0040-4020(01)83100-8. [DOI] [Google Scholar]; c Morimoto H.; Tsubogo T.; Litvinas N. D.; Hartwig J. F. Angew. Chem., Int. Ed. 2011, 50, 3793–3798. 10.1002/anie.201100633. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Litvinas N. D.; Fier P. S.; Hartwig J. F. Angew. Chem., Int. Ed. 2012, 51, 536–539. 10.1002/anie.201106668. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Popov I.; Lindeman S.; Daugulis O. J. Am. Chem. Soc. 2011, 133, 9286–9289. 10.1021/ja2041942. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Qi Q.; Shen Q.; Lu L. J. Am. Chem. Soc. 2012, 134, 6548–6551. 10.1021/ja301705z. [DOI] [PubMed] [Google Scholar]; g Zhang B.; Mück-Lichtenfeld C.; Daniliuc C. G.; Studer A. Angew. Chem., Int. Ed. 2013, 52, 10792–10795. 10.1002/anie.201306082. [DOI] [PubMed] [Google Scholar]; h Nagase M.; Kuninobu Y.; Kanai M. J. Am. Chem. Soc. 2016, 138, 6103–6106. 10.1021/jacs.6b01753. [DOI] [PubMed] [Google Scholar]
- a Kuninobu Y.; Nagase M.; Kanai M. Angew. Chem., Int. Ed. 2015, 54, 10263–10266. 10.1002/anie.201505335. [DOI] [PubMed] [Google Scholar]; b Kawamura S.; Sodeoka M. Angew. Chem., Int. Ed. 2016, 55, 8740–8743. 10.1002/anie.201604127. [DOI] [PubMed] [Google Scholar]; c Su X.; Huang H.; Yuan Y.; Li Y. Angew. Chem., Int. Ed. 2017, 56, 1338–1341. 10.1002/anie.201608507. [DOI] [PubMed] [Google Scholar]; d Kawamoto T.; Sasaki R.; Kamimura A. Angew. Chem., Int. Ed. 2017, 56, 1342–1345. 10.1002/anie.201608591. [DOI] [PubMed] [Google Scholar]
- a Feiring A. E. J. Fluorine Chem. 1984, 24, 191–203. 10.1016/S0022-1139(00)85203-3. [DOI] [Google Scholar]; b Koshechko V. G.; Kiprianova L. A.; Fileleeva L. I. Tetrahedron Lett. 1992, 33, 6677–6678. 10.1016/S0040-4039(00)61016-X. [DOI] [Google Scholar]; c Boiko V. N.; Shchupak G. M. J. Fluorine Chem. 1994, 69, 207–212. 10.1016/0022-1139(94)03132-0. [DOI] [Google Scholar]; d Kiss L. E.; Rábai J.; Varga L.; Kövesdi I. Synlett 1998, 1998, 1243–1245. 10.1055/s-1998-1913. [DOI] [Google Scholar]; Kawaguchi S.-i.; Minamida Y.; Ohe T.; Nomoto A.; Sonoda M.; Ogawa A. Angew. Chem., Int. Ed. 2013, 52, 1748–1752. 10.1002/anie.201207383. [DOI] [PubMed] [Google Scholar]
- a Nagib D. A.; Scott M. E.; MacMillan D. W. C. J. Am. Chem. Soc. 2009, 131, 10875–10877. 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Herrmann A. T.; Smith L. L.; Zakarian A. J. Am. Chem. Soc. 2012, 134, 6976–6979. 10.1021/ja302552e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Woźniak Ł.; Murphy J. J.; Melchiorre P. J. Am. Chem. Soc. 2015, 137, 5678–5681. 10.1021/jacs.5b03243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Matsubara S.; Mitani M.; Utimoto K. Tetrahedron Lett. 1987, 28, 5857–5860. 10.1016/S0040-4039(01)81073-X. [DOI] [Google Scholar]; b Lishchynskyi A.; Grushin V. V. J. Am. Chem. Soc. 2013, 135, 12584–12587. 10.1021/ja407017j. [DOI] [PubMed] [Google Scholar]; c Serizawa H.; Aikawa K.; Mikami K. Org. Lett. 2014, 16, 3456–3459. 10.1021/ol501332g. [DOI] [PubMed] [Google Scholar]; d Saijo H.; Ohashi M.; Ogoshi S. J. Am. Chem. Soc. 2014, 136, 15158–15161. 10.1021/ja5093776. [DOI] [PubMed] [Google Scholar]; e Lishchynskyi A.; Mazloomi Z.; Grushin V. V. Synlett 2014, 26, 45–50. 10.1055/s-0034-1379497. [DOI] [Google Scholar]; f Aikawa K.; Nakamura Y.; Yokota Y.; Toya W.; Mikami K. Chem. - Eur. J. 2015, 21, 96–100. 10.1002/chem.201405677. [DOI] [PubMed] [Google Scholar]; g Lai Y.-L.; Lin D.-Z.; Huang J.-M. J. Org. Chem. 2017, 82, 597–605. 10.1021/acs.joc.6b02613. [DOI] [PubMed] [Google Scholar]
- a Li Z.; García-Domínguez A.; Nevado C. J. Am. Chem. Soc. 2015, 137, 11610–11613. 10.1021/jacs.5b07432. [DOI] [PubMed] [Google Scholar]; b Domański S.; Chaładaj W. ACS Catal. 2016, 6, 3452–3456. 10.1021/acscatal.6b00777. [DOI] [Google Scholar]; c Li Z.; Merino E.; Nevado C. Top. Catal. 2017, 10.1007/s11244-017-0743-y. [DOI] [Google Scholar]
- a Yajima T.; Jahan I.; Tonoi T.; Shinmen M.; Nishikawa A.; Yamaguchi K.; Sekine I.; Nagano H. Tetrahedron 2012, 68, 6856–6861. 10.1016/j.tet.2012.06.028. [DOI] [Google Scholar]; b Lin Q.-Y.; Xu X.-H.; Qing F.-L. J. Org. Chem. 2014, 79, 10434–10446. 10.1021/jo502040t. [DOI] [PubMed] [Google Scholar]; c Sladojevich F.; McNeill E.; Bçrgel J.; Zheng S.-L.; Ritter T. Angew. Chem., Int. Ed. 2015, 54, 3712–3716. 10.1002/anie.201410954. [DOI] [PubMed] [Google Scholar]; d Straathof N. J. W.; Cramer S. E.; Hessel V.; Noël T. Angew. Chem., Int. Ed. 2016, 55, 15549–15553. 10.1002/anie.201608297. [DOI] [PubMed] [Google Scholar]
- a Hu C.-M.; Qiu Y.-L. J. Fluorine Chem. 1991, 55, 113–115. 10.1016/S0022-1139(00)81261-0. [DOI] [Google Scholar]; b Long Z.-Y.; Chen Q.-Y. J. Org. Chem. 1999, 64, 4775–4782. 10.1021/jo9900937. [DOI] [PubMed] [Google Scholar]
- a Ishihara T.; Kuroboshi M.; Okada Y. Chem. Lett. 1986, 15, 1895–1896. 10.1246/cl.1986.1895. [DOI] [Google Scholar]; b Huang W.-Y.; Hu L.-Q. J. Fluorine Chem. 1989, 44, 25–44. 10.1016/S0022-1139(00)84369-9. [DOI] [Google Scholar]; c Takeyama Y.; Ichinose Y.; Oshima K.; Utimoto K. Tetrahedron Lett. 1989, 30, 3159–3162. 10.1016/S0040-4039(00)99190-1. [DOI] [Google Scholar]; d Abou-Ghazaleh B.; Laurent Ph.; Blancou H.; Commeyras A. J. Fluorine Chem. 1994, 68, 21–24. 10.1016/0022-1139(93)02977-M. [DOI] [Google Scholar]; e Nakamura T.; Yorimitsu H.; Shinokubo H.; Oshima K. Synlett 1998, 1998, 1351–1352. 10.1055/s-1998-1985. [DOI] [Google Scholar]; f Jennings M. P.; Cork E. A.; Ramachandran P. V. J. Org. Chem. 2000, 65, 8763–8766. 10.1021/jo001318c. [DOI] [PubMed] [Google Scholar]; g Amato C.; Naud C.; Calas P.; Commeyras A. J. Fluorine Chem. 2002, 113, 55–63. 10.1016/S0022-1139(01)00464-X. [DOI] [Google Scholar]; h Motoda D.; Kinoshita H.; Shinokubo H.; Oshima K. Adv. Synth. Catal. 2002, 344, 261–265. . [DOI] [Google Scholar]; i Tsuchii K.; Imura M.; Kamada N.; Hirao T.; Ogawa A. J. Org. Chem. 2004, 69, 6658–6665. 10.1021/jo0495889. [DOI] [PubMed] [Google Scholar]; j Takagi T.; Kanamori T. J. Fluorine Chem. 2011, 132, 427–429. 10.1016/j.jfluchem.2011.03.001. [DOI] [Google Scholar]; k Slodowicz M.; Barata-Vallejo S.; Vázquez A.; Nudelman N. S.; Postigo A. J. Fluorine Chem. 2012, 135, 137–143. 10.1016/j.jfluchem.2011.10.002. [DOI] [Google Scholar]; l Xu T.; Cheung C. W.; Hu X. Angew. Chem., Int. Ed. 2014, 53, 4910–4914. 10.1002/anie.201402511. [DOI] [PubMed] [Google Scholar]; m Choi S.; Kim Y. J.; Kim S. M.; Yang J. W.; Kim S. W.; Cho E. J. Nat. Commun. 2014, 5, 4881. 10.1038/ncomms5881. [DOI] [PubMed] [Google Scholar]; n Beniazza R.; Atkinson R.; Absalon C.; Castet F.; Denisov S. A.; McClenaghan N. D.; Lastécouères D.; Vincent J.-M. Adv. Synth. Catal. 2016, 358, 2949–2961. 10.1002/adsc.201600501. [DOI] [Google Scholar]
- a Alonso P.; Pardo P.; Galván A.; Fañanás F. J.; Rodríguez F. Angew. Chem., Int. Ed. 2015, 54, 15506–15510. 10.1002/anie.201508077. [DOI] [PubMed] [Google Scholar]; b Wu J.-J.; Xu J.; Zhao X. Chem. - Eur. J. 2016, 22, 15265–15269. 10.1002/chem.201603975. [DOI] [PubMed] [Google Scholar]; c Al-huniti M. H.; Lepore S. D. Org. Lett. 2014, 16, 4154–4157. 10.1021/ol501852n. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Suero M. G.; Bayle E. D.; Collins B. S. L.; Gaunt M. J. J. Am. Chem. Soc. 2013, 135, 5332–5335. 10.1021/ja401840j. [DOI] [PubMed] [Google Scholar]; e Wang X.; Studer A. J. Am. Chem. Soc. 2016, 138, 2977–2980. 10.1021/jacs.6b00869. [DOI] [PubMed] [Google Scholar]; f Tomita R.; Koike T.; Akita M. Angew. Chem., Int. Ed. 2015, 54, 12923–12927. 10.1002/anie.201505550. [DOI] [PubMed] [Google Scholar]
- Reviews:; a Zhdankin V. V.; Stang P. J. Chem. Rev. 2008, 108, 5299–5358. 10.1021/cr800332c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yoshimura A.; Zhdankin V. V. Chem. Rev. 2016, 116, 3328–3435. 10.1021/acs.chemrev.5b00547. [DOI] [PubMed] [Google Scholar]
- a Celik M.; Alp C.; Coskun B.; Gültekin M. S.; Balci M. Tetrahedron Lett. 2006, 47, 3659–3663. 10.1016/j.tetlet.2006.03.137. [DOI] [Google Scholar]; b Egami H.; Shimizu R.; Sodeoka M. Tetrahedron Lett. 2012, 53, 5503–5506. 10.1016/j.tetlet.2012.07.134. [DOI] [Google Scholar]; c Egami H.; Shimizu R.; Usui Y.; Sodeoka M. J. Fluorine Chem. 2014, 167, 172–178. 10.1016/j.jfluchem.2014.05.009. [DOI] [Google Scholar]; d Janson P. G.; Ghoneim I.; Ilchenko N. O.; Szabó K. J. Org. Lett. 2012, 14, 2882–2885. 10.1021/ol3011419. [DOI] [PubMed] [Google Scholar]; e Wang Y.; Jiang M.; Liu J.-T. Adv. Synth. Catal. 2014, 356, 2907–2912. 10.1002/adsc.201400320. [DOI] [Google Scholar]; f Hari D. P.; Waser J. J. Am. Chem. Soc. 2016, 138, 2190–2193. 10.1021/jacs.6b00278. [DOI] [PubMed] [Google Scholar]
- ArI(Rf)OTf:; a Umemoto T.; Kuriu Y. Tetrahedron Lett. 1981, 22, 5197–5200. 10.1016/S0040-4039(01)92458-X. [DOI] [Google Scholar]; b Umemoto T.; Kuriu Y.; Shuyama H.; Miyano O.; Nakayama S.-I. J. Fluorine Chem. 1982, 20, 695–698. 10.1016/S0022-1139(00)82296-4. [DOI] [Google Scholar]; c Umeoto T.; Kuriu Y.; Nakayama S.-i. Tetrahedron Lett. 1982, 23, 1169–1172. 10.1016/S0040-4039(00)87051-3. [DOI] [Google Scholar]; d Umemoto T.; Kuriu Y.; Miyano O. Tetrahedron Lett. 1982, 23, 3579–3582. 10.1016/S0040-4039(00)87675-3. [DOI] [Google Scholar]; e Umemoto T.; Gotoh Y. Bull. Chem. Soc. Jpn. 1986, 59, 439–445. 10.1246/bcsj.59.439. [DOI] [Google Scholar]
- For SET reduction of hypervalent iodine (III) reagents, see:; a Dohi T.; Ito M.; Morimoto K.; Iwata M.; Kita Y. Angew. Chem., Int. Ed. 2008, 47, 1301–1304. 10.1002/anie.200704495. [DOI] [PubMed] [Google Scholar]; b Dohi T.; Ito M.; Yamaoka N.; Morimoto K.; Fujioka H.; Kita Y. Angew. Chem., Int. Ed. 2010, 49, 3334–3337. 10.1002/anie.200907281. [DOI] [PubMed] [Google Scholar]; c Wang X.; Ye Y.; Zhang S.; Feng J.; Xu Y.; Zhang Y.; Wang J. J. Am. Chem. Soc. 2011, 133, 16410–16413. 10.1021/ja207775a. [DOI] [PubMed] [Google Scholar]; d Parsons A. T.; Buchwald S. L. Angew. Chem., Int. Ed. 2011, 50, 9120–9123. 10.1002/anie.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Mejía E.; Togni A. ACS Catal. 2012, 2, 521–527. 10.1021/cs300089y. [DOI] [Google Scholar]; f Li Y.; Studer A. Angew. Chem., Int. Ed. 2012, 51, 8221–8224. 10.1002/anie.201202623. [DOI] [PubMed] [Google Scholar]; g Zhang B.; Mück-Lichtenfeld C.; Daniliuc C. G.; Studer A. Angew. Chem., Int. Ed. 2013, 52, 10792–10795. 10.1002/anie.201306082. [DOI] [PubMed] [Google Scholar]; h Wang Y.; Zhang L.; Yang Y.; Zhang P.; Du Z.; Wang C. J. Am. Chem. Soc. 2013, 135, 18048–18051. 10.1021/ja410195j. [DOI] [PubMed] [Google Scholar]; i Moteki S. A.; Usui A.; Selvakumar S.; Zhang T.; Maruoka K. Angew. Chem., Int. Ed. 2014, 53, 11060–11064. 10.1002/anie.201406513. [DOI] [PubMed] [Google Scholar]; j Jia K.; Zhang F.; Huang H.; Chen Y. J. Am. Chem. Soc. 2016, 138, 1514–1517. 10.1021/jacs.5b13066. [DOI] [PubMed] [Google Scholar]
- a Salih A. K.; Fentiman I. S. Cancer Treat. Rev. 2001, 27, 261–273. 10.1053/ctrv.2001.0235. [DOI] [PubMed] [Google Scholar]; b Jordan V. C. J. Med. Chem. 2003, 46, 883–908. 10.1021/jm020449y. [DOI] [PubMed] [Google Scholar]
- a Studer A.; Curran D. P. Nat. Chem. 2014, 6, 765–773. 10.1038/nchem.2031. [DOI] [PubMed] [Google Scholar]; b Studer A.; Curran D. P. Angew. Chem., Int. Ed. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- a Lemay A. B.; Vulic K. S.; Ogilvie W. W. J. Org. Chem. 2006, 71, 3615–3618. 10.1021/jo060144h. [DOI] [PubMed] [Google Scholar]; b Ide M.; Yauchi Y.; Shiogai R.; Iwasawa T. Tetrahedron 2014, 70, 8532–8538. 10.1016/j.tet.2014.09.075. [DOI] [Google Scholar]; c Yauchi Y.; Ide M.; Shiogai R.; Chikugo T.; Iwasawa T. Eur. J. Org. Chem. 2015, 2015, 938–943. 10.1002/ejoc.201403450. [DOI] [Google Scholar]; d Sproul K. C.; Chalifoux W. A. Org. Lett. 2015, 17, 3334–3337. 10.1021/acs.orglett.5b01558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


