Abstract
Background
The heavy metal arsenic is widely distributed in nature and posses a serious threat to organism’s health. However, little is known about the arsenic-induced inflammatory response in the brain tissues of birds and the relationship and mechanism of the inflammatory response. The purpose of this study was to explore the effects of dietary arsenic on the expression of inflammatory cytokines in the brains of Gallus gallus.
Results
Seventy-two 1-day-old male Hy-line chickens were divided into a control group, a low arsenic trioxide (As2O3)-treated (7.5 mg/kg) group, a middle As2O3-treated (15 mg/kg) group, and a high As2O3-treated (30 mg/kg) group. Arsenic exposure caused obvious ultrastructural changes. The mRNA levels of the transcription factor nuclear factor-κB (NF-κB) and of pro-inflammatory cytokines, including inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2), and prostaglandin E synthase (PTGEs), in chicken brain tissues (cerebrum, cerebellum, thalamus, brainstem and myelencephalon) on days 30, 60 and 90, respectively, were measured by real-time PCR. The protein expression of iNOS was detected by western blot. The results showed that after being treated with As2O3, the levels of inflammatory-related factor NF-κB and pro-inflammatory cytokines in chicken brain tissues increased (P < 0.05).
Conclusions
Arsenic exposure in the chickens triggered host defence and induced an inflammatory response by regulating the expression of inflammatory-related genes in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon. These data form a foundation for further research on arsenic-induced neurotoxicity in Gallus gallus.
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1066-8) contains supplementary material, which is available to authorized users.
Keywords: Arsenic, NF-κB, Inflammatory cytokines, Brain tissues, Chickens
Background
Arsenic is one of the most toxic substances from the natural environment and is classified as a human carcinogen (Group I) [1]. It is widely distributed in natural sources (earth crust, air, soil), anthropogenic sources (insecticides, feed additives, industrial waste), and in drugs and poisons in both organic and inorganic forms [2]. Arsenic reacts with environmental oxygen, chlorine, and sulfur, generating more toxic, soluble inorganic compounds (AsO4 3− and AsO3 3−), and posses a serious threat to organism health [3]. Dermal exposure to toxic trivalent or pentavalent arsenic compounds can produce skin cancer, melanosis, and dorsum [4]. Inhalation of arsenic-contaminated air can affect the respiratory system and can cause laryngitis, rhinitis, and pulmonary diseases [5, 6]. Arsenic-contaminated water can be absorbed by the digestive system and leads to gastritis, abdominal pain, and anorexia. Moreover, selenium deficiency can cause muscular dystrophy in various species, arsenic can also damage the liver, kidneys, heart, and reproductive and nervous systems [7–12]. Arsenic can traverse the blood brain barrier and accumulate in different regions of the brain, making it a target organ of arsenic toxicity and suggesting a role for it in neurological diseases [13, 14]. Because of their toxic effects, pollution of the environment with arsenic and arsenic compound attracts public attention [15].
Previous studies showed that the development of toxicity or alteration in cytokines level induced by molybdenum, cadmium, selenium and lead, which was assessed by evaluating mRNA expression and western blot [16–18]. Developing brain tissue is vulnerable to toxic arsenic [19], and arsenic causes histopathological changes to developing brain tissue (unpublished data). Several reports have indicated that acute or chronic exposure to inorganic arsenic causes neural injury [20]. However, little is known regarding whether arsenic-induced neural injury will result in an inflammatory response in the brain tissues of birds. One of the hallmarks of the inflammatory response is the production of pro-inflammatory mediators, which are needed to repair injured tissues [21, 22]. Nuclear transcription factor-κB (NF-κB) is attached to regulatory proteins named inhibitors of κB (IκB) and kept inactive in the cytosol [23]. IκBα and IκBβ are the main proteins involved in NF-κB activation and sustained activation, respectively. NF-κB is activated under inflammation stimulation and then moves to the nucleus, recognizes the promoter region and regulates the transcription of the pro-inflammatory genes inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) [24–26]. iNOS is a member of the NOS family and is widely distributed in a diverse number of nerve cells, whereas COX-2 is mainly found in specific neurons [27, 28]. Neither is generally expressed in resting nerve cells, and they are only expressed to take part in inflammatory responses under diverse pathological conditions, such as Alzheimer’s disease, ischaemia, and neurodegenerative disorders [29–31]. iNOS and COX-2 have vital roles in the pathophysiology of inflammation because they produce NO and prostaglandins (PGE), respectively [32–34]. Low concentrations of NO are sufficient to maintain physiological functions; however, elevated NO exerts genotoxic harm on the host [32]. Research on the inflammatory mediators NF-κB, iNOS, COX-2, and prostaglandin E synthase (PTGEs) in chickens treated with arsenic trioxide (As2O3) may contribute to the understanding of the possible inflammatory mechanisms of heavy metal arsenic in the nervous system of birds.
We explored the effects of arsenic on the mRNA and protein levels of the main inflammatory-related mediators in the brain tissues of Hy-line chickens to answer the question of whether arsenic induced an inflammatory response in chicken brain tissues, specifically the cerebrum, cerebellum, thalamus, brainstem and myelencephalon, by affecting the expression of inflammatory cytokines.
Methods
Reagents
RNAiso Plus and PrimeScript™RT reagent Kit were purchased from TaKaRa (Dalian, Liaoning, China). FastStart Universal SYBR Green Master was purchased from Roche (Indianapolis, IN, USA). SDS Lysis Buffer, Enhanced BCA Protein Assay Kit and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody were purchased from Beyotime (Shanghai, China). A horseradish peroxidase (HRP)-labelled goat anti-rabbit IgG was purchased from Beijing Biosynthesis Biotechnology Co., LTD (Beijing, China). The iNOS antibody was kindly provided by Professor Xu (Northeast Agricultural University, China).
Animals and experimental design
Procedures used in the present study were authorized by the Institutional Animal Care and Use Committee of Northeast Forestry University (Harbin, China) (UT-31; 20 June 2014). The Hy-line chicken models were created according to our previous research [35]. In short, 72 1-day-old healthy Hy-line chickens, purchased from Weiwei Co. Ltd., Harbin, China., were randomly divided into four groups (18 cocks per group): a control group, a low (7.5 mg/kg) As2O3-treated group (L group), a middle (15 mg/kg) As2O3-treated group (M group), and a high (30 mg/kg) As2O3-treated group (H group), which arsenic doses of dietary were daily administration by adding As2O3 into the food to make supplements uniformed according to the chicken median lethal doses (LD50) of 0, 1/80, 1/40, 1/20, respectively. The composition of the diet is: Maize, grains 421 g/kg; Wheat, grains 120 g/kg; Full fat soy 180 g/kg; Pea 100 g/kg; Wheat bran 80 g/kg; Limestone 80 g/kg; Dicalcium phosphate 15 g/kg and Sodium chloride 4 g/kg. This diet met the minimum requirements for energy and nutrients for chicken and without influencing results according to Nisianakis et al. [36]. Food and water were provided ad libitum. During the experiments, all chickens were injected with sodium pentobarbital to abate stress. Six brain tissue samples (cerebrum, cerebellum thalamus, brainstem and myelencephalon) were taken on day 30, 60 and 90, excised and then rinsed with ice-cold sterilized deionized water, and promptly frozen in liquid nitrogen until required.
Ultrastructural observations
For electron microscopy, brain tissue specimens were fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 3 h at 4 °C, washed in the same buffer for 1 h at 4 °C and post-fixed with 1% osmium tetroxide in sodium phosphate buffer for 1 h at 4 °C. The tissues were then dehydrated in a graded series of ethanol starting at 50% ethanol for 10 min at a time and then were immersed twice in propylene oxide. The tissue specimens were embedded in araldite. Ultrathin sections were stained with Mg-uranyl acetate and lead citrate for evaluation using a transmission electron microscope.
Primers
Primers used in the present study to detect inflammatory cytokine expression in chickens treated with As2O3 are shown in Additional file 1 [26]. GAPDH was considered a housekeeping gene and was used in this study as an internal reference.
Total RNA isolation and reverse transcription
Total RNA from chicken brain tissues was isolated using RNAiso Plus (Takara, China). The concentration and purity were analysed by measuring the absorbance at 260 and 280 nm on a spectrophotometer (Ultrospec 1100 pro, Amersham Biosciences, China). The first-strand cDNA was synthesized using the PrimeScriptTMRT reagent Kit (TaKaRa, China) according to the manufacturer’s instructions. The single chain cDNA was diluted tenfold with sterile ddH2O and stored at −80 °C before use.
Quantitative real-time PCR
Quantitative real-time PCR was performed on a BIOER LineGene 9600 Real-Time PCR System (Hangzhou, China). Reactions contained 5 μL of the SYBR Green Master Mix (Roche, USA), 1 μL of diluted cDNA, 1 μL of each primer (10 μM) and 2 μL of ddH2O water. The reaction conditions were set at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A single peak could be seen in the melting curve. The relative abundance of mRNA was calculated using the 2-∆∆Ct method and normalized to the mean expression of GAPDH [37].
Western blot analysis
Total protein from chicken brain tissues was extracted using SDS Lysis Buffer (Beyotime, China). The concentrations of the protein extracts were measured and calculated using the Enhanced BCA Protein Assay Kit (Beyotime, China). Equal amounts of protein from each extract were subjected to 12% SDS-PAGE gel electrophoresis. Separated proteins were transferred to nitrocellulose (NC) membranes in Tris-glycine buffer for 1 h at 100 mA. The NC membranes were blocked with 5% skim milk at 37 °C and 50 rpm for 4 h and incubated overnight with the diluted iNOS primary antibody (1:1000, provided by Dr. Xu) and GAPDH antibody (1:1000, Beyotime, China) followed by a 1 h incubation with a horse-radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000, Bioss, Beijing) at 37 °C and 50 rpm. The signals were detected by X-ray film (Kodak, USA), and the corresponding protein expression levels were calculated according to greyscale values of the iNOS and GADPH bands.
Statistical analyses
GraphPad Prism 5 statistical software was used to analyse the data. When a significant value (P < 0.05) was obtained by one-way ANOVA, further analysis was carried out. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey’s honest significant difference test for post hoc multiple comparisons. Data are expressed as the mean ± SD of 6 observations.
Results
All treatment groups showed no mortality during our experiments when compared with the controls. We observed no significantly differences between the feed intake, water intake and the body weight of As2O3-treated group and the control group.
Ultrastructural changes
The brain tissues from the control groups showed a normal ultrastructure with cells that had smooth rounded nuclei, intact nuclear membranes, normally distributed chromatin, and integrated mitochondria with normal cristae (Fig. 1A-E). Arsenic treatment caused extensive injury of the brain tissues. The mitochondria in brain tissues of the arsenic groups were swollen and vacuolated with degeneration or loss of cristae. The cells showed typical chromatin condensation and margination, fusion of nuclear membrane, and shrinkage of their nuclei. In addition, the nuclei and organelles of some cells were unclear (Fig. 1a-e).
Fig. 1.
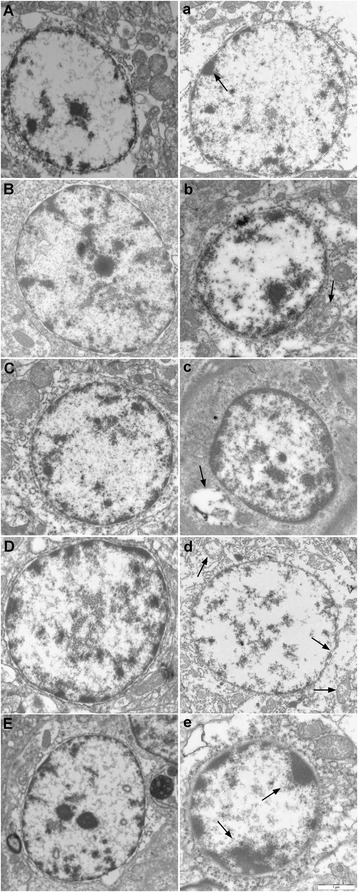
Ultrastructural observations in the brain tissues of chickens. Panels A, B, C, D and E were the histology of the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissue in the control group, respectively. Panels a, b, c, d and e represented the histology of the cerebrum, cerebellum, thalamus, brainstem, and myelencephalon tissue in the As2O3 treated groups at 90 d (H groups)
Effects of As2O3 on NF-κB mRNA levels in the brain tissues of chickens
The NF-κB mRNA levels in the brain tissues treated with As2O3 are shown in Fig. 2. The NF-κB levels increased in a dose-dependent manner in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissues compared with the control group (P < 0.05). The NF-κB mRNA levels also increased in a time-dependent manner except the L group of cerebellum, which increased first and then decreased (P < 0.05).
Fig. 2.
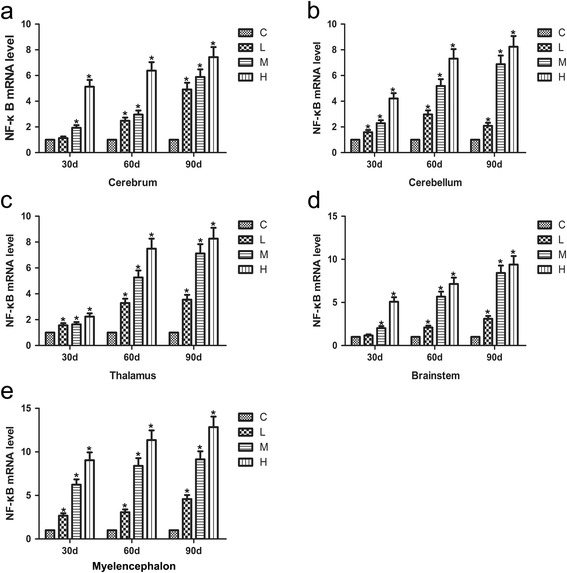
Effects of As2O3 on the NF-κB mRNA levels in the brain tissues. a-e represented the NF-κB mRNA levels in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissue, respectively. Each value represented the means ± SD of six individuals. The bars with a star at the same sampling time were significantly different (P < 0.05)
Effects of As2O3 on the iNOS mRNA levels in the brain tissues of chickens
The mRNA levels of iNOS were displayed in Fig. 3. The iNOS mRNA levels were found to be significantly increased in a time-dependent manner after As2O3 treatment in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissues compared with the control chickens (P < 0.05). The mRNA levels of iNOS also increased in a dose-dependent manner except for the H group in the cerebellum, which was lower than that of the corresponding M group at 90 d (P < 0.05).
Fig. 3.
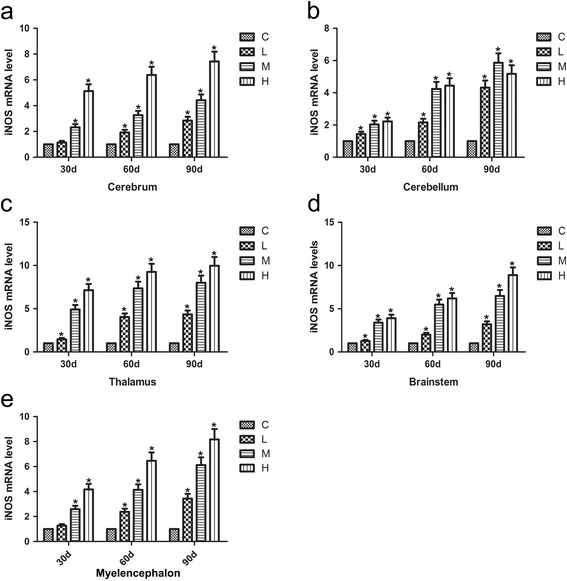
Effects of As2O3 on the iNOS mRNA levels in the brain tissues. a-e represented the iNOS mRNA levels in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissue, respectively. Each value represented the means ± SD of six individuals. The bars with a star at the same sampling time were significantly different (P < 0.05)
Effects of As2O3 on the COX-2 mRNA levels in the brain tissues of chickens
The COX-2 mRNA levels in the cerebellum, thalamus and brainstem tissues of chickens treated with As2O3 increased in a time- and dose-dependent manner compared with the controls (Fig. 4, P < 0.05). However, the COX-2 mRNA levels of the H group was lower than that of the M group in the cerebrum at 90 d, whereas the COX-2 mRNA levels in the L and M groups increased in a time- and dose-dependent fashion (P < 0.05). The COX-2 mRNA levels of the H group were slightly lower than those of the corresponding M group in the myelencephalon tissue at 30 d, whereas the COX-2 levels were found to be obviously increased in a dose-dependent manner at 60 d and 90 d (P < 0.05).
Fig. 4.
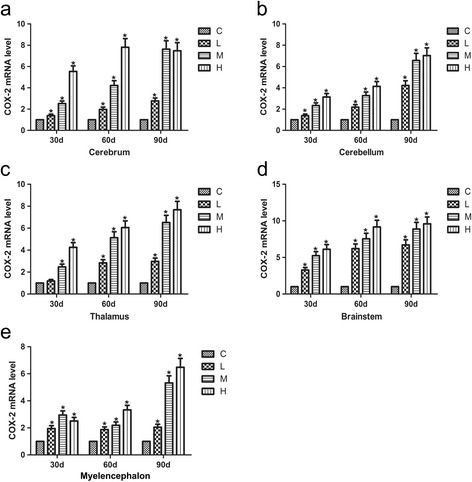
Effects of As2O3 on the COX-2 mRNA levels in the brain tissues. a-e represented the COX-2 mRNA levels in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissue, respectively. Each value represented the means ± SD of six individuals. The bars with a star at the same sampling time were significantly different (P < 0.05)
Effects of As2O3 on the mRNA levels of PTGEs in chicken brain tissue
The mRNA levels of PTGEs increased in a time- and dose-dependent fashion in the cerebrum, cerebellum, and thalamus of chickens exposed to As2O3 compared with the controls (Fig. 5, P < 0.05). In the brainstem, the L and M groups showed an increasing time- and dose-dependent trend (P < 0.05); however, at 60 d, the H group had slightly increased mRNA levels of PTGEs compared with the L group (P < 0.05). In the myelencephalon, all groups except the H group showed a dose-dependent increasing trend (P < 0.05) in the mRNA levels of PTGEs. The H group had lower mRNA levels of PTGEs at 90 d than at both the 30 and 60 d time points (P < 0.05).
Fig. 5.
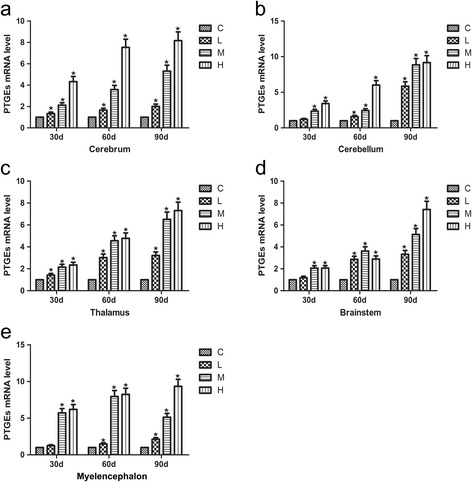
Effects of As2O3 on the PTGEs mRNA levels in the brain tissues. a-e represented the PTGEs mRNA levels in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissue, respectively. Each value represented the means ± SD of six individuals. The bars with a star at the same sampling time were significantly different (P < 0.05)
Western blot analysis of the iNOS levels
As2O3 treatment for a period of 90 days resulted in a significant increase (P < 0.05) in the protein expression of iNOS as detected by western blot (Fig. 6). The results revealed that iNOS protein expression was significantly increased in the cerebellum, thalamus, brainstem and myelencephalon tissues in a dose-dependent manner compared with the control group (P < 0.05). iNOS protein levels increased at different levels in diverse tissues. Compared with the control group, the iNOS protein levels in the cerebrum tissues of the As2O3-treated groups increased at 30 d, decreased at 60 d, and slightly increased at 90 d (P < 0.05) compared with controls.
Fig. 6.
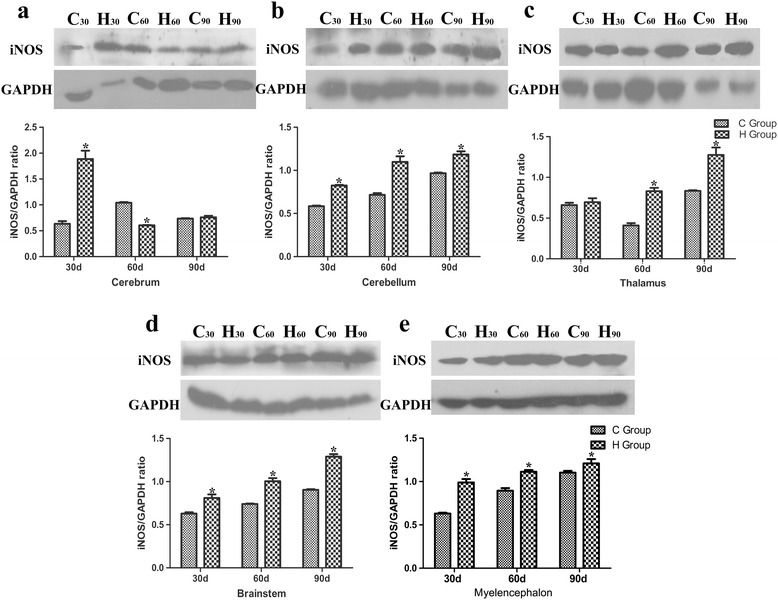
Effects of As2O3 on the iNOS protein expressions in the brain tissues. a-e represented the western blot results of the expression of the iNOS and GADPH proteins and iNOS/GADPH ratio in the cerebrum, cerebellum, thalamus, brainstem and myelencephalon tissue, respectively. C30 and H30 represented the C and H groups at 30 d, respectively, the notations for the C60, H60, C90 and H90 groups are similar. Each value represented the means ± SD of six individuals. The bars with a star at the same sampling time were significantly different (P < 0.05)
Discussion
As a natural component of the environment, animals easily access to relatively low levels of arsenic by eating food, breathing air, and drinking water [38]. The respiratory, cardiovascular gastrointestinal, haematological, renal, dermal, reproductive, and neurological toxicity of arsenic have been recorded for centuries [2]. Therefore, it is valuable to research how environmental arsenic exposure affects organism health, particularly at low levels. One of the risk factors of neurological toxicity is arsenic. The acute or chronic exposure to inorganic arsenic causes arsenic-associated neurotoxicity in humans that cause behavioural alterations in turn [39, 40]. In this study, we performed an ultrastructure assay of chicken brain tissues and found that arsenic trioxide exposure caused typical features of injury such as fusion of the nuclear membrane, nucleus shrinkage, chromatin condensation, and margination. The As2O3-induced neural injury could further trigger host defences, such as an inflammatory response. Therefore, we investigated the effect of As2O3 on the expression of inflammatory cytokines in the brain tissues of chickens.
When organisms are exposed to heavy metals, NF-κB level increases, interact with reactive oxygen species (ROS) [41] and accelerate the generation of inflammatory cytokines [42]. In our study, the expression of NF-κB was assessed by qRT-PCR, and we confirmed that NF-κB expression was significantly increased in a time- and dose-dependent manner except in the cerebrum and brainstem tissues from the As2O3-treated L group (P < 0.05). The expression of NF-κB was similar trend in a time- and dose-dependent manner in five brain tissues. Increased NF-κB activity has been found in the brain tissues of patients with Alzheimer’s disease and takes part in the neurodegenerative process [43]. Our results showed that arsenic activated NF-κB expression in the brain tissues of chickens, which might further induce the expression of other pro-inflammatory genes such as TNF-α, IL-6, iNOS, and COX-2 involved in the inflammatory process.
NOS expression has a wide range of distribution and has been found in endothelial cells, glial cells, neurons, and perivascular nerves [27, 28]. iNOS is a member of the NOS family and is expressed in inflammatory cells and nerve cells including astrocytes and microglia [44–46]. Thus, iNOS is well known to be abundant in brain tissue [47, 48]. In our study, we examined iNOS mRNA and protein levels by qRT-PCR and western blot, respectively. The iNOS mRNA levels were significantly increased in the brain tissues of the As2O3-treated groups except for the cerebellum tissues of the H group and the cerebrum tissues of the L group (P < 0.05), suggesting that iNOS might play a role in inducing brain tissue inflammation upon arsenic exposure. In the cerebrum, there was a fluctuation in protein expression that may have been related to post transcriptional regulation of gene expression [49]. Madrigal et al. reported that the iNOS level could be decreased in the brain cortex of animals treated with an NF-κB inhibitor [50]. This provides further evidence that in our study, the activation of NF-κB through arsenic exposure induced iNOS production in different brain tissues.
In addition, it is believed that COX-2 is of primary importance in the inflammatory response [51]. Several studies have shown that kainic acid can lead to the induction of COX-2 expression and neuronal apoptosis. Excitotoxin induces neuronal death in vitro and is accompanied by a selective elevation in the mRNA level of COX-2. Nonsteroidal anti-inflammatory drugs cause the contents of COX to vary in vivo [52]. These observations demonstrate that the expression of COX-2 may be involved in the pathway leading to neuronal death. COX-2 expression and the subsequent prostaglandin E2 (PTGE2) production are both used as prognostic markers of inflammation [53]. Additionally, these two markers are regarded as targets of therapeutic intervention during the inflammatory response. In the tissues damage, enzymes of iNOS and cyclooxygenase-2 (COX-2) could induce the generation of prostaglandin E synthase (PTGEs) [34]. And following the initiation of COX-2 expression, TNF-α could induce the activation of NF-κB in the COX-2 promoter [54]. Consistent with these previous studies, the mRNA levels of COX-2 and the PTGEs were up-regulated in time- and dose-dependent manners in the brain tissues from As2O3-treated chickens (P < 0.05) compared with the control group, especially myelencephalon. The results illustrated that NF-κB activation also up-regulated the expression of iNOS, COX-2 and the PTGEs to take part in an arsenic-induced inflammatory response in the brain tissues of chickens.
Conclusion
In conclusion, we demonstrated that arsenic exposure in chickens affected the expression of inflammatory cytokines in their brain tissues. Arsenic could trigger host defence and induce an inflammatory response in the brain tissues of chickens. The mRNA levels of NF-κB, iNOS, COX-2 and PTGEs and the protein levels of iNOS were significantly up-regulated in the brain tissues from As2O3–treated chickens compared with the controls. The mechanisms of neurotoxicity induced by arsenic could lead to inflammatory response in chicken brain tissues.
Acknowledgements
We thank American Journal Experts for critical modification of this manuscript. At the same time, we thank Dr. Xu Shiwen (College of Animal Medicine, Northeast Agricultural University, Harbin, China) for his supply for iNOS primary antibody.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31672619), the Fundamental Research Funds for the Central Universities (Grant No. 2572016EAJ5) and the Natural Science Foundation of Heilongjiang Province (Grant No. C2015061).
Availability of data and materials
All data pertaining to this study supporting these findings is contained within the manuscript and additional supporting files.
Authors’ contributions
XS performed the experiment and drafted the manuscript. YH contributed in experiment design and manuscript revision. YG, XS, YH carried out the statistical analysis. SL helped in conceived of the study, and participated in its design. HZ and YW carried the sampling and histological preparation. JZ, MW conceived the idea, designed the experiment, and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
Ethics treatment of animals and procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Northeast Forestry University (Harbin, China) (UT-31; 20 June 2014).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- As2O3
Arsenic trioxide
- COX-2
Cyclooxygenase-2
- iNOS
Inducible NO synthase
- IκB
inhibitors of κB
- NF-κB
Nuclear transcription factor-κB
- PTGE2
Prostaglandin E2
- PTGEs
Prostaglandin E synthase
- ROS
Reactive oxygen species
Additional file
Primers used for quantitative real-time PCR. (DOC 33 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1066-8) contains supplementary material, which is available to authorized users.
Contributor Information
Xiao Sun, Email: sunxiao@nefu.edu.cn.
Ying He, Email: heying@nefu.edu.cn.
Ying Guo, Email: yingguo0601@nefu.edu.cn.
Siwen Li, Email: lisiwen@nefu.edu.cn.
Hongjing Zhao, Email: zhaohongjing@nefu.edu.cn.
Yu Wang, Email: wangyu@nefu.edu.cn.
Jingyu Zhang, Phone: +86-15804633233, Email: zhangjingyu@foxmail.com.
Mingwei Xing, Phone: +86-13904605509, Email: xingmingwei@nefu.edu.cn.
References
- 1.Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL. Arsenic exposure and the induction of human cancers. J Toxicol. 2011;2011:431287. doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58(1):201–235. doi: 10.1016/S0039-9140(02)00268-0. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Yamashita S, Ushijima T, Takumi S, Sano T, Michikawa T, et al. Genome-wide analysis of DNA methylation changes induced by gestational arsenic exposure in liver tumors. Cancer Sci. 2013;104(12):1575–85. doi: 10.1111/cas.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt KM, Srivastava RK, Elmets CA, Athar M. The mechanistic basis of arsenicosis: pathogenesis of skin cancer. Cancer Lett. 2014;354(2):211–219. doi: 10.1016/j.canlet.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milton AH, Hasan Z, Rahman A. Chronic arsenic poisoning and respiratory effects in Bangladesh. J Occup Health. 2001;43:136–140. doi: 10.1539/joh.43.136. [DOI] [Google Scholar]
- 6.Mazumder DN, Haque R, Ghosh N, De BK, Santra A, Chakraborti D, et al. Arsenic in drinking water and the prevalence of respiratory effects in West Bengal. India. Int J Epidemiol. 2000;29(6):1047–52. doi: 10.1093/ije/29.6.1047. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Zhao P, Guo G, Guo Y, Tian L, Sun X, et al. Arsenic trioxide attenuates NF-kappaB and cytokine mRNA levels in the livers of cocks. Biol Trace Elem Res. 2016;170(2):432–7. [DOI] [PubMed]
- 8.Giberson A, Vaziri ND, Mirahamadi K, Rosen SM. Hemodialysis of acute arsenic intoxication with transient renal failure. Arch Intern Med. 1976;136(11):1303–1304. doi: 10.1001/archinte.1976.03630110065017. [DOI] [PubMed] [Google Scholar]
- 9.Nordstrom S, Beckman L, Nordenson I. Occupational and environmental risks in and around a smelter in northern Sweden. V. Spontaneous abortion among female employees and decreased birth weight in their offspring. Hereditas. 1979;90(2):291–296. doi: 10.1111/j.1601-5223.1979.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 10.Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, et al. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7(2):117–24. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Yao HD, Wu Q, Zhang ZW, Zhang JL, Li S, Huang JQ, et al. Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of se-deficient chicks. J Nutr. 2013;143(5):613–9. doi: 10.3945/jn.112.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao HD, Wu Q, Zhang ZW, Li S, Wang XL, Lei XG, et al. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim Biophys Acta. 2013;1830(4):3112–20. doi: 10.1016/j.bbagen.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraja TN, Desiraju T. Effects on operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intake. Hum Exp Toxicol. 1994;13(5):353–356. doi: 10.1177/096032719401300511. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez VM, Jimenez-Capdeville ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145(1):1–18. doi: 10.1016/S0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 15.Han FX, Kingery WL, Selim HM, Gerard PD, Cox MS, Oldham JL. Arsenic solubility and distribution in poultry waste and long-term amended soil. Sci Total Environ. 2004;320(1):51–61. doi: 10.1016/S0048-9697(03)00441-8. [DOI] [PubMed] [Google Scholar]
- 16.Cao H, Gao F, Xia B, Zhang M, Liao Y, Yang Z, et al. Alterations in trace element levels and mRNA expression of Hsps and inflammatory cytokines in livers of duck exposed to molybdenum or/and cadmium. Ecotoxicol Environ Saf. 2016;125:93–101. [DOI] [PubMed]
- 17.Sun GX, Chen Y, Liu CP, Li S, Fu J. Effect of selenium against lead-induced damage on the gene expression of heat shock proteins and inflammatory cytokines in peripheral blood lymphocytes of chickens. Biol Trace Elem Res. 2016;172(2):474–480. doi: 10.1007/s12011-015-0602-2. [DOI] [PubMed] [Google Scholar]
- 18.Sheng PF, Jiang Y, Zhang ZW, Zhang JL, Li S, Zhang ZQ, et al. The effect of se-deficient diet on gene expression of inflammatory cytokines in chicken brain. Biometals. 2014;27(1):33–43. doi: 10.1007/s10534-013-9682-7. [DOI] [PubMed] [Google Scholar]
- 19.Rice D, Barone SJ. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalwani S, Dogra TD, Bhardwaj DN, Sharma RK, Murty OP, Vij A. Study on arsenic level in ground water of Delhi using hydride generator accessory coupled with atomic absorption spectrophotometer. Indian J Clin Biochem. 2004;19(2):135–140. doi: 10.1007/BF02894273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67(2):161–172. doi: 10.1016/S0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen CJ, Ou YC, Lin SY, Liao SL, Chen SY, Chen JH. Manganese modulates pro-inflammatory gene expression in activated glia. Neurochem Int. 2006;49(1):62–71. doi: 10.1016/j.neuint.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6(11):441–448. doi: 10.1016/S1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7(12):1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 25.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen LJ, Sun BH, Qu JP, Xu S, Li S. Avermectin induced inflammation damage in king pigeon brain. Chemosphere. 2013;93(10):2528–2534. doi: 10.1016/j.chemosphere.2013.09.058. [DOI] [PubMed] [Google Scholar]
- 27.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy S, Simmons ML, Agullo L, Garcia A, Feinstein DL, Galea E, et al. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 1993;16(8):323–8. doi: 10.1016/0166-2236(93)90109-Y. [DOI] [PubMed] [Google Scholar]
- 29.Pasinetti GM. Cyclooxygenase and inflammation in Alzheimer's disease: experimental approaches and clinical interventions. J Neurosci Res. 1998;54(1):1–6. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 30.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 31.Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107(3):247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res. 1995;339(2):73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 33.Tunctan B, Uludag O, Altug S, Abacioglu N. Effects of nitric oxide synthase inhibition in lipopolysaccharide-induced sepsis in mice. Pharmacol Res. 1998;38(5):405–411. doi: 10.1006/phrs.1998.0381. [DOI] [PubMed] [Google Scholar]
- 34.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480–481:243–68. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 35.Xing M, Zhao P, Guo G, Guo Y, Zhang K, Tian L, et al. Inflammatory factor alterations in the gastrointestinal tract of cocks overexposed to arsenic trioxide. Biol Trace Elem Res. 2015;167(2):288–99. doi: 10.1007/s12011-015-0305-8. [DOI] [PubMed] [Google Scholar]
- 36.Nisianakis P, Giannenas I, Gavriil A, Kontopidis G, Kyriazakis I. Variation in trace element contents among chicken, turkey, duck, goose, and pigeon eggs analyzed by inductively coupled plasma mass spectrometry (ICP-MS) Biol Trace Elem Res. 2009;128(1):62–71. doi: 10.1007/s12011-008-8249-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao FQ, Zhang ZW, Wang C, Zhang B, Yao HD, Li S, et al. The role of heat shock proteins in inflammatory injury induced by cold stress in chicken hearts. Cell Stress Chaperones. 2013;18(6):773–83. doi: 10.1007/s12192-013-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123(2):305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji JS, Garry MR, Perez V, Chang ET. Low-level arsenic exposure and developmental neurotoxicity in children: a systematic review and risk assessment. Toxicology. 2015;337:91–107. doi: 10.1016/j.tox.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Vahidnia A, Romijn F, Tiller M, van der Voet GB, de Wolff FA. Arsenic-induced toxicity: effect on protein composition in sciatic nerve. Hum Exp ToxicoL. 2006;25(11):667–674. doi: 10.1177/0960327106070671. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Nie S, Huang D, Xie M. Nonylphenol regulates cyclooxygenase-2 expression via Ros-activated NF-kappaB pathway in sertoli TM4 cells. Environ Toxicol. 2015;30(10):1144–1152. doi: 10.1002/tox.21987. [DOI] [PubMed] [Google Scholar]
- 42.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179(2):503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745(3):287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 45.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 46.Di Monte DA, Royland JE, Anderson A, Castagnoli K, Castagnoli NJ, Langston JW. Inhibition of monoamine oxidase contributes to the protective effect of 7-nitroindazole against MPTP neurotoxicity. J Neurochem. 1997;69(4):1771–1773. doi: 10.1046/j.1471-4159.1997.69041771.x. [DOI] [PubMed] [Google Scholar]
- 47.Araki T, Tanji H, Fujihara K, Kato H, Itoyama Y. Increases in [3H]FK-506 and [3H]L-N(G)-nitro-arginine binding in the rat brain after nigrostriatal dopaminergic denervation. Metab Brain Dis. 1999;14(1):21–31. doi: 10.1023/A:1020605429535. [DOI] [PubMed] [Google Scholar]
- 48.Muramatsu Y, Kurosaki R, Watanabe H, Michimata M, Matsubara M, Imai Y, et al. Cerebral alterations in a MPTP-mouse model of Parkinson's disease--an immunocytochemical study. J Neural Transm (Vienna). 2003;110(10):1129–44. [DOI] [PubMed]
- 49.Casper I, Nowag S, Koch K, Hubrich T, Bollmann F, Henke J, et al. Post-transcriptional regulation of the human inducible nitric oxide synthase (iNOS) expression by the cytosolic poly(A)-binding protein (PABP) Nitric Oxide. 2013;33:6–17. doi: 10.1016/j.niox.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, et al. Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem. 2001;76(2):532–8. doi: 10.1046/j.1471-4159.2001.00108.x. [DOI] [PubMed] [Google Scholar]
- 51.O'Banion MK. COX-2 and Alzheimer's disease: potential roles in inflammation and neurodegeneration. Expert Opin Investig Drugs. 1999;8(10):1521–1536. doi: 10.1517/13543784.8.10.1521. [DOI] [PubMed] [Google Scholar]
- 52.Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Zhang R, Wang T, Jiang H, Guo M, Zhou E, et al. Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-kappaB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation. 2014;37(2):478–85. doi: 10.1007/s10753-013-9761-5. [DOI] [PubMed] [Google Scholar]
- 54.Chen CC, Sun YT, Chen JJ, Chiu KT. TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J Immunol. 2000;165(5):2719–2728. doi: 10.4049/jimmunol.165.5.2719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data pertaining to this study supporting these findings is contained within the manuscript and additional supporting files.


