Abstract
Background
Enterocytozoon bieneusi is the dominant specie of microsporidia which can infect both anthroponotic and zoonotic species. The golden snub-nosed monkey is an endangered primate which can also infect by E. bieneusi. To date, few genetic data on E. bieneusi from golden snub-nosed monkeys has been published. Therefore, to clarify the prevalence and genotypes of E. bieneusi in captive golden snub-nosed monkeys is necessary to assess the potential for zoonotic transmission.
Result
We examined 160 golden snub-nosed monkeys from six zoos in four cities in China, using PCR and comparative sequence analysis of the ribosomal internal transcribed spacer (ITS). The overall prevalence of E. bieneusi was 46.2% (74/160); while the prevalence was 26.7%, 69.1%, 69.4% and 33.3% in Shanghai Zoo, Shanghai Wild Animal Park, Tongling Zoo, and Taiyuan Zoo respectively (P = 0.006). A total of seven E. bieneusi genotypes were found that included four known (D, J, CHG1, and CHG14) and three new (CM19–CM 21) genotypes. The most common genotype was D (54/74, 73.0%), followed by J (14/74, 18.9%); other genotypes were restricted to one or two samples. Phylogenetic analysis revealed that genotype D belonged to the previously-characterized Group 1, with zoonotic potential; whereas genotypes J, CHG1, CHG14 and CM19–CM 21 clustered in the previously-characterized Group 2, the so-called cattle host specificity group.
Conclusions
The findings of high prevalence of zoonotic E. bieneusi genotypes D and J in golden snub-nosed monkeys suggest that golden snub-nosed monkeys may be the reservoir hosts for human microsporidiosis, and vice versa.
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1084-6) contains supplementary material, which is available to authorized users.
Keywords: Microsporidia, Molecular characterization, Phylogeny, Nonhuman primates
Background
Microsporidia is a relatively diverse clade of unicellular fungi, living as obligate intracellular pathogens, comprising over 1300 species in at least 160 genera [1]. To date, 14 species of microsporidian pathogens have been diagnosed in humans [2], among which Enterocytozoon bieneusi is dominant [3]. Manifestations of microsporidiosis caused by E. bieneusi include stubborn chronic diarrhea, abdominal pain and weight loss, in immunocompromised people, such as those infected with HIV, organ transplant recipients, cancer patients, and diabetics; while in immunocompetent individuals E. bieneusi causes self-limiting diarrhea and malnutrition, and is sometimes asymptomatic [4–6]. E. bieneusi has also been detected in variety of animal hosts, including mammals, birds, rodents and reptiles [7, 8].
Since the first identification of E. bieneusi in an AIDS patient by Desportes and others in 1985, more than 240 genotypes of the species have been reported from numerous animal hosts, based on sequence analysis of the internal transcribed spacer (ITS) region of the ribosomal RNA (rRNA) gene [8–14]. Phylogenetic analysis of ITS sequences has revealed the presence of eight genotype groups [15]. More than 94% of the identified ITS genotypes of E. bieneusi constitute a large group with zoonotic potential, designated as Group 1, while the rest are divided into several host-adapted groups, designated as Groups 2 to 8 [10, 15, 16]. While the molecular phylogeny of these strains is well-studied, the full range of host diversity, including reservoirs and potential zoonotic transmission, remain unresolved issues [17].
The golden snub-nosed monkey is classified as a Category I protected species in China [18]. The species is listed as Category Endangered by the International Union for Conservation of Nature (IUCN), as its numbers have declined by more than 50% in the last 3 generations (approximately 40 years), because of habitat loss. This decline is continuing, though in some areas the populations are declining at a lower rate [19]. In an attempt to improve population numbers and protect the species’ diversity, some golden snub-nosed monkeys have been captured and placed in zoos [20]. Several recent studies have focused on E. bieneusi in nonhuman primates (NHPs) in China and in Kenya, with 43 E. bieneusi ITS genotypes reported from various NHP species [15, 17, 21]. However, very limited genetic data are available on E. bieneusi from golden snub-nosed monkeys. The present study clarifies the prevalence of E. bieneusi in captive golden snub-nosed monkeys, as well as assessing the potential for zoonotic transmission.
Methods
Study sites and sampling
The study was conducted over a 5-month period (June to November 2015), during that period of time fecal specimens of golden snub-nosed monkeys were collected from Beijing Zoo (116°33′E, 39°94′N), Beijing Wildlife Park (116°33′E, 39°49′N), Shanghai Zoo (121°36′E, 31°19′N), Shanghai Wild Animal Park (121°72′E, 31°05′N), Taiyuan Zoo (112°57′E, 37°91′N) and Tongling Zoo (117°85′E, 30°82′N) (Fig. 1). Some of the monkeys live in single-individual cages; but the majority live as family units in large cages or houses, there are 2 to 4 monkeys in one family unit. The accommodation usually includes play equipment to occupy the animals. To avoid direct contact with visitors, cages are fenced and surrounded by ditches. All the monkeys are usually fed with multi-grain bread twice a day, in the morning and afternoon, with eggs, nuts, seasonal fruits and vegetables as snacks.
Fig. 1.
Locations of zoos at which specimens were collected in this study. The figure was generated using the softwares of Chinamap 2.42, Microsoft PowerPoint 2003 and Adobe Photoshop CS6
A total of 160 fresh fecal specimens were collected from the six zoos in this study (Table 1 and Additional file 1: Table S1 ). All the specimens were collected with the help of local animal attendants, to minimize possible social disruption to the monkeys. Where monkeys were housed individually, fresh fecal deposits were collected in the early morning, as the floors of animal houses were cleaned each evening. For monkeys that were kept in family units in houses during the day, fecal specimens were collected from houses where they spent the night. As the animal handlers described, almost all the monkeys only have one excretion during a whole night, and there are distinct differences among feces from different family members, such as color, smell, size, viscosity, hardness and so on. To avoid duplicate sampling of animals, the fecal deposits were collected for once according to their characteristics. All the specimens were placed into labeled clean zip-lock bags, transported to the Laboratory of Veterinary Parasitology, Henan Agricultural University, in a cooler with ice packs, transferred in water into a 50 ml centrifuge tube, sieved through a 6.5 cm diameter sieve with a pore size of 375 μm (to avoid cross-contamination between samples, the sieve was washed twice with distilled water), and then concentrated by centrifugation. The concentrated fecal specimens were stored in 2.5% potassium dichromate solution at 4 °C prior to DNA extraction.
Table 1.
Prevalence and ITS genotype distribution of E. bieneusi in Golden snub-nosed monkeys in different zoos in China
| Study location | No. of specimens | No. (%) of positive specimens | ITS genotypes (no. of specimens) | |
|---|---|---|---|---|
| Known | Novel | |||
| Beijing Wildlife Park | 25 | 0 | ||
| Beijing Zoo | 8 | 0 | ||
| Shanghai Zoo | 15 | 4(26.7) | J(2) | CM19(1), CM20(1) |
| Shanghai Wild Animal Park | 55 | 38(69.1) | D(33), CHG1(1), J(3), CHG14(1) | |
| Tongling Zoo, Anhui Province | 36 | 25(69.4) | D(21), J(3) | CM21(1) |
| Taiyuan Zoo, Shanxi Province | 21 | 7(33.3) | J(6) | CM19(1) |
DNA extraction
The stored fecal specimens were washed three times with distilled water after centrifugation, to remove the potassium dichromate. Genomic DNA was extracted using the E.Z.N.A.R Stool DNA kit (Omega Biotek Inc., Norcross, USA), following the manufacturer’s protocol. The extracted DNA was stored at −20 °C prior to PCR analysis.
PCR amplification
The presence of E. bieneusi was detected using nested PCR amplification of a 389 bp nucleotide fragment of the ribosomal RNA gene, which included 76 bp of the 3′-end of SSU rRNA gene, the full 243 bp of the ITS region, and 70 bp of the 5′-region of the 5.8S rRNA gene [22]. Outer primers were EBITS3 (5′-GGTCATAGGGATGAAGAG-3′) and EBITS4 (5′-TTCGAGTTCTTTCGCGCTC-3′); EBITS1 (5′-GCTCTGAATATCTATGGCT-3′) and EBITS2.4 (5′-ATCGCCGACGGATCCAAGTG-3′) were used as nested primers for secondary PCR. The cycling parameters were as previously described [23]. Each specimen was analyzed twice, with 1 μl of extracted DNA as template for each PCR, using an Applied Biosystems 2720 Thermal Cycler (Applied Biosystems, Foster City, USA). KOD-Plus enzyme (Toyobo Co. Ltd., Osaka, Japan) was used for PCR amplification. A negative control without DNA was included in all PCRs. Secondary PCR products were examined after 1% agarose gel electrophoresis and staining with GelRed (Biotium Inc., Hayward, CA).
Nucleotide sequencing and genetic proximity analysis
All positive secondary PCR products were purified using Montage PCR filters (Millipore, Bedford, MA), and sequenced with the secondary PCR primers, by Sinogenomax Biotechnology Co. Ltd. (Beijing, China), using a BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA). The accuracy of nucleotide sequences was confirmed by bidirectional sequencing, and by sequencing additional PCR products if necessary.
Sequences were aligned with reference sequences that had been downloaded from GenBank, using Clustal × 2.1 (http://www.clustal.org/). To assess the phylogenetic relationships between ITS genotypes of E. bieneusi obtained in the present study and those downloaded from Genbank, a phylogenetic tree was constructed using the Neighbor-Joining(NJ) algorithm, based on a matrix of evolutionary distances calculated using the Kimura 2-parameter model, in MEGA 6.0 (http://www.megasoftware.net/). Confidence in the NJ tree was estimated using a bootstrap analysis with 1000 replicates. Nucleotide sequences obtained in this study have been deposited in GenBank under the accession numbers KU604929–KU604934 and KU685395.
Statistical analysis
Infection rates were compared using a chi-square test, with differences considered statistically significant at P < 0.05.
Results
Occurrence and genotypes of E. bieneusi
A total of 160 fecal specimens were examined for E. bieneusi by PCR, with 74 (46.2%) specimens being positive for E. bieneusi. The pathogen was detected in four zoos, including Shanghai Zoo (26.7% of specimens), Shanghai Wild Animal Park (69.1%), Tongling Zoo (69.4%), and Taiyuan Zoo (33.3%), of the six zoos enrolled in this study (Table 1). Differences in prevalence were statistically significant among different zoos (P = 0.006). Phylogenetic analysis showed the sequences came from seven distinct E. bieneusi genotypes, including four known genotypes (D, J, CHG1, and CHG14) and three new genotypes (named CM19, CM20 and CM21 in this study). As shown in Table 1, genotype D (54/74, 73.0%) was dominant, followed by genotype J (14/74, 18.9%); CM19 was found in two specimens (2/74, 2.7%), while the remaining four genotypes were seen in only one specimen each (1/74, 1.4%).
Phylogenetic analysis and genetic proximity
Phylogenetic analysis revealed that genotype D belonged to the previously-described Group 1 (Fig. 2). New genotypes CM19 and CM20 differed from genotype CHG20 (KP262375) in Single Nucleotide Polymorphisms (SNPs) at ITS nucleotide positions 177 (an A to G change), 181 (T to C), 261 (G deletion), 268 (G insertion) and 270 (A to G); while CM20 has another SNP at position 177 (A to G). Compared to genotype CHG3 (KP262389), genotype CM21 had one SNP at position 265 (G to T). Genotypes J, CHG1, CHG14 and the three new genotypes (CM19, CM20, and CM21) formed Group 2 in our tree, which has previously been described as the so-called cattle host specificity group (Fig. 2 and Additional file 2: Table S2).
Fig. 2.
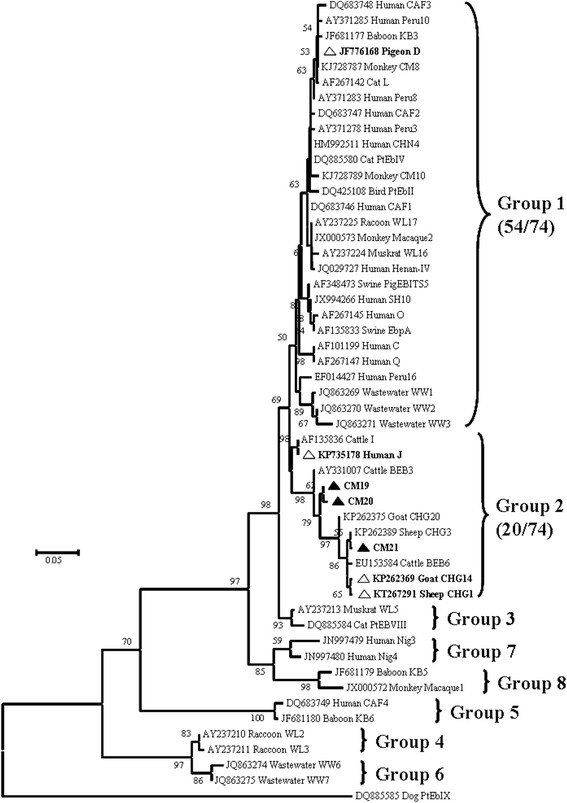
Neighbor-Joining tree of E. bieneusi ITS genotypes. The tree was rooted with GenBank sequence DQ885585. Bootstrap values greater than 50% from 1000 replicates are shown on nodes. Each sequence from GenBank is identified by its accession number, host origin, and genotype designation. The group terminology for the clusters is based on previous papers by Thellier and Breton [6], and Karim et al. [15]. Known and new genotypes identified in this study are indicated by open and filled triangles, respectively
Discussion
In this study, E. bieneusi was found in 46.2% of golden snub-nosed monkeys examined in six zoos in China. The prevalence was higher than that previously reported for golden snub-nosed monkeys in Sichuan (3.5%, 1/29) and Shaanxi (9.5%, 6/63) provinces in China [21, 24]. All of the fecal specimens examined in this study were sampled from six zoos distributed in four locations (Beijing, Shanghai, Anhui Province and Shanxi Province). The infection rates of E. bieneusi in monkeys differed among the four zoos that included positive fecal samples. Similar results (11.4%–29.8% prevalence) have previously been reported in different NHP species from various parts of China [15, 17, 21, 24–26]. Perceived rates of infection are influenced by many factors, including the need for laboratory diagnosis, sample sizes, animal management practices, infection intensity of the pathogen, climate and geography. As a parasite with zoonotic potential, E. bieneusi has been proved to have the probability to transmit across different NHP species or between animals and animal handlers [27–29]. So the prevalence also maybe influenced by other NHPs living nearby or the animal handlers. As an endangered species which needs more care and protection, the snub-nosed monkeys are usually confined away from other NHP species in all the four zoos that included positive fecal samples. Thus an inference was made that the other NHPs contributed little to the high prevalence in this study. Although the fecal samples of animal handlers were not involved in this study, nevertheless, the transmission probability between monkeys and animal handlers is undeniable.
The zoonotic genotype D, which was the commonest in this study, has been observed frequently in other NHPs, including orangutan, gibbon, macaque and baboon [15, 17, 21, 24–26]. Genotype D has also been identified as the most prevalent genotype in humans, in human wastewater, and in many other animal species (cattle, pigs, sheep, dogs, cats, horses, beavers, otters, muskrats, raccoons, pigeons, falcons and foxes) worldwide [3, 8, 17, 29–34]. For example, a study examining 33 stool samples from human immunodeficiency virus (HIV)-infected adult patients in Thailand showed that genotype D was the most common, found in 36.4% (12 of 33) of the samples [29]. In a recent study, a total of 23 ITS genotypes were obtained from wastewater in Shanghai, Nanjing, Wuhan and Qingdao cities, with genotype D being the most prevalent, found in 279 of 338 (82.5%) positive samples [34]. These results demonstrate that interspecies transmission of genotype D poses a zoonotic risk, as well as being of public health significance already within the human population. The second commonest genotype found in this study, J, is most often found in cattle, though it has been reported as being in humans in Jilin province, China [35]. We have also found this genotype in patients from Henan Province, China (unpublished data). These findings suggest that genotype J may be transmissible from golden snub-nosed monkeys to humans, or vice versa.
The three new genotypes (CM19–CM21), CHG1 and CHG14 were all clustered in the previously-described Group 2, along with the zoonotic genotype J. Although these genotypes fall into a group that has previously been considered cattle-specific, genotype J clearly has the ability to infect humans. Thus, these three new genotypes belonging to Group 2 may also have the ability to cause human microsporidiosis.
Conclusion
Our study demonstrates a high prevalence of E. bieneusi genotypes D and J in golden snub-nosed monkey kept in zoos in China. As these genotypes are common to humans and animals, golden snub-nosed monkeys may serve as potential reservoir hosts for zoonotic E. bieneusi genotypes.
Additional files
Distribution of positive samples in different zoo. (XLS 29 kb)
Nucleotide substitutions among all sequences found in this study versus the reported genotype J (KU557671). (XLS 49 kb)
Acknowledgments
We want to thank all staffs who got involved in sample collection in this study at Beijing Zoo, Beijing Wildlife Park, Shanghai Zoo, Shanghai Wild Animal Park, Taiyuan Zoo and Tongling Zoo.
Funding
This study was supported, in part, by the National Natural Science Foundation of China (U1404327), the Key Program of the National Natural Science Foundation of China (31330079), and the Key National Science and Technology Specific Projects (2012ZX10004220–001).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files (Additional file 1: Table S1 and Additional file 2: Table S2).
Authors’ contributions
Conceived and designed the experiments: LXZ. Performed the experiments: FCY, YYW, TYL, SMZ, RJW and SHH. Analyzed the data: FCY, JTW, HLZ and JKC. Wrote the manuscript: FCY, CSN and LXZ. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The present study was carried out in accordance with the Law of the People’s Republic of China on the Protection of Wildlife of adopted in 1988. The research protocol was reviewed and approved by the Research Ethics Committee of Henan Agricultural University. Specimens were collected after acquiring the permission of owners of the zoos. No animals were injured during this procedure.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- HIV
Human immunodeficiency virus
- ITS
Internal transcribed spacer
- IUCN
The International Union for Conservation of Nature
- NHPs
Nonhuman primates
- rRNA
Ribosomal RNA
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12917-017-1084-6) contains supplementary material, which is available to authorized users.
Contributor Information
Fuchang Yu, Email: yufuchang456@qq.com.
Yayun Wu, Email: 420090729@qq.com.
Tongyi Li, Email: zoolty@126.com.
Jianke Cao, Email: 215263029@qq.com.
Jiantang Wang, Email: 923874567@qq.com.
Suhui Hu, Email: 996067061@qq.com.
Huili Zhu, Email: 888julia@163.com.
Sumei Zhang, Email: smzhang2815@henau.edu.cn.
Rongjun Wang, Email: wrj-1978@163.com.
Changshen Ning, Email: nnl1986@163.com.
Longxian Zhang, Phone: 86-371-63555387, Email: zhanglongxian8999@foxmail.com, Email: zhanglx8999@henau.edu.cn, Email: zhanglx8999@gmail.com.
References
- 1.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19(5):485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Didier ES, Weiss LM. Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis. 2011;24(5):490–495. doi: 10.1097/QCO.0b013e32834aa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matos O, Lobo ML, Xiao L. Epidemiology of Enterocytozoon bieneusi Infection in Humans. J Parasitol Res. 2012; doi:10.1155/2012/981424. [DOI] [PMC free article] [PubMed]
- 4.Mor SM, Tumwine JK, Naumova EN, Ndeezi G, Tzipori S. Microsporidiosis and malnutrition in children with persistent diarrhea. Uganda Emerg Infect Dis. 2009;15(1):49–52. doi: 10.3201/eid1501.071536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sak B, Brady D, Pelikánová M, Květoňová D, Rost M, Kostka M, et al. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol. 2011;49(3):1064–70. doi: 10.1128/JCM.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thellier M, Breton J. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite. 2008;15(3):349–358. doi: 10.1051/parasite/2008153349. [DOI] [PubMed] [Google Scholar]
- 7.Karim MR, Yu F, Li J, Li J, Zhang L, Wang R, et al. First molecular characterization of enteric protozoa and the human pathogenic microsporidian, Enterocytozoon bieneusi, in captive snakes in China. Parasitol Res. 2014;113(8):3041–8. [DOI] [PubMed]
- 8.Santín M, Fayer R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci. 2011;90(3):363–371. doi: 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Desportes I, Charpentier YL, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, et al. Occurrence of a New Microsporidan: Enterocytozoon bieneusi ng, n. sp., in the Enterocytes of a Human Patient with AIDS1. J Protozool. 1985;32(2):250–4. [DOI] [PubMed]
- 10.Henriques-Gil N, Haro M, Izquierdo F, Fenoy S, del Águila C. Phylogenetic approach to the variability of the microsporidian Enterocytozoon bieneusi and its implications for inter-and intrahost transmission. Appl Environ Microbiol. 2010;76(10):3333–3342. doi: 10.1128/AEM.03026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Huang J, Karim MR, Zhao J, Dong H, Ai W, et al. Zoonotic Enterocytozoon bieneusi genotypes in Pere David’s deer (Elaphurus davidianus) in Henan. China Exp Parasitol. 2015;155:46–8. [DOI] [PubMed]
- 12.Zhao W, Zhang W, Yang D, Zhang L, Wang R, Liu A. Prevalence of Enterocytozoon bieneusi and genetic diversity of ITS genotypes in sheep and goats in China. Infect Genet Evol. 2015;32:265–270. doi: 10.1016/j.meegid.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z, Wang R, Zhao W, Qi M, Zhao J, Zhang L, et al. Genotyping and subtyping of Giardia and Cryptosporidium isolates from commensal rodents in China. Parasitology. 2015;142(6):800–6. [DOI] [PubMed]
- 14.Fiuza VR, Oliveira FC, Fayer R, Santin M. First report of Enterocytozoon bieneusi in pigs in Brazil. Parasitol Int. 2015;64(4):18–23. doi: 10.1016/j.parint.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Karim MR, Wang R, Dong H, Zhang L, Li J, Zhang S, et al. Genetic polymorphism and zoonotic potential of Enterocytozoon bieneusi from nonhuman primates in China. Appl Environ Microbiol. 2014;80(6):1893–8. [DOI] [PMC free article] [PubMed]
- 16.Guo Y, Alderisio KA, Yang W, Cama V, Feng Y, Xiao L. Host specificity and source of Enterocytozoon bieneusi genotypes in a drinking source watershed. Appl Environ Microbiol. 2014;80(1):218–225. doi: 10.1128/AEM.02997-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim MR, Dong H, Li T, Yu F, Li D, Zhang L, et al. Predomination and new genotypes of Enterocytozoon bieneusi in captive nonhuman primates in zoos in China: high genetic diversity and zoonotic significance. PLoS One. 2015;10(2):e0117991. [DOI] [PMC free article] [PubMed]
- 18.Wen R. Research for the Species of Rhinopithecus and Their Distribution in Ancient and Modern Times. Chinese J Nature. 2003;25(1):41–48. [Google Scholar]
- 19.Yongcheng L, Richardson M. Rhinopithecus roxellana. In: The IUCN Red List of Threatened Species 2008. the International Union for Conservation of Nature. 2008. http://www.iucnredlist.org/details/39829/0. Accessed 18 Feb 2016.
- 20.Yu Z. A Comparative Analysis of the Reproductive Fitness of a Captive Population of Golden Snub-Nosed Monkey. Chinese J Wildlife. 2011;32(2):69–72. [Google Scholar]
- 21.Du SZ, Zhao GH, Shao JF, Fang YQ, Tian GR, Zhang LX, et al. Qi M, Yu SK. Cryptosporidium spp., Giardia intestinalis, and Enterocytozoon bieneusi in Captive Non-Human Primates in Qinling Mountains. Korean J Parasitol. 2015;53(4):395–402. [DOI] [PMC free article] [PubMed]
- 22.Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW, Xiao L. Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol. 2003;69(8):4495–4501. doi: 10.1128/AEM.69.8.4495-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckholt MA, Lee JH, Tzipori S. Prevalence of Enterocytozoon bieneusi in Swine: an 18-Month Survey at a Slaughterhouse in Massachusetts. Appl Environ Microbiol. 2002;68(5):2595–2599. doi: 10.1128/AEM.68.5.2595-2599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karim MR, Wang R, He X, Zhang L, Li J, Rume FI, et al. Multilocus sequence typing of Enterocytozoon bieneusi in nonhuman primates in China. Vet Parasitol. 2014;200(1–2):13–23. [DOI] [PubMed]
- 25.Li W, Kiulia NM, Mwenda JM, Nyachieo A, Taylor MB, Zhang X, et al. Cyclospora papionis, Cryptosporidium hominis, and human-pathogenic Enterocytozoon bieneusi in captive baboons in Kenya. J Clin Microbiol. 2011;49(12):4326–9. [DOI] [PMC free article] [PubMed]
- 26.Ye J, Xiao L, Li J, Huang W, Amer SE, Guo Y, et al. Occurrence of human-pathogenic Enterocytozoon bieneusi, Giardia duodenalis and Cryptosporidium genotypes in laboratory macaques in Guangxi. China Parasitol Int. 2014;63(1):132–7. [DOI] [PubMed]
- 27.Karim MR. Molecular epidemiology and multilocus typing of Croptosporidium spp., Giardia duodenalis and Enterocytozoon bieneusi in nonhuman primates in China. Zhengzhou: Henan Agricultural University; 2014. [Google Scholar]
- 28.Ye P. The Research on Gastrointestinal Parasites in Provisioned and Wild-raiding in Sichuan Snub-nosed monkeys (Rhinopithecus roxellanain) in Autumn and Winter. Changsha: South University of Forestry and Technology; 2013. [Google Scholar]
- 29.Wan Q, Xiao L, Zhang X, Li Y, Lu Y, Song M, et al. Clonal Evolution of Enterocytozoon bieneusi Populations in Swine and Genetic Differentiation in Subpopulations between Isolates from Swine and Humans. PLoS Negl Trop Dis. 2016;10(8):e0004966. [DOI] [PMC free article] [PubMed]
- 30.Leelayoova S, Subrungruang I, Suputtamongkol Y, Worapong J, Petmitr PC, Mungthin M. Identification of genotypes of Enterocytozoon bieneusi from stool samples from human immunodeficiency virus-infected patients in Thailand. J Clin Microbiol. 2006;44(8):3001–3004. doi: 10.1128/JCM.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori H, Mahittikorn A, Thammasonthijarern N, Chaisiri K, Rojekittikhun W, Sukthana Y. Presence of zoonotic Enterocytozoon bieneusi in cats in a temple in central Thailand. Vet Parasitol. 2013;197(3–4):696–701. doi: 10.1016/j.vetpar.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Mori H, Mahittikorn A, Watthanakulpanich D, Komalamisra C, Sukthana Y. Zoonotic potential of Enterocytozoon bieneusi among children in rural communities in Thailand. Parasite. 2013;20:14. doi: 10.1051/parasite/2013014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santín M, Cortés Vecino JA, Fayer R. A zoonotic genotype of Enterocytozoon bieneusi in horses. J Parasitol. 2010;96(1):157–161. doi: 10.1645/GE-2184.1. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Zhang W, Yang Z, Liu A, Zhang L, Yang F, et al. Genotyping of Enterocytozoon bieneusi in Farmed Blue Foxes (Alopex lagopus) and Raccoon Dogs (Nyctereutes procyonoides) in China. PLoS One. 2015;10(11):e0142611. [DOI] [PMC free article] [PubMed]
- 35.Li N, Xiao L, Wang L, Zhao S, Zhao X, Duan L, et al. Molecular surveillance of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi by genotyping and subtyping parasites in wastewater. PLoS Negl Trop Dis. 2012;6(9):e1809. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of positive samples in different zoo. (XLS 29 kb)
Nucleotide substitutions among all sequences found in this study versus the reported genotype J (KU557671). (XLS 49 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files (Additional file 1: Table S1 and Additional file 2: Table S2).


