Abstract
Apart from transplant, there are no satisfactory therapies for the severe depression in contractility in familial dilated cardiomyopathy (DCM). Current heart failure treatments that act by increasing contractility involve signaling cascades that alter calcium homeostasis and induce arrhythmias. Omecamtiv mecarbil is a promising new inotropic agent developed for heart failure that may circumvent such limitations. Omecamtiv is a direct cardiac myosin activator that promotes and prolongs the strong myosin–actin binding conformation to increase the duration of systolic elastance. We tested the effect of omecamtiv on Ca2+ sensitivity of myofilaments of a DCM mouse model containing a tropomyosin E54K mutation. We compared tension and ATPase activity of detergent-extracted myofilaments with and without treatment with 316 nM omecamtiv at varying pCa values. When transgenic myofilaments were treated with omecamtiv, the pCa50 for activation of tension increased from 5.70 ± 0.02 to 5.82 ± 0.02 and ATPase activity increased from 5.73 ± 0.06 to 6.07 ± 0.04. This significant leftward shift restored Ca2+ sensitivity to levels no longer significantly different from controls. Proteomic studies lacked changes in sarcomeric protein phosphorylation. Our data demonstrate that omecamtiv can potentially augment cardiac contractility in DCM by increasing Ca2+ sensitivity. The use of direct myosin activators addresses functional defects without incurring the adverse side effects of Ca2+-dependent treatments.
Keywords: ATPase activity, sarcomere, Ca-sensitization
INTRODUCTION
There is ample evidence that familial dilated cardiomyopathy (DCM) is linked commonly to sarcomeric mutations inducing a depression in response to Ca2+.1 Yet, despite this knowledge, the ultimate cause of the disorder therapies is lacking. We have investigated whether a novel sarcomere activator, omecamtiv mecarbil, is able to increase response to Ca2+ in myofilaments from a DCM mouse model expressing a mutant tropomyosin (TM). The specific mutation identified in α-TM results in a localized charge reversal by replacement of an acidic residue with a basic one (Glu54Lys).2 The resulting positive charge causes a disruption in the electrostatic actin-TM interaction and regulatory mechanism.2,3 In a previous study, transgenic mice were generated that express mutant α-TM Glu54Lys in the adult hearts so that the effect on cardiac development and function could be addressed.4 Consistent with the phenotype seen in human DCM, these mice exhibited a dilation of the ventricles and decreased left ventricular (LV) fractional shortening, as well as impaired systolic and diastolic functions.2,4 Notably, this transgenic model presented significantly decreased myofilament Ca2+ sensitivity, agreeing with previous studies that indicate alterations in TM function and sensitivity deficiencies are associated with the Glu54Lys mutation.4–7
Devastating mutations such as α-TM (Glu54Lys) that decrease cardiac contractility require intensive treatment, yet effective pharmacological options are limited. Many current therapies aim to block neurohormonal activation using renin–angiotensin pathway inhibitors, and/or β-adrenergic or aldosterone blockers, rather than to restore contractile function.8 Inotropic drugs, such as phosphodiesterase inhibitors and β-adrenergic receptor agonists, work by activating second messenger signaling pathways to increase the intracellular calcium, which indirectly increases systolic function. Unfortunately, leading hypotheses on these drugs suggest that they increase heart rate (HR), cause arrhythmias, and create maladaptive Ca2+-dependent signaling.9–11
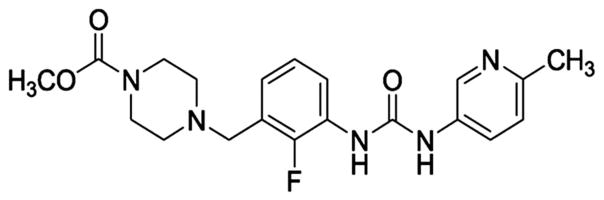
A recent and novel approach to improve systolic function may address these limitations by activating cardiac myosin directly using the compound omecamtiv mecarbil, formerly called CK-1827452 (Cytokinetics, Inc, South San Francisco, CA) (Fig. 1). It directly binds to and activates the S1 domain of myosin and accelerates the transition of the actin–myosin complex from the weakly bound state to the strongly bound force-producing state. Ultimately, this increases the number of myosin heads interacting with the thin filament during systole, while reducing the rate of nonproductive adenosine triphosphate (ATP) hydrolysis.12–14 In isolated ventricular cardiac myocytes, omecamtiv significantly increases contractility in the absence of changes to the calcium transient.15 In vivo studies of mongrel dogs with HF, induced by myocardial infarction and rapid ventricular pacing, show significant improvements in cardiac function after acute infusion with omecamtiv.16,17 There were marked increases in wall thickness and fractional shortening in the absence of changes in loading conditions. Omecamtiv also produced increases in stroke volume and cardiac output, while coincidently lowering heart rate (HR). Importantly, the improvements in systolic function were observed without changes to myocardial energetics.17
FIGURE 1.
Chemical structure of omecamtiv mecarbil.
In this study, we sought to investigate the effects of omecamtiv mecarbil on myofilaments from nontransgenic controls and the DCM mutant α-TM Glu54Lys transgenic mouse model. We determined myofilament tension and myofibrillar ATPase activity as a function of free Ca2+ concentration. This approach provided additional insight into the proposed mechanism of omecamtiv and the sarcomeric response to direct activation. Our findings support the proposed hypothesis that omecamtiv may provide inotropic support not only in acquired HF but also in the depressed contractility associated with DCM.
METHODS
Animal Model of DCM
All experiments were conducted in accordance with the University of Illinois at Chicago Animal Care and Use Committee. The DCM mutant α-TM54 transgenic mice were generated as previously described using the FVB/N strain.4
Isolation of Cardiac Myofibrils
Four-month-old female transgenic (TM54) and non-transgenic (NTG) mice were anesthetized with a 200 mg/kg ketamine per 20 mg/kg xylazine mixture.18 The hearts were excised and immediately prepared fresh using 30–40 mg tissue as previously described but with minor modifications.19,20 The tissue was first homogenized in a 2 mL dounce homogenizer in 1 mL of standard relax buffer (10 mM imidazole pH 7.2, 75 mM KCl, 2 mM MgCl2, 2 mM EDTA, and 1 mM NaN3) with collagenase type I (Worthington Biochemical Corp) at 1 mg/mL. After centrifugation and removal of the supernatant, the pellets were washed twice in standard buffer with 1% vol/vol Triton X-100 and then again in standard relax buffer only to wash out the triton. The last wash was in 1 mL of a modified A-70 buffer (87.5 mM NaCl, 12.5 mM MgCl2, 50 mM MOPS, pH 7.0, 2 mM NaN3), after which the pellet was resuspended in the modified A-70 buffer at a 1:20 ratio.21 All standard relax buffers contained both protease and phosphatase inhibitors at a 1:100 dilution. A DC assay (Bio-Rad, Hercules, CA) was used to determine protein concentration.
Isolation of Detergent-extracted Skinned Fiber Bundles
Four-month-old male and female mice were heparinized, anesthetized, and hearts extracted. Left ventricular papillary muscles were then isolated, dissected into fiber bundles approximately 200 μm in width and 3–4 mm in length, and detergent extracted in a high relaxing (HR) solution (10 mM EGTA, 41.89 mM K-Prop, 100 mM BES, 6.75 mM MgCl2, 6.22 mM Na2ATP, 10 mM Na2CrP, 5 mM NaN3, pH 7.0) with 1% vol/vol Triton X-100 for 3–4 hours at 4°C. The HR solution was then replaced with HR solution without Triton X-100. Free Ca2+ concentrations were calculated using WEBMAXC Standard and ranged from pCa [−log (Ca2+)] values of 8.0 to 4.5. Free Ca2+ concentrations were generated by mixing varying ratios of HR solution with HR solution containing 9.99 mM CaCl2.
Measurement of ATPase Activity
Myofibril protein suspensions were diluted to 0.5 mg/mL in the modified A-70 buffer. Myofibrils were added to the reaction tubes so that final conditions were 20 μg total protein, 35 mM NaCl, 5 mM MgCl2, 1 mM EGTA, and 20 mM MOPS, pH 7.0 with varied CaCl2 concentrations ranging from pCa values of 7.7 to 4.6. For dose–response experiments, omecamtiv mecarbil was added to the reaction tubes, containing either NTG or transgenic myofibrils, at logarithmic concentrations ranging from 0 to 31.6 μM. A pCa value of 7.031 was used for all dosing experiments. The half-maximal effective concentration (EC50) value was used to select an appropriate dose of 316 nM. Either 316 nM omecamtiv or vehicle (0.667% DMSO) was administered to the myofibrils in each reaction tube. We determined myofibrillar ATPase activity at 25°C by beginning the reaction with 2.5 mM ATP and subsequently quenching the reaction with 0.2 M perchloric acid at 10°C at various times. Liberation of inorganic phosphate was measured after a 30-minute incubation at 25°C using a malachite green assay read by spectrophotometric absorbance at 655 nm.22
Measurement of the Force-Ca2+ Relationship
Fibers bundles were mounted between a micromanipulator and a force transducer and bathed in HR solution containing 0.06% DMSO. The sarcomere length was set to 2.3 μm using He-Ne laser diffraction and kept constant throughout the experiment. The fibers were initially contracted at saturating Ca2+ concentration and the width and diameter measured along 3 points. Fibers were then subjected to sequential increases in Ca2+ concentration containing 0.06% DMSO, and their developed force was recorded on a chart recorder. Using the same fiber bundle, a second curve was recorded at the same Ca2+ concentrations containing 316 nM omecamtiv mecarbil, 0.06% DMSO, after a 5-minute preincubation of HR containing 316 nM omecamtiv mecarbil, 0.06% DMSO. All experiments were performed at 22°C.21,23,24
Gel Electrophoresis
Myofibrils from reaction tubes at pCa value 6.0 were kept from ATPase assays and further solubilized in sample buffer (8 M urea, 2 M thiourea, 0.05 M Tris-HCl pH 6.8, 75 mM DTT, 3% SDS, 0.05% bromophenol blue).25 An RC-DC assay (Bio-Rad) was used to determine concentrations, and 3 μg protein was loaded onto a 12% SDS-PAGE26 gel and run at 200 V constant voltage for 80 minutes. When finished running, the gel was placed in a fix solution (50% methanol, 10% acetic acid) overnight and then stained with PRO-Q Diamond Stain (Invitrogen) following the manufacturer’s protocol. The gel was imaged on a Typhoon 9410 imager using a CY3 filter set and subsequently stained with Coomassie blue to visualize total protein.
Data and Statistical Analysis
The tension and ATPase data were fit using the following modified Hill’s equation, which extends from the previous equation but allows for a variable: Y = Bottom + (Top−Bottom)/(1 + 10(pCa50−pCa)−Hillslope), where Y is the ATPase activity, Bottom is the minimum ATPase value, Top is the maximum ATPase value, pCa50 is the pCa at which 50% of the maximum value is reached, pCa is the given value, and the Hillslope describes the steepness of the curve. Tension was calculated as force per cross-sectional area. Isometric tension measurements were plotted as a function of pCa and fit by a nonlinear least squares regression analysis to the Hill’s equation. A 2-way analysis of variance with a Bonferroni’s post hoc method was used to compare maximum, minimum, max tension, pCa50, and Hill slope values with one another. An unpaired t test with Welch’s correction was used for analyzing a specific point on the ATPase curve against another. The Pro-Q data were analyzed using a 2-way analysis of variance, with a Newman–Keuls post hoc method. All values are presented as mean ± standard error of the mean (SEM) with a level of significance set at P < 0.05.
RESULTS
Myofibrillar ATPase Activity
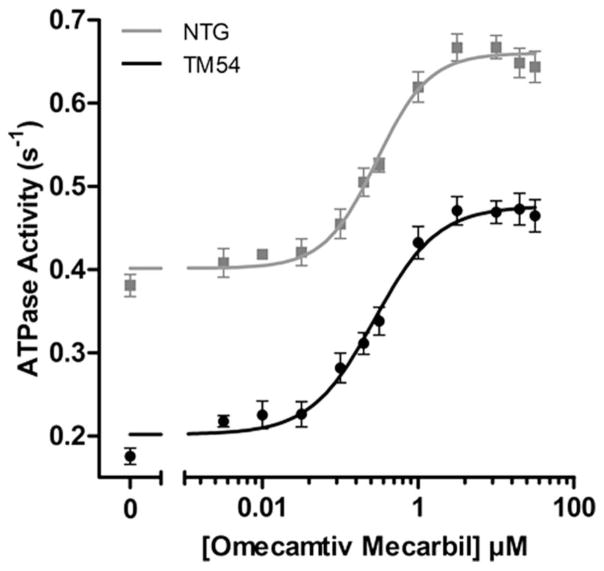
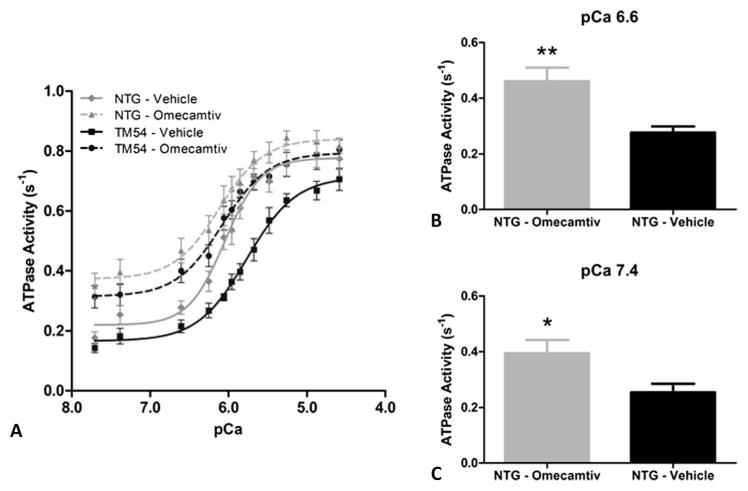
To determine an appropriate dosage of omecamtiv, dose–response experiments were performed in both the TM54 and NTG mice (Fig. 2), giving EC50 values of 271 and 292 nM, respectively. These values were not significantly different; however, significant differences were seen in the minimum and maximum between the 2 groups. A concentration of 316 nM was selected and used to observe the direct effects of omecamtiv on myofilament response to Ca2+. We compared ATPase activity of isolated myofibrillar preparations with and without treatment of 316 nM omecamtiv (Fig. 3A). The pCa50 for NTG-Vehicle controls was 6.01 ± 0.03 seconds−1 (n = 14) compared with 5.73 ± 0.06 seconds−1 (n = 8) for TM54-Vehicle myofibrils (Table 1), presenting a significant rightward shift and desensitization to Ca2+ in the TM54 samples that is consistent with previous studies.9 When TM54 myofibrils were subjected to 316 nM omecamtiv, the pCa50 increased to 6.07 ± 0.04 seconds−1 (n = 11), demonstrating a significant leftward shift in ATPase activity. This increased pCa50 in TM54 after treatment with omecamtiv brings Ca2+ responsiveness near that of the NTG-Vehicle, in which the pCa50 values between the 2 were not significantly different. Additionally, the pCa50 of NTG-Omecamtiv also increased to 6.14 ± 0.05 seconds−1 (n = 11) but was not significantly different from before treatment, and thus, t tests were used to analyze specific pCa values between NTG-Vehicle and NTG-Omecamtiv. Significant differences in the ATPase rates at pCa 6.6 (Fig. 3B) and pCa 7.4 (Fig. 3C) were observed. There were also significant changes in the minimum ATPase rates, specifically in the NTG-Vehicle, which increased from 0.18 ± 0.02 to 0.35 ± 0.04 seconds−1. The Hill’s coefficients were not significantly different between any groups.
FIGURE 2.
Dose dependence of omecamtiv mecarbil. Stimulation of NTG and DCM TM54 myofibrils at pCa 7.0. NTG EC50 = 0.29 ± 0.06 μM (n = 5) and TM54 EC50 = 0.27 ± 0.07 μM (n = 9). All ATPase values are given as mean ± SEM and are significantly lower in TM54 versus NTG myofibrils. P < 0.05.
FIGURE 3.
Effect of omecamtiv mecarbil on pCa-ATPase activity relations of (A) TM54 and NTG myofibrils treated with both DMSO (vehicle) and 316 nM omecamtiv mecarbil over varying pCa values. ATPase values are given as mean ± SEM (n = 8– 14 hearts). B, Comparison of ATPase activity in NTG-Omecamtiv versus NTG-Vehicle myofibrils at pCa 6.6. C, Comparison of ATPase activity in NTG-Omecamtiv versus NTG-Vehicle myofibrils at pCa 7.4. Histograms present rates as mean ± SEM. *P < 0.05, **P < 0.01.
TABLE 1.
Assessment of Myofibrillar ATPase Activity and Cooperativity
| Groups | Min (1/s) | Max (1/s) | pCa50 | Hill Slope | n |
|---|---|---|---|---|---|
| TM54-Vehicle | 0.14 ± 0.02 | 0.71 ± 0.04 | 5.73 ± 0.06 | 1.39 ± 0.22 | 8 |
| TM54-Omecamtiv | 0.31 ± 0.04*† | 0.81 ± 0.03 | 6.07 ± 0.04‡ | 1.47 ± 0.33 | 11 |
| NTG-Vehicle | 0.18 ± 0.02 | 0.76 ± 0.04 | 6.01 ± 0.03‡ | 1.92 ± 0.38 | 14 |
| NTG-Omecamtiv | 0.35 ± 0.04†‡ | 0.82 ± 0.03 | 6.14 ± 0.05‡ | 1.51 ± 0.39 | 11 |
Values are mean ± SEM.
P < 0.05.
P < 0.05 versus NTG-Vehicle.
P < 0.0002 versus TM54-Vehicle.
Skinned Fiber Tension Measurements
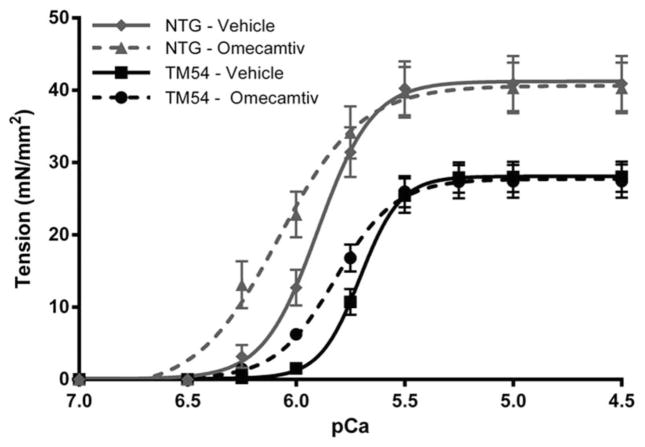
Compared with NTG-Vehicle fibers, TM54-Vehicle fiber bundles display a significant decrease in Ca2+ sensitivity of tension, as measured by the pCa of half-maximal tension (pCa50), and maximal tension (Fig. 4). On treatment with 316 nM omecamtiv, TM54 fibers show a significant increase in pCa50 compared with TM54-Vehicle fibers (5.70 ± 0.02 vs. 5.82 ± 0.02), which was no longer significantly different from NTG-Vehicle fibers (Table 2). Omecamtiv had a similar effect on NTG fibers, significantly increasing the pCa50 in NTG-Omecamtiv fibers compared with NTG-Vehicle (5.91 ± 0.03 vs. 6.05 ± 0.03). In both groups, the addition of omecamtiv significantly decreased the Hill’s slope, indicating a decrease in cooperativity of the fibers. As observed in a previous study from our laboratory, maximal tension was significantly decreased in the TM54-Vehicle fibers compared with those from NTG-Vehicle4; however, omecamtiv had no effect on maximal tension generation in either group.
FIGURE 4.
Tension-pCa relation in skinned fiber bundles from TM54 and NTG myofilaments treated with DMSO (vehicle) and 316 nM omecamtiv mecarbil over varying pCa values. Tension-pCa relations are presented as mean ± SEM (n = 9–11 fibers).
TABLE 2.
Assessment of the Force-calcium Relationship in Detergent-extracted Fiber Bundles
| Groups | Max Tension, mN/mm2 | pCa50 | Hill Slope | n |
|---|---|---|---|---|
| TM54-Vehicle | 28.08 ± 2.10 | 5.70 ± 0.02 | 5.20 ± 0.13 | 11 |
| TM54-Omecamtiv | 27.43 ± 2.28 | 5.82 ± 0.02* | 3.28 ± 0.10† | 10 |
| NTG-Vehicle | 40.93 ± 3.83*‡ | 5.91 ± 0.03† | 3.73 ± 0.18† | 10 |
| NTG-Omecamtiv | 40.32 ± 3.51*‡ | 6.05 ± 0.03†§¶ | 2.85 ± 0.16†|| | 9 |
Values are mean ± SEM.
P < 0.05.
P < 0.0001 versus TM54-Vehicle.
P < 0.05.
P < 0.0001 versus TM54-Omecamtiv.
P < 0.01.
P < 0.001 versus NTG-Vehicle.
Phosphorylation of Sarcomeric Proteins
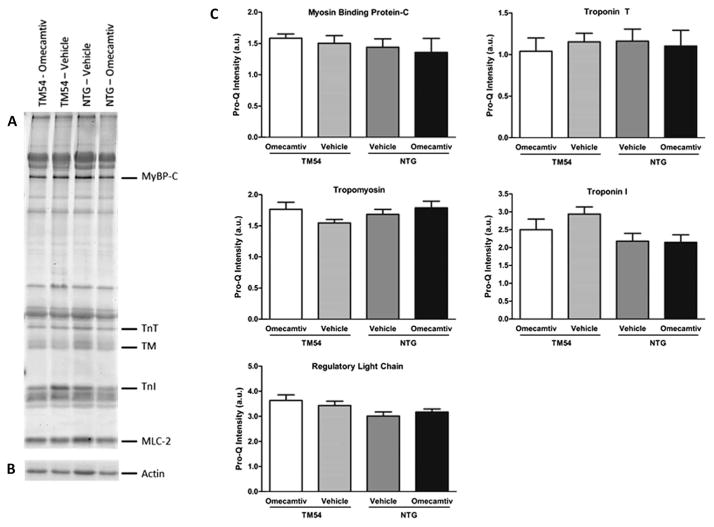
To determine whether the mechanism for increased Ca2+ sensitivity of ATPase in the omecamtiv-treated TM54 samples involved a change in phosphorylation of myofilament proteins, we used a proteomic analysis. Figure 5A shows a representative image from the Pro-Q Diamond gel staining and actin from a Coomassie stained gel used for normalization (Fig. 5B). Results indicated no changes in the phosphorylation of sarcomeric proteins (Fig. 5C), specifically in myosin-binding protein C, troponin T, TM, troponin I, and regulatory light chain. Thus, our data indicate that protein phosphorylation levels do not account for differences seen in ATPase activity. This also supports the current mechanistic model that omecamtiv is a direct cardiac myosin S1 activator.
FIGURE 5.
The effect of omecamtiv mecarbil on myofilament protein phosphorylation. A, Representative image of Pro-Q Diamond stained gel specific for myofilament proteins of interest. +, Peppermint stick standard; MLC-2, regulatory light chain; MyBP-C, myosin-binding protein C; TnI, troponin I; TnT, troponin T. B, A region of interest from a Coomassie stained gel showing actin, which was used for normalization. C, Histograms displaying the relative phosphorylation levels in arbitrary units (a.u.), presented as mean ± SEM (n = 5–6, no significant differences).
DISCUSSION
Our data are the first to test whether omecamtiv mecarbil is a potential inotropic agent therapeutically effective in familial DCM. Our data also provide support for the mechanism of omecamtiv mecarbil as a direct cardiac myosin activator that improves Ca2+ response depressed by a thin filament mutation affecting the actin–myosin interaction. Omecamtiv treatment restored the Ca2+ sensitivity of tension and ATPase activity of myofilaments from DCM α-TM54 hearts to NTG levels without modifying the phosphorylation of myofilament proteins.
Previous studies have tested the effects of the Ca2+-sensitizing agent, pimobendan, in a model of DCM linked to a deletion mutant of TnT.27,28 In this model, pimobendan reversed the reduced myofilament Ca2+ sensitivity, prevented myocardial remodeling, and prolonged survival.28 Yet, a subsequent study determined that pimobendan treatment in this model induced an increased risk of sudden cardiac death.27,28 Moreover, pimobendan induced triggered activity in cardiomyocytes isolated from the DCM mice, likely due to early or delayed depolarization. This increased risk of arrhythmias most likely arises from an elevated cytoplasmic Ca2+ induced by phosphodiesterase III (PDEIII) inhibition.29 Similarly, the Ca2+-sensitizer levosimendan shows slight improvements to Ca2+ sensitivity yet also increased cAMP levels and phosphorylation of several sarcomeric and Ca2+-handling proteins. Although it has been shown to act preferably as a Ca2+ sensitizer, at high concentrations levosimendan will inhibit PDE III activity, contributing to the observed positive inotropy.30 Increases in cAMP levels can have downstream effects on protein kinase A (PKA) that increase phosphorylation of proteins controlling Ca2+ homeostasis. In contrast, omecamtiv mecarbil was specifically screened with the criteria that it improves cardiac function without inhibiting PDE III or inducing changes in the Ca2+ transient.13
Omecamtiv specifically improves cardiac function by activating the S1 domain of cardiac myosin and was shown to bind to purified cardiac myosin S1 at a 1.6 ± 0.3 μM affinity, with one molecule of omecamtiv binding per cardiac myosin S1. Tandem mass spectrometry identified a peptide labeled with an omecamtiv mecarbil analog at Ser148, which resides in a region where the converter domain and relay helix converge at the base of the lever arm.15 These findings were consistent with previous crystallographic studies that suggest the positions of such structural elements can affect chemical transitions in the nucleotide binding pocket and ultimately the motion of the lever arm to generate a power stroke.31,32 The proposed binding site of omecamtiv allows for allosteric modulation of the cardiac myosin motor, and on binding, an increase in the number of myosin heads, which interact with the actin filament in a force-producing manner.
An important aspect of our data is that in the case of ATPase activity, the relative effect of omecamtiv in increasing myofibrillar response to Ca2+ was greater in preparations from DCM hearts than from controls. Compared with vehicle controls, omecamtiv increased the pCa50 for activation of DCM myofibrils by 0.34 pCa units, whereas with NTG myofibrils, an increase of 0.13 pCa units. In a study comparing the effect of omecamtiv in controls and dog hearts in systolic failure, there was an effect of omecamtiv on controls, which was nearly the same in the failing hearts.15 Similarly, when investigating the effects of omecamtiv in a loaded model using skinned fibers, the difference in pCa50 after treatment with omecamtiv was 0.12 pCa units in TM54 fibers compared with 0.14 pCa units in NTG. Thus, our data suggest that although the effect of omecamtiv is robust in myofibrillar ATPase measurements, once the sarcomeres are loaded and under physiological constraints, the relative effect of treatment is similar between NTG and TM54 groups. In the case of the DCM myofibrils, submaximum tension (Fig. 4)4 and ATPase activity (Figs. 3A and 4) are depressed, thus indicating that relatively more cross-bridges are available for recruitment by omecamtiv. Omecamtiv is shown to restore Ca2+ sensitivity in both systems presented here to levels no longer significantly different from NTG myofilaments, whereas maximum tension remained unchanged in both groups after omecamtiv. Although improvements in maximal tension generation would be beneficial to the TM54 model of DCM, the results fit not only with the TM54 mutation but also with the mechanism of omecamtiv. As described earlier, the mutation involves a localized charge reversal from an acidic glutamic acid residue to a basic lysine residue, resulting in a disruption of the actin-TM interaction with fewer cross-bridges reacting with the thin filament at full Ca2+ activation. Omecamtiv directly targets the myosin S1 head, promoting entry of cross-bridges into the force generating state. Although omecamtiv improves the response to Ca2+, it does not target the thin filament, and it is apparent that its mechanism does not involve any direct conformational changes that could restore the actin-TM interaction in the TM54 model. We therefore think our studies not only extend the therapeutic application of myosin activators but also add a new insight into the effects of this mode of myofilament activation in cardiac disorders.
This is the first study to demonstrate the effect of omecamtiv mecarbil on skinned fiber tension and myofibrillar ATPase activity in a DCM model, the α-TM54 mutant, and presents significant changes that may attenuate the dysfunction present in live models, such as augmenting contractility and restoring the cardiac structure. Our findings help to stress the potential of omecamtiv mecarbil as a promising treatment for heart failure and convey the importance of stimulating sarcomeric activity as a new therapeutic approach.33
CONCLUSIONS
There is a significant interest in the activation of myofilament proteins as a therapeutic approach to treating HF. Our results emphasize the potential benefits of omecamtiv mecarbil, a direct cardiac myosin activator in familial DCM, and provide additional insight into aspects of its mechanism. The data presented here indicate that omecamtiv increases myofilament ATPase activity, in both a loaded and unloaded system using a mutant DCM model of HF, by which Ca2+ sensitivity is improved to NTG levels and no changes in phosphorylation levels of sarcomeric proteins are observed. Our findings suggest that the use of direct myosin activators provides unique advantages in the ability to address contractile defects of a DCM model without eliciting adverse side effects associated with PDE III inhibition. Future studies could involve in vivo treatment with omecamtiv and successive measurement of changes to the phosphorylation of sarcomeric and calcium handling proteins, which may provide insight into any additional effects and modifications.
Acknowledgments
Supported by NIH Grant PO1 HL 62426 (Project 1, Core C); AHA Grant 15PRE22180010 (to D.M.R.).
The authors thank Chad Warren for his helpful insights and support when designing experiments.
Footnotes
R. J. Solaro is a member of the Scientific Advisory Board of Cytokinetics, Inc. The other authors report no conflicts of interest.
References
- 1.Towbin JA. Inherited cardiomyopathies. Circ J. 2014;78:2347–2356. doi: 10.1253/circj.cj-14-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson TM, Kishimoto NY, Whitby FG, et al. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J Mol Cell Cardiol. 2001;33:723–732. doi: 10.1006/jmcc.2000.1339. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan AD, Stewart M. The 14-fold periodicity in alpha-tropomyosin and the interaction with actin. J Mol Biol. 1976;103:271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- 4.Rajan S, Ahmed RP, Jagatheesan G, et al. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101:205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- 5.Chang AN, Harada K, Ackerman MJ, et al. Functional consequences of hypertrophic and dilated cardiomyopathy-causing mutations in alpha-tropomyosin. J Biol Chem. 2005;280:34343–34349. doi: 10.1074/jbc.M505014200. [DOI] [PubMed] [Google Scholar]
- 6.Mirza M, Marston S, Willott R, et al. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem. 2005;280:28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- 7.Mirza M, Robinson P, Kremneva E, et al. The effect of mutations in alpha-tropomyosin (E40K and E54K) that cause familial dilated cardiomyopathy on the regulatory mechanism of cardiac muscle thin filaments. J Biol Chem. 2007;282:13487–13497. doi: 10.1074/jbc.M701071200. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228–238. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 9.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113:305–315. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–1816. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 11.Petersen JW, Felker GM. Inotropes in the management of acute heart failure. Crit Care Med. 2008;36(1 suppl):S106–S111. doi: 10.1097/01.CCM.0000296273.72952.39. [DOI] [PubMed] [Google Scholar]
- 12.Teerlink JR. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail Rev. 2009;14:289–298. doi: 10.1007/s10741-009-9135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik FI, Morgan BP. Cardiac myosin activation part 1: from concept to clinic. J Mol Cell Cardiol. 2011;51:454–461. doi: 10.1016/j.yjmcc.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Solaro RJ. CK-1827452, a sarcomere-directed cardiac myosin activator for acute and chronic heart disease. IDrugs. 2009;12:243–251. [PubMed] [Google Scholar]
- 15.Malik FI, Hartman JJ, Elias KA, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komamura K, Shannon RP, Pasipoularides A, et al. Alterations in left ventricular diastolic function in conscious dogs with pacing-induced heart failure. J Clin Invest. 1992;89:1825–1838. doi: 10.1172/JCI115787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen YT, Malik FI, Zhao X, et al. Improvement of cardiac function by a cardiac myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522–527. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 18.Fish R, Danneman PJ, Brown M, et al. Anesthesia and Analgesia in Laboratory Animals. Amsterdam, The Netherlands: Elsevier Science; 2011. [Google Scholar]
- 19.Solaro RJ, Pang DC, Briggs FN. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971;245:259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- 20.Layland J, Cave AC, Warren C, et al. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005;19:1137–1139. doi: 10.1096/fj.04-2519fje. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Solaro RJ. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem. 2006;281:13471–13477. doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 22.Kodama T, Fukui K, Kometani K. The initial phosphate burst in ATP hydrolysis by myosin and subfragment-1 as studied by a modified malachite green method for determination of inorganic phosphate. J Biochem. 1986;99:1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- 23.Wolska BM, Keller RS, Evans CC, et al. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express beta-tropomyosin. Circ Res. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]
- 24.Takeda S, Kobayashi T, Taniguchi H, et al. Structural and functional domains of the troponin complex revealed by limited digestion. Eur J Biochem. 1997;246:611–617. doi: 10.1111/j.1432-1033.1997.00611.x. [DOI] [PubMed] [Google Scholar]
- 25.Yates LD, Greaser ML. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983;168:123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- 26.Fritz JD, Swartz DR, Greaser ML. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal Biochem. 1989;180:205–210. doi: 10.1016/0003-2697(89)90116-4. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka M, Morimoto S, Murayama T, et al. Stage-dependent benefits and risks of pimobendan in genetic dilated cardiomyopathy mice with progressive heart failure. Br J Pharmacol. 2015;172:2369–2382. doi: 10.1111/bph.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du CK, Zhan DY, Morimoto S. In vivo effects of propyl gallate, a novel Ca(2+) sensitizer, in a mouse model of dilated cardiomyopathy caused by cardiac troponin T mutation. Life Sci. 2014;109:15–19. doi: 10.1016/j.lfs.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Parissis JT, Rafouli-Stergiou P, Paraskevaidis I, Mebazaa A. Levosimendan: from basic science to clinical practice. Heart Fail Rev. 2009;14:265–275. doi: 10.1007/s10741-008-9128-4. [DOI] [PubMed] [Google Scholar]
- 30.Edes I, Kiss E, Kitada Y, et al. Effects of Levosimendan, a cardiotonic agent targeted to troponin-c, on cardiac-function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic-reticulum in guinea-pig heart. Circ Res. 1995;77:107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney HL, Houdusse A. Structural and functional insights into the myosin motor mechanism. Annu Rev Biophys. 2010;39:539–557. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 32.Holmes KC, Angert I, Kull FJ, et al. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 33.Arteaga GM, Kobayashi T, Solaro RJ. Molecular actions of drugs that sensitize cardiac myofilaments to Ca2+ Ann Med. 2002;34:248–258. doi: 10.1080/078538902320322510. [DOI] [PubMed] [Google Scholar]