Abstract
Background & objectives:
Multidrug-resistant Salmonellae have emerged worldwide as also in India. The aim of this study was to study the antimicrobial susceptibility pattern of Salmonella enterica serovars isolated at a tertiary care hospital in northern India.
Methods:
A total of 106 S. enterica serovars isolated from various clinical samples from January 2011 to June 2012 were tested for antimicrobial susceptibility by Kirby-Bauer disk diffusion method. The minimum inhibitory concentration (MIC) of ciprofloxacin, chloramphenicol and ceftriaxone was determined both by agar dilution method and E-test for all the isolates.
Results:
Salmonella Typhi (73.6%) was the predominant isolate followed by S. Paratyphi A (15.1%), S. Typhimurium (9.4%) and S. Enteritidis (1.9%). Of these, 34 (32.1%) were resistant to ciprofloxacin (MIC ≥1 μg/ml by agar dilution) with MIC90 of ciprofloxacin for S. Typhi, S. Paratyphi A and S. Typhimurium being 32, 4 and 1 μg/ml, respectively. All the isolates were sensitive to chloramphenicol (MIC ≤8 μg/ml) and ceftriaxone (MIC ≤1 μg/ml). Disk diffusion method showed high susceptibility rates to cefotaxime (100%), azithromycin (93.4%) and co-trimoxazole (97.2%). Nalidixic acid resistance was seen in 105 (99.1%) isolates. Of the nalidixic acid-resistant strains, only 34 (32.3%) were found to be resistant to ciprofloxacin (MIC ≥1 μg/ml).
Interpretation & conclusions:
This study showed an alarming increase in MIC to quinolones and re-emergence of susceptibility to conventional antibiotics among Salmonellae.
Keywords: Antimicrobial susceptibility, MIC, re-emergence, resistance, Salmonella
Antimicrobial therapy is the mainstay of treatment of invasive salmonellosis. In the last two decades, multidrug-resistant (MDR) Salmonella enterica serovars have emerged worldwide, thereby reducing the available therapeutic options1. Furthermore, strains with decreased susceptibility to fluoroquinolones have been documented in the Indian subcontinent2,3. Thus, the present study was conducted to determine the antimicrobial profiles of S. enterica serovars isolated from clinical samples in a tertiary care hospital in north India.
Material & Methods
The study was conducted in the Department of Microbiology, Government Medical College and Hospital, Chandigarh, India. A total of 106 consecutive, non-duplicate clinical isolates of S. enterica serovars isolated from blood, pus and stool samples of patients from January 2011 to June 2012 were included in the study. Antimicrobial susceptibility pattern of the isolates was determined by Kirby-Bauer's disk diffusion method4 on Mueller-Hinton agar using following commercial antibiotic discs (Hi-media, Mumbai): ampicillin (10 μg), chloramphenicol (15 μg), co-trimoxazole (1.25/23.75 μg), ciprofloxacin (5 μg), nalidixic acid (30 μg), azithromycin (15 μg), cefotaxime (30 μg) and ceftriaxone (30 μg)4. The minimum inhibitory concentration (MIC) of three drugs, i.e. ciprofloxacin, chloramphenicol and ceftriaxone, was determined both by agar dilution method and E-test (AB Biodisk Solna, Sweden). A standard strain of Escherichia coli (ATCC 25922) was used as quality control and was included with each batch of tests. The results of disk diffusion and MIC were interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines4. As for azithromycin, no zone diameter interpretive standard was recommended by CLSI, so the British Society for Antimicrobial Chemotherapy (BSAC) guidelines were used5. The results were analyzed by Chi-square test using the SPSS software version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). To see agreement between two methods, kappa test of agreement was applied.
Results & Discussion
Of the 106 isolates, Salmonella Typhi was the predominant isolate (n=78, 73.6%) followed by S. Paratyphi A (n=16, 15.1%), S. Typhimurium (n=10, 9.4%) and S. Enteritidis (n=2, 1.9%). Of these isolates, 104 (98.1%) were from blood culture of patients, one from pus from psoas abscess of a patient (S. Typhi) and one from stool of a patient suffering from chronic diarrhoea (S. Typhimurium). Majority of the Salmonella serovars were isolated from patients in the age group of 12-45 yr (57/106, 53.7%) followed by 1-12 yr age group (47/106, 44.3%). Further, there were two isolates of S. Typhi from neonates with septicaemia.
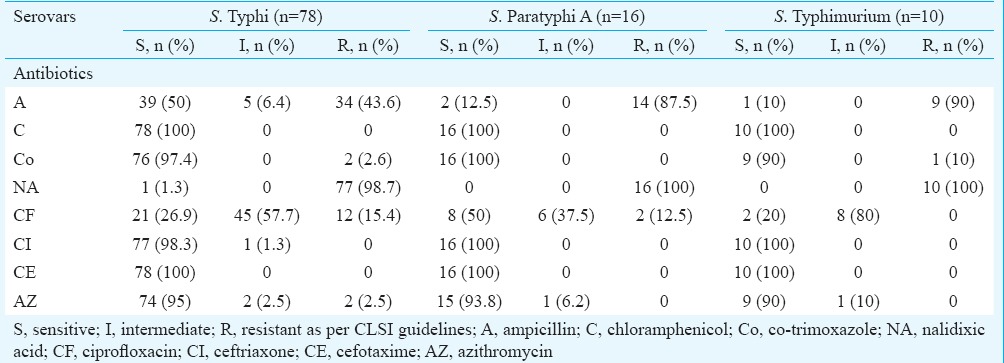
The antibiogram of 106 isolates of Salmonella to various antimicrobials tested by disk diffusion is shown in Table I4. Complete susceptibility was observed to chloramphenicol and cefotaxime. Furthermore, high rates of susceptibility were seen to ceftriaxone (99.1%), azithromycin (93.4%) and co-trimoxazole (97.2%). The susceptibility to ampicillin varied among the Salmonella serovars with high rates of resistance to ampicillin in S. Paratyphi A (87.5%) and S. Typhimurium (90%) as compared to S. Typhi (43.6%). Regarding ciprofloxacin, majority of the serovars were intermediate susceptible (S. Typhi 57.7%, S. Paratyphi A 37.5%, S. Typhimurium 80% and S. Enteritidis 50%), but for 10 isolates of Typhi and two of Paratyphi A which were completely resistant to ciprofloxacin on disk diffusion. Nalidixic acid resistance was seen in 105 (99.1%) isolates. Of the 105 nalidixic acid-resistant (NAR) strains, six were susceptible (MIC ≤0.06), 65 were intermediate susceptible (MIC 0.12-1.0) and 34 were resistant (MIC ≥1) to ciprofloxacin.
Table I.
Antimicrobial susceptibility patterns of Salmonella serovars by Kirby-Bauer disk diffusion method
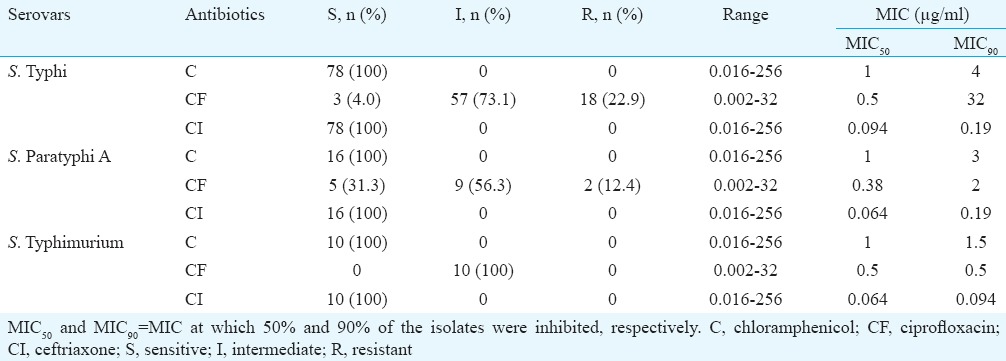
The MICs of isolates to chloramphenicol, ciprofloxacin and ceftriaxone as determined by agar dilution and E-test method are shown in Tables II and III, respectively. Complete susceptibility of all the isolates to chloramphenicol and ceftriaxone was determined by both the methods. Regarding ciprofloxacin, majority of the isolates were found to be in the intermediate range of MIC (0.12-1.0 μg/ml) as determined by both the methods. MIC90 of ciprofloxacin was found to be 32, 4 and 1 μg/ml for S. Typhi, S. Paratyphi A and S. Typhimurium, respectively, by agar dilution. E-test for ciprofloxacin found the MIC90 to be 32, 2 and 0.5 μg/ml for S. Typhi, S. Paratyphi A and S. Typhimurium, respectively. A total of 34 isolates were found to be resistant (MIC ≥1 μg/ml) to ciprofloxacin by agar dilution while with E-test 20 isolates were found to be resistant to ciprofloxacin.
Table II.
Minimum inhibitory concentration (MIC) for Salmonella isolates determined by agar dilution method
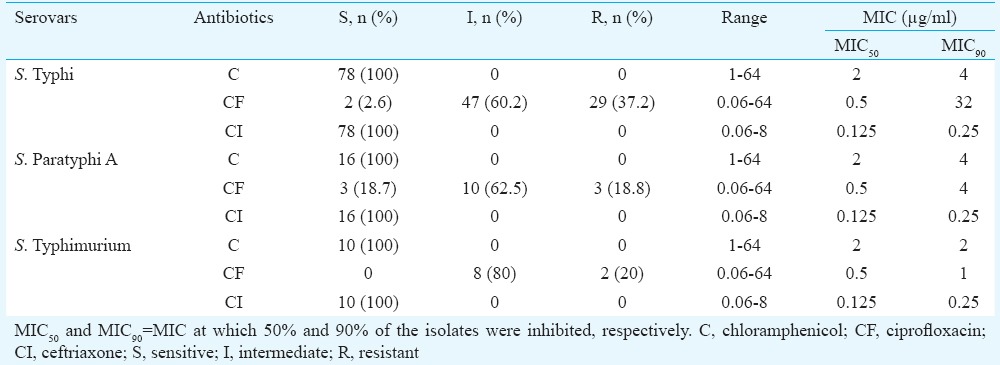
Table III.
Minimum inhibitory concentration (MIC) for Salmonella isolates determined by E-test method
Significant agreement was found between antimicrobial susceptibility determined by all the three methods for chloramphenicol and ceftriaxone. However, regarding ciprofloxacin, low agreement (kappa value 0.25) was found between susceptibility by disk diffusion and agar dilution method, while moderate agreement was found between MIC determined by agar dilution and E-test method (kappa value of 0.659) and between disk diffusion and E-test method (kappa value of 0.426).
S. Typhi has been the predominant isolate in an earlier study from north India6. In this single centre study also, S Typhi was the major isolate followed by S. Paratyphi A. The highest incidence of salmonellosis occurs in the 5-19 yr age group. After age 20, the incidence falls, likely due to acquisition of immunity from clinical or subclinical infection7. However, in our study, majority of isolates were obtained from patients in age group of 12-45 yr. Further, two isolates of S. Typhi were isolated from neonates with septicaemia. Although rare, but similar reports of S. Typhi implicated as a cause of septicaemia, especially among the young and malnourished infants, have been documented from India8.
The emergence of multidrug resistant strains of Salmonella in the last two decades has led to the use of fluoroquinolones and third-generation cephalosporins as the first-line drugs for the treatment of invasive salmonellosis. However, in the recent past, efficacy of ciprofloxacin in the treatment of salmonellosis has been seriously jeopardized9,10. Our study also documented a decreased susceptibility to ciprofloxacin in majority of the strains. This can be attributed to the overuse of ciprofloxacin in treating enteric fever and other acute febrile illnesses. MIC determination by agar dilution method detected most of the resistant strains of ciprofloxacin in our study. This suggests the need for routine testing of MIC of ciprofloxacin in all cases of invasive salmonellosis. Like in previous studies11, our study also observed discrepancy in MIC of ciprofloxacin as determined by E-test and agar dilution.
NA resistant strains of Salmonella, which are believed to be a marker of low-level resistance and treatment failure to ciprofloxacin, were found to be 99.1 per cent, of which only 32.3 per cent were found to be resistant to ciprofloxacin on MIC testing. Similar finding has been reported previously which suggests poor predictive value of nalidixic acid resistance for ciprofloxacin resistance12. This study also documents re-emergence of chloramphenicol susceptibility with all the isolates being completely susceptible (100%) to chloramphenicol. Similar findings of increased susceptibility to chloramphenicol have been documented by others13,14. Thus, the possibility of reuse of chloramphenicol for the treatment of enteric fever can be considered, provided therapy is monitored for its bone marrow toxicity. The present study showed high-sensitivity rates to co-trimoxazole (97.2%) and azithromycin (93.4%) which could become effective alternate therapy for salmonellosis. Further, high resistance to ampicillin was also seen which was in contrast to studies from different parts of India, which showed high rates of sensitivity to the drug among S. Typhi and S. Paratyphi A isolates10,12. All our isolates were sensitive to ceftriaxone and cefotaxime contrary to studies which reported resistance to ceftriaxone10,15.
The major limitations of the study were small sample size and short duration of time. The samples were obtained from a tertiary care centre only, not from peripheral health centres. The clinical outcome of patients with salmonellosis was not analyzed. Also, molecular characterization of ciprofloxacin-resistant strains could not be determined as the isolates were not archived.
In conclusion, our study showed an increase in resistance to fluoroquinolones, complete sensitivity to ceftriaxone and a re-emergence of chloramphenicol, co-trimoxazole sensitivity at our centre. This changing susceptibility pattern of S. enterica serovars over time necessitates continuous surveillance of antibiogram of Salmonella isolates to rationalize the treatment protocols for invasive salmonellosis and prevent emergence of resistant strains.
Footnotes
Conflicts of Interest: None.
References
- 1.Harish BN, Menezes GA. Antimicrobial resistance in typhoidal Salmonellae. Indian J Med Microbiol. 2011;29:223–9. doi: 10.4103/0255-0857.83904. [DOI] [PubMed] [Google Scholar]
- 2.Lakshmi V, Ashok R, Susmita J, Shailaja VV. Changing trends in the antibiograms of Salmonella isolates at a tertiary care hospital in Hyderabad. Indian J Med Microbiol. 2006;24:45–8. doi: 10.4103/0255-0857.19894. [DOI] [PubMed] [Google Scholar]
- 3.Geetha VK, Yugendran T, Srinivasan R, Harish BN. Plasmid-mediated quinolone resistance in typhoidal salmonellae: A preliminary report from South India. Indian J Med Microbiol. 2014;32:31–4. doi: 10.4103/0255-0857.124292. [DOI] [PubMed] [Google Scholar]
- 4.Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI document M100-S22. Wayne, PA: CLSI; 2012. Clinical and Laboratory Standards Institute. [Google Scholar]
- 5.British Society for Antimicrobial Chemotherapy. BSAC Methods of Antimicrobial Susceptibility Testing, Version 11.1. BSAC Document. UK: 2012. [Google Scholar]
- 6.Mohanty S, Gaind R, Sehgal R, Chellani H, Deb M. Neonatal sepsis due to Salmonella Typhi and Paratyphi A. J Infect Dev Ctries. 2009;3:633–8. doi: 10.3855/jidc.557. [DOI] [PubMed] [Google Scholar]
- 7.Manchanda V, Bhalla P, Sethi M, Sharma VK. Treatment of enteric fever in children on the basis of current trends of antimicrobial susceptibility of Salmonella enterica serovar Typhi and Paratyphi A. Indian J Med Microbiol. 2006;24:101–6. doi: 10.4103/0255-0857.25182. [DOI] [PubMed] [Google Scholar]
- 8.Mohanty S, Renuka K, Sood S, Das BK, Kapil A. Antibiogram pattern and seasonality of Salmonella serotypes in a North Indian tertiary care hospital. Epidemiol Infect. 2006;134:961–6. doi: 10.1017/S0950268805005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagshetty K, Channappa ST, Gaddad SM. Antimicrobial susceptibility of Salmonella Typhi in India. J Infect Dev Ctries. 2010;4:70–3. doi: 10.3855/jidc.109. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary A, Gopalakrishnan R, Nambi PS, Ramasubramanian V, Ghafur KA, Thirunarayan MA. Antimicrobial susceptibility of Salmonella enterica serovars in a tertiary care hospital in Southern India. Indian J Med Res. 2013;137:800–2. [PMC free article] [PubMed] [Google Scholar]
- 11.Capoor MR, Rawat D, Nair D, Hasan AS, Deb M, Aggarwal P, et al. In vitro activity of azithromycin, newer quinolones and cepalosporins in ciprofloxacin resistant Salmonella causing enteric fever. J Med Microbiol. 2007;56:1490–4. doi: 10.1099/jmm.0.47353-0. [DOI] [PubMed] [Google Scholar]
- 12.Ray P, Sharma J, Marak RS, Garg RK. Predictive efficacy of nalidixic acid resistance as a marker of fluoroquinolone resistance in Salmonella enterica var Typhi. Indian J Med Res. 2006;124:105–8. [PubMed] [Google Scholar]
- 13.Kumar Y, Sharma A, Mani KR. Re-emergence of susceptibility to conventionally used drugs among strains of Salmonella Typhi in central West India. J Infect Dev Ctries. 2011;5:227–30. doi: 10.3855/jidc.1310. [DOI] [PubMed] [Google Scholar]
- 14.Harish BN, Menezes GA. Preserving efficacy of chloramphenicol against typhoid fever in a tertiary care hospital, India. Regional Health Forum. 2011;15:92–6. [Google Scholar]
- 15.Elumalai S, Muthu G, Selvam RE, Ramesh S. Detection of TEM-, SHV- and CTX-M-type ß-lactamase production among clinical isolates of Salmonella species. J Med Microbiol. 2014;63:962–7. doi: 10.1099/jmm.0.068486-0. [DOI] [PubMed] [Google Scholar]


