Abstract
The use of three dimensional in vitro systems in cancer research is a promising path for developing effective anticancer therapies. The aim of this study was to engineer a functional 3-D in vitro model of normal and cancerous cervical tissue.Normal epithelial and immortalized cervical epithelial carcinoma cell lines were used to construct 3-D artificial normal cervical and cervical cancerous tissues. De-epidermised dermis (DED) was used as a scaffold for both models. Morphological analyses were conducted by using hematoxylin and eosin staining and characteristics of the models were studied by analyzing the expression of different structural cytokeratins and differential protein marker Mad1 using immunohistochemical technique.Haematoxylin and eosin staining results showed that normal cervical tissue had multi epithelial layers while cancerous cervical tissue showed dysplastic changes. Immunohistochemistry staining results revealed that for normal cervix model cytokeratin 10 was expressed in the upper stratified layer of epithelium while cytokeratin 5 was expressed mainly in the middle and basal layer. Cytokeratin 19 was weakly expressed in a few basal cells. Cervical cancer model showed cytokeratin 19 expression in different epithelial layers and weak or no expression for cytokeratin 5 and cytokeratin 10. Mad1 expression was detected in some suprabasal cells.The 3-D in vitro models showed stratified epithelial layers and expressed the same types and patterns of differentiation marker proteins as seen in corresponding in vivo tissue in either normal cervical or cervical cancerous tissue. Findings imply that they can serve as functional normal and cervical cancer models.
Keywords: cervical cancer, MAX dimerisation protein 1, cytokeratins, three dimensional in vitro models
Introduction
In vitro studies are commonly used to mimic the physiologic environment of tumors[1-2] at early stages of drug development. When cells are grown as monolayers in conventional two-dimensional (2-D) models, they lack the natural three-dimensional (3-D) tissue in vivo characteristics[3]. 2-D cell cultures can only provide some approximate information of normal and cancer tissues due to the highly unnatural geometric and mechanical limitations imposed on cells[4]. This means that cells grown in conventional 2-D culture conditions commonly fail to mimic tissue structure and functions, and consequently do not provide information about the way cancer cells interact with the extracellular matrix (ECM) and its complex environment interactions that exist in human cancers[5]. Under conventional culture conditions, keratinocytes grow as monolayers and are not able to grow and differentiate in stratified squamous epithelium as observed in the normal human cervix, but only reach an incomplete terminal differentiation[6-7]. Animal models established in immunocompromised mice reconstitute conditions observed in vivo mimicking the physiologic microenvironment of cervical carcinoma, but these may also show false effects on tumor progression and molecular mechanisms of the disease due to the differences between mice and humans[5]. To overcome the above difficulties, in vitro 3-D tumor models with different human cells have been progressively explored to enable accurate human tissue reproduction[8]. 3-D in vitro models have an important role in tumor biology and provide important insights into cancer research. They enhance our understanding of tissue organization, cellular differentiation and provide us a better understanding of tumor behavior. The 3-D ECM and its receptors can promote normal epithelial polarity and differentiation[9]. Various techniques have been developed for the construction of 3-D in vitro tumor models, such as cell-seeding 3-D scaffolds[10], hydrogel embedding[11], microfluidic chips[12] or cell patterning[13]. Tissue engineering developments have further improved the diversity and quality of 3-D in vitro models which take them one step closer to the in vivo situation. However, each of the represented models has its advantages and limitations. One model is organotypic epithelial “raft” culture system that allows proliferation and full differentiation of keratinocyte monolayers by culturing cells on collagen gels at the air-liquid interface[14-16]. Normal keratinocytes grown in this model stratify and fully differentiate in a similar way to normal squamous epithelium[16]. Another model that mimics the native state in the skin is the system where keratinocytes are grown on a de-epidermized or devitalized dermis, on which cells are able to grow at the air–liquid interface[17-20]. The latter model is considered more physiologically relevant as the cell's growing conditions are similar to the in vivo situation, and the diffusion of nutrients from the underlying dermis into the epidermis can be observed[16]. In addition, the de-epidermised dermal (DED) scaffold uses a human acellular dermis to construct a new multilayered epidermis to preserve the basement membrane which is critical for keratinocytes attachment in vitro [21].
3-D in vitro systems closely recapitulate the 3-D organization of cells and ECM as seen in in vivo tissues. To mimic the human cervix in vitro , i.e., its multilayered tissue organization and its interactions with ECM, a 3-D in vitro models of normal and cancer cervix were developed and the method used in this current research was adopted and modified from DED model of human skin[21-22]. The de-epidermised dermis was used as a scaffold for both in vitro models. This system is an inexpensive alternative to other commercially available 3-D in vitro models and a viable alternative to animal testing. It can be widely used to assess the efficacy of drugs, to estimate dosage of new therapies and to develop tumor vaccines. In this study, a list of biomarkers was used to confirm and validate the reliability of proposed in vitro systems. Cytokeratin biomarkers were used to assess the reliability of the 3-D in vitro model of the cervix. As in cervical cancer tissues, the pattern of cytokeratins is often disorganized[23], a differentiation biomarker MAX dimerisation protein 1 (Mad 1) was used to assist in evaluating the 3-D cervical cancer model.
Materials and methods
The immortalized human keratinocytes cell line NTERT and the cervical cancer cell line C33A were purchased from ATCC (ATCC, UK). C33A cell lines were grown in RPMI (Gibco Invitrogen, UK) media supplemented with 10% fetal bovine serum (FBS, BioSera, UK) and 1% penicillin/streptomycin antibiotic (Sigma, UK). NTERT cells were grown in cytokeratinocyte-SFM medium with L-glutamine, without calcium chloride (Gibco Invitrogen, UK) supplemented with cytokeratinocyte-SFM supplement human recombinant epidermal growth factor (2.5 μg/L) and bovine pituitary extract (BPE) (25 mg/L) (Gibco Invitrogen). Both cell lines were grown at 37°C in a humidified atmosphere of 5% CO2 with the media being changed every 2-3 days as required. To create the desired microenvironment for cells to enable them to grow and differentiate to the required phenotype, estradiol (Sigma, UK) at physiologic concentration level of 1 μmol/L[24] was added to the 3-D in vitro models of normal cervix and cervical cancer. Estradiol can promote differentiation in cervical epithelial cells[25-26] and can also increase proliferation and inhibit apoptosis in cervical cancers[27-31].
Human skin (Euro Skin Bank, Netherlands) was first incubated in sterile PBS for 48 hours and then the epidermis was removed using a scalpel. The remaining dermis was cut in small 1.5 × 1.5 cm2 pieces and washed three times in sterile PBS. Dermal scaffolds were incubated for 24 hours with different media according to the cell line used. Human keratinocyte NTERT cells and cervical cancer C33A cells were respectively seeded inside a metal ring on the top of dermal scaffolds, and left for 2 days to attach ( Fig. 1A ). Rings were then removed and dermal scaffolds were raised and placed on metal meshes for another four to six weeks at the air–liquid interface to allow cell stratification and differentiation ( Fig. 1B ). At least three dermal scaffolds were prepared for each cell line used. After three weeks samples were washed in PBS and fixed in 4% paraformaldehyde for 48 hours at 4°C. After fixation, samples were processed, wax embedded and cut into 6 mm sections for staining.
Fig.1.
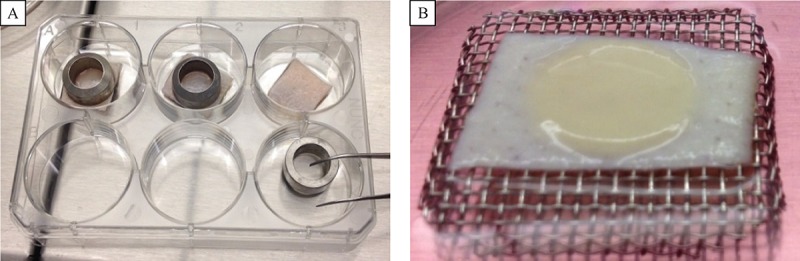
The 3-D in vitro model of the cervix.
Slides were stained using standard immunohistochemistry (IHC) methods published elsewhere[32]. In brief, paraffin embedded tissue blocks were cut into 6μm sections, dewaxed and rehydrated through graded alcohol to water. Sections were then either stained with hematoxylin and eosin, or incubated with primary antibodies ( Table 1 ) followed by avidin biotin complex (ABC) staining methods dehydration, clearing and mounting in DePeX (BHD, UK). Images were captured using the Image-Pro Express 6.3 program. Results were graded by two researchers independently and then recorded according to the number of positive cells in the tissues studied. “+” represents 1 to 5 positive cells; “++” represents 5 to 20 positive cells and “+++” represents 20 or more positive cells. Sections where no staining was detected were classified as negative (–ve).
Tab.1.
Antibodies used in immunochemical staining
| 1° Ab | Ig type | Concentration | Antigen retrieval | Serum | 2 ° Ab |
| Anti-cytokeratin [CK5] (Rabbit monoclonal)(Abcam) | IgG | 1 μg/mL | Citrated buffer x1, heated for 20 minutes | horse | Biotinylated anti-rabbit IgG |
| Anti-cytokeratin 10 [CK10] (Mouse monoclonal)(Abcam) | IgG1 | 1 μg/mL | Citrated buffer x1, heated for 20 minutes | horse | Biotinylated anti-mouse IgG |
| Anti-cytokeratin 19 [CK19] (Mouse monoclonal)(Abcam) | IgG2a | 1 μg/mL | Citrated buffer x1, heated for 20 minutes | horse | Biotinylated anti-mouse IgG |
| Mad1 (Rabbit polyclonal)(Santa Cruz Biotechnology) | IgG | 2 μg/mL | 0.1% Triton-100, 10 minutes | horse | Biotinylated anti-rabbit IgG |
| Isotypic control(Goat anti-mouse) (Abcam) | IgG1 | 5 μg/mL10 μg/mL | Citrated buffer x1, heated for 20 minutes0.1% Triton-100, 10 minutes | horse | Biotinylated anti-goat IgG |
| Isotypic control(Goat anti-mouse) (Abcam) | IgG2a | 1.1 μg/mL38 μg/mL | 0.1% Triton-100, 20 minutes | horse | Biotinylated anti-goat IgG |
| Isotypic control(Goat anti-mouse) (Abcam) | IgG | 2 μg/mL1.53 μg/mL | 0.1% Triton-100, 20 minutes | horse | Biotinylated anti-goat IgG |
Results
NTERT cells were grown in our 3-D in vitro model and served as a control model of cervical tissue. After 4-6 weeks of incubation, human keratinocytes managed to grow on the de-epidermised dermis and formed multilayered structures. IHC analysis revealed that the NTERT model was positively stained with cytokeratin 10 with the staining seen in the upper most stratified layer of the epithelium as seen in a normal cervix. Cytokeratin 5 expression was seen mostly in either the entire cell layer or in the basal part of tissue depending on the 3-D in vitro model thickness achieved and this is consistent with the staining pattern seen in a normal cervix ( Fig. 2 ).
Cytokeratin 19 is normally seen in the basal cell layer of the normal cervix. The NTERT 3-D in vitro model sections were mostly negative for cytokeratin 19, with only weak staining for single cells found in the basal layer ( Fig. 2 ). Two negative controls were included, one of which was an internal negative control where primary antibody was omitted and the other a negative isotypic control, where primary antibody was replaced with normal IgG from the same species using the same isotype at the same concentration.
Fig.2.
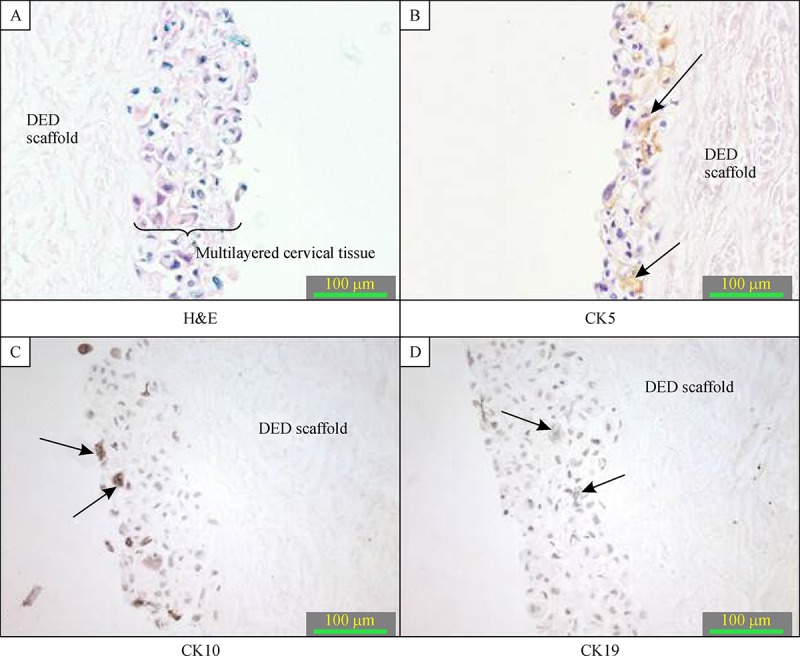
The 3-D in vitro models of normal cervix (magnification 400×).
C33A cells were then grown in our 3-D in vitro model to serve as a model of cervical cancer. Staining results showed that the cytokeratin distribution pattern resembled a typical cervix cancerous tissue, where weak or no staining for cytokeratin 10 and cytokeratin 5 was detected whereas cytokeratin 19 was expressed in different layers of artificial tissue model ( Fig. 3 ).
Expression of Mad 1 differentiation marker
MAX dimerization protein 1 (Mad1) is a transcriptional repressor that is produced in differentiating cells and was used as a differentiation marker to characterize the cell maturation and differentiation processes observed when cells were grown at a air-liquid interface in the 3-D in vitro model of cervical cancer. In the C33A 3-D in vitro model, cells in the upper, most differentiated layer were stained with Mad1 ( Fig. 4 ).
Fig.3.
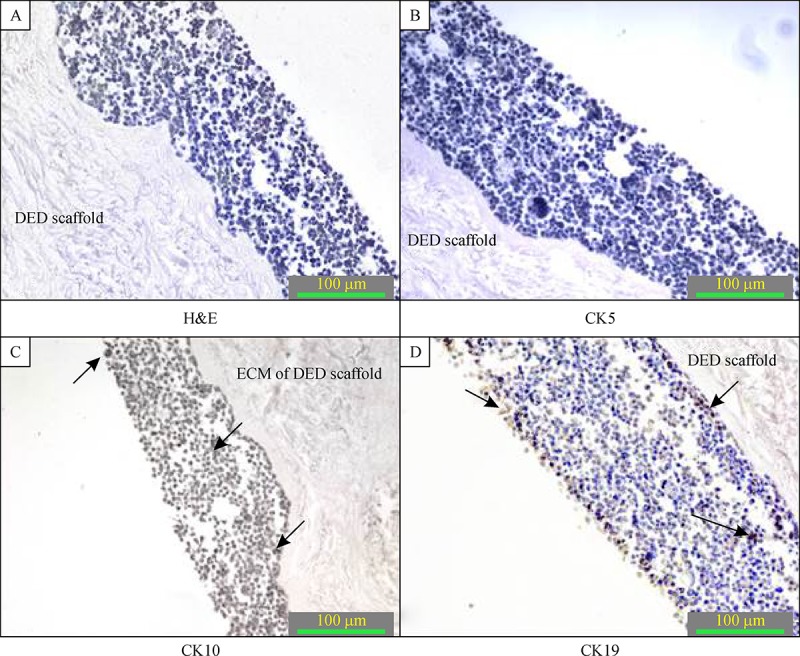
The C33A 3-D in vitro model of cervical cancer at magnification of 200×.
Fig.4.
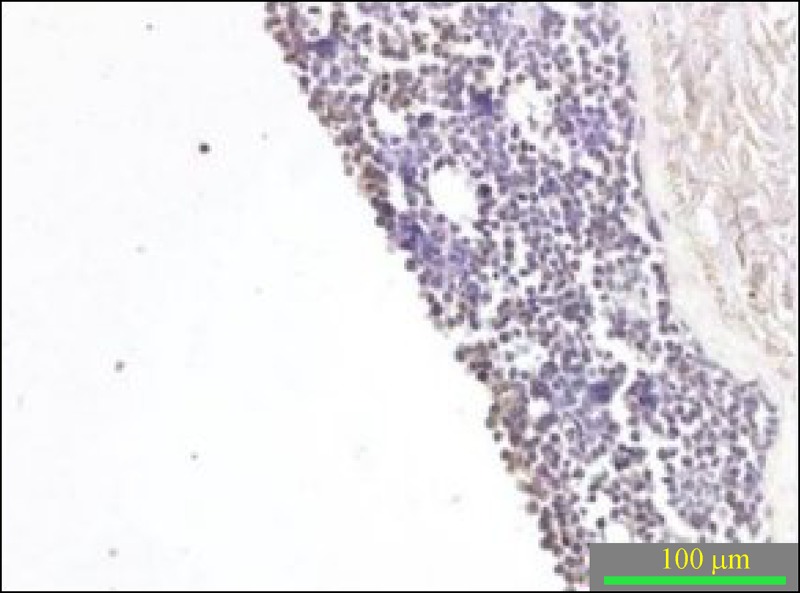
Immunohistochemical staining of Mad 1 from C33A 3-D in vitro model of cervical cancer.
Discussion
To gain a better insight into mechanisms of human cancer pathology and to find new anticancer therapies, reliable, physiologically relevant and cost effective in vitro models are needed. In this study, we have developed a 3-D in vitro model to allow in vitro reconstruction of the normal cervix and cervical cancer. Previous studies showed that under conventional culture conditions where keratinocytes were grown in plastic petri dishes or flasks as monolayers, they could only reach an incomplete terminal differentiation and were not able to form multiple cell layers as observed in the human cervix[6-7, 19]. Terminal differentiation of NTERT cells was observed in our multilayered 3-D model of normal cervix. Cultured C33A cancer cells in the proposed model resulted in significant tumor formation in a 3-D fashion, with some differentiated cells found in the most superficial layer. One of the possible reasons for the cells to be able to form tissue like structures in this in vitro model proposed in our study is that they are surrounded by a natural environment of human dermis, where nutrients are naturally transported from the underlying media and at the same time most superficial cells are exposed to the liquid air interface.
To evaluate the accuracy of our models, we compared the expression of cytokeratins 5, 10 and 19 in the created 3-D in vitro system of normal cervix with the expression of the same cytokeratins seen in normal human cervix in vivo . Cytokeratins (CK), also known as keratins (K) constitute the largest intermediate filament protein subgroup and represent a multigene family with more than 20 different types of polypeptides. They are the major structural proteins of epithelial cells and are divided into acidic type I (CK9-CK20) and basic type II (CK1-CK8) cytokeratins[33-34]. The main function of cytokeratins is to maintain the epithelial cell integrity and to form the cytoskeleton of epithelial cells[34-35]. Cytokeratins have a specific distribution pattern in epithelial tissues[36-37]. Changes in the pattern of cytokeratin expression during neoplastic transformation in the cervix have been shown in several studies[38-40]. Table 2 represents different patterns of cytokeratin expression in non-malignant and cancerous cervical tissue[23, 34, 40-47]. Of particular interest are the changes of cytokeratins 19, 10 and 5.
Tab.2.
Different patterns of cytokeratins expression in non-malignant and malignant cervical tissue, where “−” represents negative staining, “+” representsweak positive, “−/+”represents negative or weak positive, “++”represents positive and “+++” representsstrongly positive staining.
| Non- malignant tissue | Malignant tissue | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral-basal or acidic | Neutral-basal | Acidic | Neutral-basal | Acidic | |||||||
| Types of Keratin | Cytokeratin-1 | Cytokeratin-5 | Cytokeratin-10 | Cytokeratin-19 | Cytokeratin-14 | Cytokeratin-1 | Cytokeratin-5 | Cytokeratin-10 | Cytokeratin-19 | Cytokeratin-14 | |
| Expression levels of different keratins in stratified squamous epithelia of cervix | Non-keratinizing intermediate and superficial cells | + | + | + | − | ++ | −/+ | + | −/+ | +++ | + |
| Basal cells | − | +++ | − | ++ | +++ | −/+ | + | −/+ | +++ | + |
Cytokeratin 10 is a member of the type I keratin family and it is a suprabasal differentiation marker in ectocervical epithelium restricted to skin and cervix[43]. Whenever cytokeratin 10 is expressed in invasive carcinomas, it is associated with the grade of differentiation and is expressed in well-differentiated areas and keratin pearls of squamous carcinomas[23, 40, 42]. Cytokeratin 19 is an intermediate type I keratin, the smallest known acidic keratin of 40 kDa, that is not paired with a basic keratin in epithelial cells. This keratin is specifically found in the periderm, the transiently superficial layer that envelops the developing epidermis[34, 44]. Cytokeratin 19 has not been detected in the epidermis of adult human skin and its presence is restricted to the outer root sheath of the hair follicle[45-46]. This cytokeratin is found only in the basal layer of normal epithelium of the cervix and in the full thickness of metaplastic cervical epithelium[43]. Cytokeratin 5 is a neutral-basic cytokeratin expressed during differentiation of simple and stratified epithelial tissues. Cytokeratin 5 is type II keratin that is expressed mostly in the basal layer of the epidermis with its family member acidic keratin 14[47]. In this study, cytokeratin 5 and 10 expression in NTERT 3-D in vitro model corresponded with staining seen in the normal cervix. Cytokeratin 19 was weakly expressed and found only in a few NTERT cells, but the localization of these positively stained cells corresponded to its in vivo expression in the normal cervix. Moreover, we found that C33A cells when grown in our 3-D system showed cytokeratin distribution characteristic of typical cancerous tissue, where no positivity for cytokeratin 5, and weak or no positivity for cytokeratin 10 was observed, whereas cytokeratin 19 was expressed in different layers. In addition to cytokeratins, another differentiation marker, Mad 1, was used to indicate whether any differentiation process could be observed in our cervical cancer model. The Mad1 differentiation marker is a basic helix–loop–helix–leucine zipper protein that is a transcriptional repressor produced in differentiating cells[48-49]. Mad1 is detected in differentiating epithelial cells of the suprabasal layers of normal epidermis[50] and its expression extends from the spinous to superficial layer[51]. This transcriptional repressor is expressed at low levels in proliferating cells and its expression increases during differentiation of epithelial cells[50, 52]. Mad 1 expression is associated with growth arrest whereas loss of its expression is related with the progression to invasive, poorly-differentiated cancers[50, 52]. With increasing severity of dysplasia, the expression of Mad 1 is progressively shifted to more superficial layers and the immunostaining intensity is reduced[51]. In this study, the expression of Mad1 confirmed that the partial differentiation process occurred in cancer cells after culturing them in the 3-D conditions.
Advances of the presented 3-D model have enabled direct assembly of normal human keratinocytes, cervical cancer cells and ECM of human de-epidermised dermis to form in vitro like cellular models. This promising technique has offered an inexpensive solution to culture a variety of cell types under 3-D culture conditions, also enabled us to manipulate a particular cell of interest and to study specific interactions in a well-defined environment. The functionality of the proposed model may provide us better insights in human cervical cancers and create effective therapeutic designs for patients with cervical carcinoma.
Acknowledgments
This work was supported by the Middlesex University, particularly in the award of a Postgraduate Research Studentship that provided the necessary financial support for this research.
Contributor Information
Anna Karolina Zuk, Department of Natural sciences, Middlesex University, The Burroughs, Hendon, London NW4 4BT, UK.
Xuesong Wen, Department of Natural sciences, Middlesex University, The Burroughs, Hendon, London NW4 4BT, UK.
Stephen Dilworth, Department of Natural sciences, Middlesex University, The Burroughs, Hendon, London NW4 4BT, UK.
Dong Li, Department of Natural sciences, Middlesex University, The Burroughs, Hendon, London NW4 4BT, UK.
Lucy Ghali, Department of Natural sciences, Middlesex University, The Burroughs, Hendon, London NW4 4BT, UK.
References
- 1. Kim JB. Three-dimensional tissue culture models in cancer biology[J]. Semin Cancer Biol, 2005, 15(5): 365–377. [DOI] [PubMed] [Google Scholar]
- 2. Ellingsen C, Natvig I, Gaustad JV, et al. Human cervical carcinoma xenograft models for studies of the physiological microenvironment of tumors[J]. J Cancer Res ClinOncol, 2009, 135(9): 1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Padrón JM, van der Wilt CL, Smid K, et al. The multilayered postconfluent cell culture as a model for drug screening[J]. Crit Rev Oncol Hematol, 2000, 36(2-3): 141–157. [DOI] [PubMed] [Google Scholar]
- 4. Sun T, Jackson S, Haycock JW, et al. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents[J]. J Biotechnol, 2006, 122(3): 372–381. [DOI] [PubMed] [Google Scholar]
- 5. Benam KH, Dauth S, Hassell B, et al. Engineered in vitro disease models[J]. Annu Rev Pathol, 2015, 10(10): 195–262. [DOI] [PubMed] [Google Scholar]
- 6. Burdick AD, Bility MT, Girroir EE, et al. Ligand activation of peroxisome proliferator-activated receptor-beta/delta(PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes[J]. Cell Signal, 2007, 19(6): 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. York M, Griffiths HA, Whittle E, et al. Evaluation of a human patch test for the identification and classification of skin irritation potential[J]. Contact Dermatitis, 1996, 34(3): 204–212. [DOI] [PubMed] [Google Scholar]
- 8. Horning JL, Sahoo SK, Vijayaraghavalu S, et al. 3-D tumor model for in vitro evaluation of anticancer drugs[J]. Mol Pharm, 2008, 5(5): 849–862. [DOI] [PubMed] [Google Scholar]
- 9. Roskelley CD, Bissell MJ. Dynamic reciprocity revisited: a continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function[J]. Biochem Cell Biol, 1995, 73(7-8): 391–397. [DOI] [PubMed] [Google Scholar]
- 10. Fischbach C, Chen R, Matsumoto T, et al. Engineering tumors with 3D scaffolds[J]. Nat Methods, 2007, 4(10): 855–860. [DOI] [PubMed] [Google Scholar]
- 11. Szot CS, Buchanan CF, Freeman JW, et al. 3D in vitro bioengineered tumors based on collagen I hydrogels[J]. Biomaterials, 2011, 32(31): 7905–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsiao AY, Torisawa YS, Tung YC, et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids[J]. Biomaterials, 2009, 30(16): 3020–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu F, Celli J, Rizvi I, et al. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform[J]. Biotechnol J, 2011, 6(2): 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andrei G, van den Oord J, Fiten P, et al. Organotypic epithelial raft cultures as a model for evaluating compounds against alphaherpesviruses[J]. Antimicrob Agents Chemother, 2005, 49(11): 4671–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anacker D, Moody C. Generation of organotypic raft cultures from primary human keratinocytes[J]. J Vis Exp, 2012, 60(60): 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delvenne P, Hubert P, Jacobs N, Giannini SL, Havard L, Renard I, Saboulard D and Boniver J. The organotypic culture of HPV-transformed keratinocytes: an effective in vitro model for the development of new immunotherapeutic approaches for mucosal (pre)neoplastic lesions[J]. Vaccine, 200, 19 (17–19): 2557–2564. [DOI] [PubMed] [Google Scholar]
- 17. Gibbs S, Vicanová J, Bouwstra J, et al. Culture of reconstructed epidermis in a defined medium at 33 degrees C shows a delayed epidermal maturation, prolonged lifespan and improved stratum corneum[J]. Arch Dermatol Res, 1997, 289(10): 585–595. [DOI] [PubMed] [Google Scholar]
- 18. Regnier M and Darmon M. 25-Dihydroxyvitamin D3 stimulates specifically the last steps of epidermal differentiation of cultured human keratinocytes[J]. Differentiation; research in biological diversity, 1991, 47 (3): 173–188. [DOI] [PubMed] [Google Scholar]
- 19. Breiden B, Gallala H, Doering T, et al. Optimization of submerged keratinocyte cultures for the synthesis of barrier ceramides[J]. Eur J Cell Biol, 2007, 86(11-12): 657–673. [DOI] [PubMed] [Google Scholar]
- 20. Oudhoff MJ, Kroeze KL, Nazmi K, et al. Structure-activity analysis of histatin, a potent wound healing peptide from human saliva: cyclization of histatin potentiates molar activity 1,000-fold[J]. FASEB J, 2009, 23(11): 3928–3935. [DOI] [PubMed] [Google Scholar]
- 21. Chakrabarty KH, Dawson RA, Harris P, et al. Development of autologous human dermal-epidermal composites based on sterilized human allodermis for clinical use[J]. Br J Dermatol, 1999, 141(5): 811–823. [DOI] [PubMed] [Google Scholar]
- 22. Xie Y, Rizzi SC, Dawson R, et al. Development of a three-dimensional human skin equivalent wound model for investigating novel wound healing therapies[J]. Tissue Eng Part C Methods, 2010, 16(5): 1111–1123. [DOI] [PubMed] [Google Scholar]
- 23. Smedts F, Ramaekers F, Troyanovsky S, et al. Keratin expression in cervical cancer[J]. Am J Pathol, 1992, 141(2): 497–511. [PMC free article] [PubMed] [Google Scholar]
- 24. Kim CJ, Um SJ, Kim TY, et al. Regulation of cell growth and HPV genes by exogenous estrogen in cervical cancer cells[J]. Int J Gynecol Cancer, 2000, 10(2): 157–164. [DOI] [PubMed] [Google Scholar]
- 25. Abe H, Oikawa T. Effects of estradiol and progesterone on the cytodifferentiation of epithelial cells in the oviduct of the newborn golden hamster[J]. Anat Rec, 1993, 235(3): 390–398. [DOI] [PubMed] [Google Scholar]
- 26. Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod, 1998, 13(11): 3114–3120. [DOI] [PubMed] [Google Scholar]
- 27. Wang Q, Li X, Wang L, et al. Antiapoptotic effects of estrogen in normal and cancer human cervical epithelial cells[J]. Endocrinology, 2004, 145(12): 5568–5579. [DOI] [PubMed] [Google Scholar]
- 28. Wang H, Yuang F, Huang X, Zhang H, Yang S and Shi B. Effects of Estrogen and Progestogen on the Growth and Apoptosis of Human Cervical Cancer Cells[J]. U.S Chinese Journal of Lymphology and Urology, 2006, 5(2): 65–70. [Google Scholar]
- 29. Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice[J]. Proc Natl AcadSci U S A, 1996, 93(7): 2930–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elson DA, Riley RR, Lacey A, et al. Sensitivity of the cervical transformation zone to estrogen-induced squamous carcinogenesis[J]. Cancer Res, 2000, 60(5): 1267–1275. [PubMed] [Google Scholar]
- 31. Park JS, Rhyu JW, Kim CJ, et al. Neoplastic change of squamo-columnar junction in uterine cervix and vaginal epithelium by exogenous estrogen in hpv-18 URR E6/E7 transgenic mice[J]. GynecolOncol, 2003, 89(3): 360–368. [DOI] [PubMed] [Google Scholar]
- 32. Li D, Wen X, Ghali L, et al. hCGβ expression by cervical squamous carcinoma--in vivo histological association with tumour invasion and apoptosis[J]. Histopathology, 2008, 53(2): 147–155. [DOI] [PubMed] [Google Scholar]
- 33. Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease[J]. Annu Rev Biochem, 1994, 63: 345–382. [DOI] [PubMed] [Google Scholar]
- 34. Alix-Panabières C, Vendrell JP, Slijper M, et al. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer[J]. Breast Cancer Res, 2009, 11(3): R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments[J]. CurrOpin Cell Biol, 2002, 14(1): 110–122. [DOI] [PubMed] [Google Scholar]
- 36. Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells[J]. Cell, 1982, 31(1): 11–24. [DOI] [PubMed] [Google Scholar]
- 37. Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns[J]. J Cell Biol, 1990, 111(2): 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bobrow LG, Makin CA, Law S, et al. Expression of low molecular weight cytokeratin proteins in cervical neoplasia[J]. J Pathol, 1986, 148(2): 135–140. [DOI] [PubMed] [Google Scholar]
- 39. Lane EB, Alexander CM. Use of keratin antibodies in tumor diagnosis[J]. Semin Cancer Biol, 1990, 1(3): 165–179. [PubMed] [Google Scholar]
- 40. Carrilho C, Alberto M, Buane L, et al. Keratins 8, 10, 13, and 17 are useful markers in the diagnosis of human cervix carcinomas[J]. Hum Pathol, 2004, 35(5): 546–551. [DOI] [PubMed] [Google Scholar]
- 41. Smedts F, Ramaekers F, Robben H, et al. Changing patterns of keratin expression during progression of cervical intraepithelial neoplasia[J]. Am J Pathol, 1990, 136(3): 657–668. [PMC free article] [PubMed] [Google Scholar]
- 42. Smedts F, Ramaekers F, Link M, et al. Detection of keratin subtypes in routinely processed cervical tissue: implications for tumour classification and the study of cervix cancer aetiology[J]. Virchows Arch, 1994, 425(2): 145–155. [DOI] [PubMed] [Google Scholar]
- 43. Maddox P, Sasieni P, Szarewski A, et al. Differential expression of keratins 10, 17, and 19 in normal cervical epithelium, cervical intraepithelial neoplasia, and cervical carcinoma[J]. J ClinPathol, 1999, 52(1): 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heatley MK. Keratin expression in human tissues and neoplasms[J]. Histopathology, 2002, 41(4): 365–366. [DOI] [PubMed] [Google Scholar]
- 45. Stasiak PC, Purkis PE, Leigh IM, et al. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins[J]. J Invest Dermatol, 1989, 92(5): 707–716. [DOI] [PubMed] [Google Scholar]
- 46. Michel M, Török N, Godbout MJ, et al. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage[J]. J Cell Sci, 1996, 109(Pt 5): 1017–1028. [DOI] [PubMed] [Google Scholar]
- 47. Bonifas JM, Bare JW, Lynch ED, et al. Regional assignment of the human keratin 5 (KRT5) gene to chromosome 12q near D12S14 by PCR analysis of somatic cell hybrids and multicolor in situ hybridization[J]. Genomics, 1992, 13(2): 452–454. [DOI] [PubMed] [Google Scholar]
- 48. Roussel MF, Ashmun RA, Sherr CJ, et al. Inhibition of cell proliferation by the Mad1 transcriptional repressor[J]. Mol Cell Biol, 1996, 16(6): 2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amati B, Land H. Myc-Max-Mad: a transcription factor network controlling cell cycle progression, differentiation and death[J]. CurrOpin Genet Dev, 1994, 4(1): 102–108. [DOI] [PubMed] [Google Scholar]
- 50. Hurlin PJ, Foley KP, Ayer DE, et al. Regulation of Myc and Mad during epidermal differentiation and HPV-associated tumorigenesis[J]. Oncogene, 1995, 11(12): 2487–2501. [PubMed] [Google Scholar]
- 51. Zanotti S, Fisseler-Eckhoff A, Mannherz HG. Changes in the topological expression of markers of differentiation and apoptosis in defined stages of human cervical dysplasia and carcinoma[J]. GynecolOncol, 2003, 89(3): 376–384. [DOI] [PubMed] [Google Scholar]
- 52. Hurlin PJ, Quéva C, Koskinen PJ, et al. Mad3 and Mad4: novel Max-interacting transcriptional repressors that suppress c-myc dependent transformation and are expressed during neural and epidermal differentiation[J]. EMBO J, 1995, 14(22): 5646–5659. [DOI] [PMC free article] [PubMed] [Google Scholar]


