Abstract
In this study we examined the 24-hour plasma melatonin patterns of young adult (~11 years of age) and old (~24 years of age) rhesus macaques, and determined how they would be influenced by 30% caloric restriction (CR). Well-defined 24-hour plasma melatonin rhythms were observed in all the males but only the old animals showed significant attenuation of nighttime melatonin levels. Moreover, 4.5 years of CR failed to prevent the age-associated decline in plasma melatonin levels in the old males, and caused a significant decrease in the young adult males. Similar plasma melatonin rhythms were also observed in all the females but no age-related decline was detected, and 2 years of CR had no obvious effect on plasma melatonin levels. If anything, there was a trend for the CR to decrease melatonin levels in the young adult females. Taken together, the results fail to show any clear benefit of CR on plasma melatonin levels in old rhesus macaques and may even be detrimental to plasma melatonin levels in young adults.
Keywords: Aging, Caloric restriction, Circadian, Endocrine rhythms, Melatonin, Primate
1. Introduction
Clinical studies have shown that nighttime melatonin secretion decreases markedly during aging, especially in individuals with sleep disorders, although in some individuals it remains elevated (Iguchi et al., 1982; Haimov et al., 1994; Pandi-Perumal et al., 2005; Supplementary Material). Therefore, the aim of the present study was to examine age-related changes in circulating melatonin levels in male and female rhesus macaques and to determine if these hormone levels are affected by caloric restriction (CR), a well-established non-genetic manipulation that can influence longevity and can attenuate many of the biological changes associated with aging (Colman et al., 2014; Mattison et al., 2017).
2. Materials and methods
As previously described (Downs et al., 2008), the rhesus macaques used in this IACUC-approved study were housed in a temperature controlled environment (24°C), under a 12:12 hr light-dark cycle, and fed a specially formulated monkey chow (Agway, Ithaca, NY, USA), supplemented daily with fresh fruit or vegetables. The study comprised 10 young adult (10.6±0.1 years old; 9.5±0.6 kg body weight) and 10 old (26.5±0.7 years old; 7.6±0.7 kg body weight) males, and 8 young adult (11.5±0.1 years old; 6.6±0.4 kg body weight) and 12 old (22.2±0.7 years old; 7.1±0.3 kg body weight) females. Approximately half of the animals from each age group were maintained on a continuous CR diet, receiving 30% fewer calories than the control animals, which were fed at approximately ad libitum levels. The CR lasted for ~4.5 years in the males and 2 years in the females. Hourly blood samples were collected remotely from each animal for 24 hours, and the plasma assayed for melatonin by RIA (Supplementary Material).
3. Results
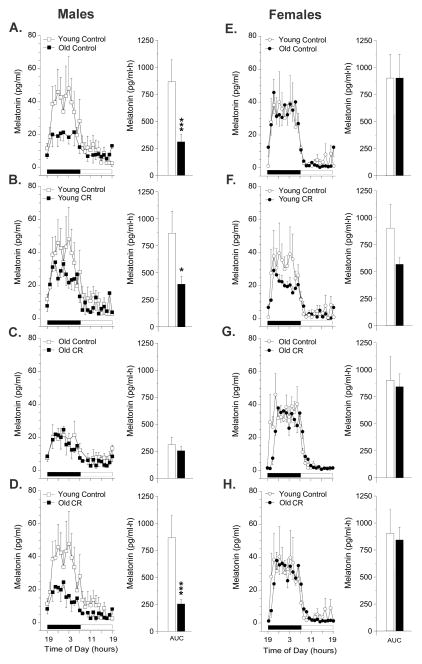
All monkeys displayed clear 24-h rhythmicity in circulating plasma melatonin concentrations, characterized by elevated melatonin levels at night and borderline detectable levels during the day. In the control males, however, plasma melatonin concentrations were significantly (p<0.001) lower in the old animals compared to the young (Fig. 1A). In addition, the young males subjected to CR had significantly (p<0.01) lower plasma melatonin concentrations, compared to age-matched controls (Fig. 1B). In contrast, CR had no effect on the melatonin levels in the old males (Fig. 1C), and after 4.5 years of treatment plasma melatonin levels in the old males still had not been restored to youthful levels (Fig. 1D). In the females, neither age nor CR had any obvious effect on plasma melatonin concentrations (Fig. 1E–H). Although the young CR females appeared to have lower melatonin concentrations than their age-matched controls (Fig. 1F), as seen in the young males (Fig. 1B), this trend was not statistically significant.
Fig. 1.
Effect CR on circulating 24-h melatonin patterns in young and old rhesus macaques. Left panels: Mean plasma 24-h melatonin profiles from (A) young control (n=5) and old control (n=6) males; (B) young control (n=5) and young CR (n=5) males; (C) old control (n=6) and old CR (n=4) males; (D) young control (n=5) and old CR (n=4) males; (E) young control (n=4) and old control (n=7) females; (F) young control (n=4) and young CR (n=4) females; (G) old control (n=7) and old CR (n=5) females; (H) young control (n=4) and old CR (n=5) females. The horizontal black and white bar on the abscissas correspond to the 12 h light:12 h dark lighting schedule. Right panels: Analyses of age, CR and age in combination with CR-related differences in the mean 24-h area under the curve (AUC) of melatonin concentrations. Data are expressed as mean ± SEM. *p < 0.05, ***p < 0.001 (Newman Keuls test).
4. Discussion
The root cause of perturbed sleep cycles in elderly humans is unknown and the therapeutic potential of exogenous melatonin unclear (Brzezinski et al., 2005). In the present study, we maintained our animals under highly controlled environmental conditions and subjected them to one of two carefully controlled diet regimens. Therefore, our examination of plasma melatonin rhythms was conducted under conditions with minimal environmental interference, and based on previous animal studies we expected to observe significant effects of CR. For example, in a previous study involving 28-month-old male rats, 40% food restriction resulted in increased circulating melatonin concentrations relative to age-matched controls (Stokkan et al., 1991). Similarly, in a previous study involving aged (>22-year-old) male and female rhesus macaques, plasma melatonin concentrations were significantly higher in long-term 30% CR animals than in age-matched controls (Roth et al., 2001). In the present study, however, 30% CR failed to elicit any obvious benefit on maintaining elevated concentrations of melatonin in the circulation. If anything, the results from our young animals were more consistent with those of Röjdmark & Wetterberg (1989), who showed a decrease in melatonin secretion in healthy men subjected to a short-term fast. Although the reason for the discrepancy between the previous studies and our findings is unclear, one possibility may stem from differences in the body weight response to the CR paradigm. For example, the 40% food restriction used in the rat study produced a significant (40%) reduction of body mass in the treated animals compared to their age-matched controls; the associated reduction in blood volume may have simply served to concentrate the melatonin in the circulation (Stokkan et al., 1991). Similarly, the 30% CR employed in the previous rhesus macaque study produced a significant (32%) reduction of body mass in the old CR males (but not in the females) compared to their age-matched controls (Roth et al., 2001). In contrast, the 30% CR employed in the present study did not lead to significant differences in mean body mass between the CR animals and their age-matched controls, in either sex. Our findings are consistent with those of Mattison et al., (2017), which also failed to detect significant changes in body weight in rhesus macaques due to CR, but did observed reduced adiposity and food intake in the CR animals. Consequently, it is plausible that any contribution of blood volume differences between experimental groups would have had minimal impact on differentially diluting plasma melatonin concentrations in the present study.
Although the old males and females used in the present study were on average only 1–2 years younger than those used in the previous rhesus macaque study (Roth et al., 2001), they had been exposed to the CR treatment for a much shorter period (i.e., 4.5 years for the males and 2 years for the females, compared to 12 years for the males and 7 years for the females in the previous study). It is possible, therefore, that in long-lived species such as the rhesus macaque beneficial effects of CR on plasma melatonin levels become evident only if the treatment starts earlier in life and is continued for a much longer period. Another possible explanation for the difference between the present results and those previously reported for the rhesus macaque (Roth et al., 2001) may stem from the different way in which the blood samples were collected. In the earlier study the monkeys were anesthetized for blood collection, and to minimize disturbance the nocturnal blood samples were collected under dim red light. In contrast, in the present study the monkeys remained un-sedated at all times and hourly blood samples were collected remotely from an adjacent room, via an indwelling vascular catheter and swivel-tether system (Urbanski, 2011).
Interestingly, the failure to detect a significant age-related decrease in plasma melatonin levels in the females reflects a similar finding for the adrenal steroid, dehydroepiandrosterone sulfate (DHEAS), observed in the same cohort of animals (Downs et al., 2008). Rhesus macaques, like humans, normally show a marked age-related decrease in circulating levels of DHEAS and the absolute levels are lower in females than in males (Sorwell et al., 2012; 2014). Consequently, it is plausible that the age differential between the young and old females in the present study (11.5 versus 22.2 years) was too small to disclose a significant age-related decline in the circulating levels of DHEAS as well as melatonin.
Taken together, the data suggest that a combination of age, duration and/or severity of the CR treatment may ultimately determine how circulating melatonin levels are affected. In monkeys, CR appears to show beneficial effects on plasma melatonin levels only if the treatment is continuously adopted for an extended period (i.e., >7 years), and only if the animals are very old (i.e., showing a naturally occurring age-associated decline, which was not at all obvious in our 22-year-old females). Furthermore, there appears to be no evidence, either from the present study or from the previous rhesus macaque study (Roth et al., 2001) that plasma melatonin levels of young adults benefit from CR; if anything, CR causes an attenuation of these levels. Therefore, although CR has been shown to delay the onset of aging-related pathologies, it is unlikely that changes in melatonin play a key role in mediating these beneficial effects. However, this does not rule out the potential benefits of supplementation in elderly individuals who have highly attenuated melatonin levels (e.g., due to excessive bright light exposure at night, or due to taking medications that suppress melatonin secretion as a side effect).
Supplementary Material
Acknowledgments
The authors are grateful to the ONPRC Division of Comparative Medicine for care of the animals. This work was supported by NIH grants: AG-019914, AG-029612, AG-036670 and OD-011092, and in part, by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Disclosure statement
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, Ford I. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimov I, Laudon M, Zisapel N, Souroujon M, Nof D, Shlitner A, Herer P, Tzischinsky O, Lavie P. Sleep disorders and melatonin rhythms in elderly people. BMJ. 1994;309:167. doi: 10.1136/bmj.309.6948.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi H, Ksto KI, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab. 1982;55:27–29. doi: 10.1210/jcem-55-1-27. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP. Melatonin and sleep in aging population. Exp Gerontol. 2005;40:911–925. doi: 10.1016/j.exger.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Röjdmark S, Wetterberg L. Short-term fasting inhibits the nocturnal melatonin secretion in healthy man. Clin Endocrinol (Oxf) 1989;30:451–457. doi: 10.1111/j.1365-2265.1989.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lesnikov V, Lesnikov M, Ingram DK, Lane MA. Dietary caloric restriction prevents the age-related decline in plasma melatonin levels of rhesus monkeys. J Clin Endocr Metab. 2001;86:3292–3295. doi: 10.1210/jcem.86.7.7655. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487e1–e13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Testosterone increases circulating dehydroepiandrosterone sulfate levels in the male rhesus. Front Endocrinol. 2014;5:101. doi: 10.3389/fendo.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Reiter RJ, Nonaka KO, Lerchl A, Yu BP, Vaughan MK. Food restriction retards aging of the pineal gland. Brain Res. 1991;545:66–72. doi: 10.1016/0006-8993(91)91270-b. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal Models of Behavioral Analysis, Neuromethods. Vol. 50. Humana Press; New York: 2011. pp. 217–235. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.