We previously showed gut microbial production of trimethylamine N-oxide (TMAO) from dietary nutrients like choline, lecithin and L-carnitine is linked to the development of cardiovascular diseases.1–3 We also recently reported plasma TMAO levels are associated with incident thrombotic event risk in subjects, and that TMAO both enhances platelet responsiveness to multiple agonists by augmenting stimulus-dependent Ca2+ signaling, and heightens thrombosis potential in animal models.4 Specifically, a role for TMAO and gut microbiota in transmitting heightened thrombosis potential in vivo was supported by both direct TMAO infusion and microbial transplantation studies4. A Western diet, rich in choline, is associated with heightened thrombosis risk; however, the effect of dietary choline on TMAO and platelet hyperresponsiveness in human subjects has not yet been reported.
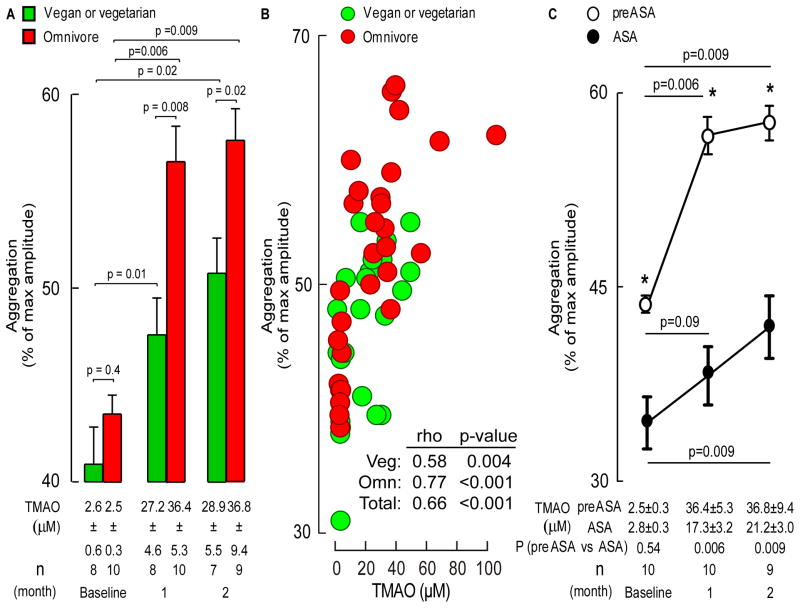
We prospectively recruited healthy vegans/vegetarians (n=8) and omnivores (n=10) with no preceding (1 month) history of antibiotics or probiotics. This is a single-center study approved by the Cleveland Clinic Institutional Review Board. After informed consent, subjects (46±5yo, 40% male, non-smokers without hypertension, diabetes or cardiovascular disease) were given oral choline supplementation (choline bisulfate 500mg twice daily, ~450mg total choline/day) for 2 months with monthly blood testing after overnight fast. Both vegan/vegetarian and omnivore alike showed significant >10-fold increases in plasma TMAO levels at both 1 and 2 month periods (p<0.01 each; Figure 1A), with corresponding enhanced platelet aggregation responses to submaximal adenosine diphosphate (ADP, 5μM) following choline supplementation (Figure 1A). Moreover, a striking dose-dependent association was observed between plasma TMAO levels and platelet function (Figure 1B). Similarly, amongst all subjects in the study, a significant association was noted between change from baseline in TMAO level versus the change from baseline in platelet aggregation (Spearman rho=0.38, p=0.03).
Figure 1. Oral choline supplementation increases fasting TMAO levels, enhances platelet aggregation, and attenuates the anti-platelet effect observed with aspirin.
A) Plasma TMAO levels and platelet aggregation in response to submaximal (5μM) ADP in vegan/vegetarian and omnivore groups. B) Correlation between plasma TMAO and platelet aggregation responses amongst the indicated groups. Spearman correlations and p values shown. C) Effect of choline supplementation on TMAO and platelet aggregation responses in omnivores in the absence versus presence of aspirin (ASA). All data shown are mean (±SEM) with the indicated number of subjects. Asterisks shown represent p<0.05 for comparison of aggregation responses off versus on ASA for the corresponding time point. P values were calculated with Wilcoxon Rank Sum test for two-group comparisons, and Wilcoxon Signed Rank Test for pairwise comparisons.
We next tested whether platelet hyper-responsiveness associated with choline supplementation and elevated TMAO was observed in the presence of aspirin. Omnivores previously examined in the absence of aspirin had a choline supplement-free washout period of at least 1 month and then were started on aspirin (81 mg each evening) for 1 month prior to a baseline evaluation, followed by 2 months of choline supplementation. Compared to baseline, choline again increased both fasting plasma TMAO levels and ADP-dependent platelet aggregation responses at 1 and 2 months of supplementation; however, both the degree of TMAO elevation and platelet hyperresponsiveness were attenuated by aspirin therapy (Figure 1C).
These studies show for the first time a direct pro-thrombotic effect of dietary choline and elevated levels of the gut microbial metabolite TMAO in humans. They also suggest the platelet hyperresponsiveness mediated by elevated TMAO can be attenuated by low dose of aspirin. Importantly, they suggest that elevated levels of the gut microbe-generated metabolite TMAO may overcome the anti-platelet effects of low-dose aspirin – a hypothesis that warrants further investigation, particularly in subjects at high cardiovascular risk. An unanticipated finding was that low dose aspirin partially reduced choline supplement-dependent rise in TMAO. While the mechanism for this is unknown, aspirin has been reported to alter the composition of the gut microbial community5. Finally, aspirin use in primary prevention subjects has recently been debated. The present studies, coupled with published studies linking heightened TMAO levels with thrombotic event risk4, suggest studies are warranted to explore if low dose aspirin is beneficial amongst subjects with elevated TMAO and no clear contraindications to aspirin.
Acknowledgments
Sources of Funding: This research was supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, R01DK106000, R01HL126827).
Footnotes
Disclosures: Drs. Hazen and Wang are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics; Dr. Hazen is a paid consultant for Esperion and P&G; has received research funds from P&G, Pfizer Inc., Roche Diagnostics, and Takeda; and also reports he may receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, Siemens, Esperion, and Frantz Biomarkers, LLC. Dr. Wang reports he may receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab. The other authors have reported that they have no relevant relationships to disclose.
References
- 1.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers MA, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016:178, e1–e9. doi: 10.1016/j.cmi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]