Abstract
Context:
Total pancreatectomy with islet auto transplantation (TPIAT) is a treatment for medically refractory chronic pancreatitis that can prevent postsurgical diabetes in some patients. Predictors of insulin independence are needed for appropriate patient selection and counseling.
Objective:
To explore glycemic predictors of insulin independence after TPIAT.
Design:
A prospective cohort of patients.
Methods:
We investigated 34 patients undergoing TPIAT from 2011-2016 at Johns Hopkins Hospital, all had a 75-g oral glucose tolerance test (OGTT) administered prior to their TPIAT. The primary outcome was insulin independence 1 year after TPIAT.
Results:
Ten of 34 (29%) patients were insulin independent 1 year after TPIAT. All patients with impaired fasting glucose and/or impaired glucose tolerance preoperatively were insulin dependent at 1 year. In age-adjusted regression analyses, fasting glucose ≤ 90 mg/dL [odds ratio (OR) = 6.56; 1.11 to 38.91; P = 0.04], 1-hour OGTT glucose ≤ 143 mg/dL (OR = 6.65; 1.11 to 39.91; P = 0.04), and 2-hour OGTT glucose ≤ 106 mg/dL (OR = 11.74; 1.46 to 94.14; P = 0.02) were significant predictors of insulin independence. In receiver operating characteristic analyses, homeostatic model assessment of β-cell function (HOMA-β) was the most robust predictor of insulin independence [area under the curve (AUC) = 0.88; 0.73 to 1.00].
Conclusions:
Normal preoperative glucose status and lower fasting and postchallenge OGTT glucose values are significant predictors of insulin independence after TPIAT. Higher islet function (HOMA-β) was the strongest predictor. OGTT testing may be a useful tool to aid in patient counseling prior to TPIAT and should be further investigated.
We comprehensively investigated glycemic predictors of insulin independence after TPIAT using OGTT. Lower OGTT glucose levels and higher HOMA-β were predictors of insulin independence.
Total pancreatectomy with islet autotransplantation (TPIAT) is a surgical procedure increasingly used for patients with refractory abdominal pain secondary to chronic pancreatitis. In this procedure, the explanted pancreas is digested and the tissue, including islets, is infused into the patients’ portal vein (1). Postsurgical rates of insulin independence have been reported up to 50% (2). Even so, there is considerable variability among studies, likely related to variable definitions of insulin independence and length of follow-up (3–8). A recent meta-analysis of 362 patients from 5 studies found an average 1-year insulin independence rate of 28.4% (9). The procedure is generally well tolerated, with similar mortality to total pancreatectomy alone. However, TPIAT may occasionally result in additional morbidity for some patients, including bleeding, portal vein thrombosis, or septicemia from contaminated islet preparations (10). Additionally, there is an added cost for isolating and transplanting islet tissue. Thus, TPIAT might not be appropriate for all patients. Whereas TPIAT is performed primarily for pain control in patients with chronic pancreatitis, identifying preoperative predictors of insulin independence may facilitate improved patient selection, more effective counseling about expected glycemic outcomes after surgery, and more judicious allocation of resources.
Although the first islet autotransplantation procedure was performed almost 40 years ago (11), reliable presurgical predictors of insulin independence after TPIAT have yet to be identified. The sole predictor of insulin independence consistently reported in prior studies is higher islet yield (2, 3, 8, 12, 13), which cannot be determined prior to transplantation. Although well described, islet yield is not a perfect predictor of insulin independence, and identifying novel predictors is imperative for better patient selection. A few studies have suggested some preoperative factors as being related to postsurgical insulin independence, such as higher stimulated C-peptide after mixed meal tolerance, female sex, lower body weight, or other pancreatitis-related features (6, 8, 12–14). However, sample sizes were small and outcomes often poorly defined in these studies. To our knowledge, no study has comprehensively examined the relationship of preoperative glycemic status to insulin requirement following TPIAT. In the general population, markers of impaired β-cell function (15) and impaired glucose tolerance (16) are related to a higher risk of developing diabetes, and it is unclear whether a similar relationship may exist in TPIAT candidates.
In the present study, we sought to investigate preoperative glycemic predictors of insulin independence 1 year after TPIAT among patients without diabetes operated on at our institution from 2011 to 2016. Our hypotheses were: 1) lower preoperative fasting, 1-hour, and 2-hour oral glucose tolerance test (OGTT) glucose levels would be predictive of 1-year insulin independence; 2) higher fasting, 1-hour, and 2-hour insulin/C-peptide levels would be predictive of insulin independence; and 3) those with preoperative prediabetes compared with those with normal glucose status would be less likely to have insulin independence at 1 year after TPIAT.
Materials and Methods
Study population
Thirty-nine patients underwent TPIAT and had 1-year follow-up data available at Johns Hopkins Hospital from August 2011 to April 2016. All patients underwent preoperative glucose testing, including measures of hemoglobin A1c (HbA1c) and a 75-g OGTT at ≤3 mo before TPIAT. Of the 39 patients, we excluded 5 with a preoperative diagnosis of diabetes mellitus based on the American Diabetes Association’s diagnostic criteria for diabetes, including HbA1c ≥ 6.5% (48 mmol/mol), fasting glucose ≥ 126 mg/dL, and/or 2-hour glucose on OGTT of ≥200 mg/dL (17). Thus, 34 patients without diabetes who had 1-year TPIAT data available were included in the present analysis.
The Johns Hopkins University Institutional Review Board approved the study, and all patients gave informed consent.
Preoperative glycemic predictors
Preoperative laboratory testing included HbA1c and an OGTT in which glucose, insulin, and C-peptide values were obtained fasting, 60 minutes, and 120 minutes after an oral 75-g dextrose challenge. Impaired fasting glucose (IFG) was defined as a fasting glucose 100 to 125 mg/dL, impaired glucose tolerance (IGT) as 2-hour glucose on OGTT of 140 to 199 mg/dL, and at high risk for diabetes as HbA1c 5.7% to 6.4% (39 to 47 mmol/mol), all according to American Diabetes Association criteria for prediabetes. Fasting glucose and insulin values were used to calculate homeostatic model assessment of insulin resistance [glucose (mg/dL) × insulin (mIU/L)/405] and homeostatic model assessment of β-cell function (HOMA-β; [insulin (mIU/L) × 360]/[(glucose (mg/dL)] – 63), with the latter being a measure of percentage β-cell function (18). Levels of glucose, insulin, and C-peptide at all time points of the OGTT were evaluated as predictors of insulin independence.
Operative procedure
Patients underwent total pancreatectomy with on-site isolation and islet transplantation into the portal vein during the same surgery. Islet counting was performed using an automatic counter in 29 of 34 cases (85%), and manual counting was used in the final 2 cases. In 3 instances, counts could not be obtained due to technical difficulties with the automatic counter. Following the procedure, all patients were admitted to a surgical intensive care unit, managed with intravenous insulin infusion with tight goal glucose of 100 to 120 mg/dL, similar to other centers (19). At postoperative day 3 patients were transitioned to subcutaneous basal insulin therapy with additional prandial and correction insulin added as needed. Diabetes education was given and insulin doses were adjusted as necessary to achieve glucose goals similar to other patients with diabetes. Daily morning C-peptide measurements were obtained. All patients were discharged on subcutaneous insulin regimens including basal and/or bolus insulin for at least 3 months unless prevented by recurrent hypoglycemia, a common practice to “rest” the islets after TPIAT (3).
Postoperative care
Patients returned every 3 months until 1-year follow-up for clinical evaluation, repeat OGTT testing, and attempt to wean insulin. For patients who did not meet the diagnosis for diabetes using either OGTT or HbA1c criteria and who were also using <10 U of basal insulin, an attempt was made to wean to no insulin. If an oral agent was needed, a dipeptidyl peptidase IV inhibitor was preferred to aid in postprandial hyperglycemia.
Outcome assessment
The primary outcome for our analysis was insulin independence 1 year after TPIAT, defined as having no requirement for any insulin therapy at that time and an HbA1c <6.5%. At the 1-year follow-up, information was collected on use of insulin therapy or oral hypoglycemic agents. HbA1c was obtained for all patients. An OGTT was obtained for those who were insulin-independent. One-year determination of insulin independence was based on clinical evaluation in endocrinology outpatient clinic at the visit closest to the 12-month postoperative date, but had to be ±3 months from this date. If there was no 1-year follow-up visit by this definition, but the patient was found to be insulin-independent at a subsequent visit, they were assumed to be insulin-independent at 1 year. If the last visit documented was after 3 months but prior to the 1-year postoperative date using this definition, and they were insulin-dependent at that time, they were considered to also be insulin-dependent at 1 year.
Covariates
We collected self-reported information preoperatively on age, race, sex, and characteristics of pancreatitis. Body mass index (BMI) was calculated from data from the patient’s preoperative visit, using standard procedures.
Statistical analysis
Differences in preoperative characteristics between groups, categorized according to 1-year insulin requirement, were calculated using the Student t test for continuous variables and Fisher’s exact tests for categorical variables.
Sequential logistic regression models explored the relationship of individual glycemic predictors to the primary outcome of 1-year insulin independence: model 1 was unadjusted and model 2 was adjusted for age. A sensitivity analysis investigated further adjustment for islet yield. Receiver operating characteristics (ROC) were calculated via nonparametric estimation of the ROC curve, producing confidence intervals for the area under the ROC curve.
An analysis for the secondary outcome of “well-controlled” glycemic status was performed, defined as HbA1c <7% and insulin independence or requirement of ≤5 U of basal insulin at 1-year follow-up.
A 2-tailed P of <0.05 was used to indicate statistical significance. All analyses were performed using Stata version 14.1 (StataCorp, College Station, TX).
Results
Baseline patient characteristics according to 1-year insulin status are shown in Table 1. Twenty-nine percent of patients (10 out of 34) were completely insulin-independent at 1 year. Comparing patients that were insulin-independent vs -dependent, mean age was lower, a greater proportion were female, and mean BMI was lower. The patients were predominantly white. Among those that were insulin-independent at 1 year, preoperative prediabetes was present in 10%, and among those that were insulin-dependent at 1 year, preoperative diabetes was present in 67% (P < 0.01). Islet yield was significantly higher in the insulin-independent group.
Table 1.
Preoperative Patient Characteristics by Insulin Independence Status at 1 Year
| Variable | Total | Insulin Independent | Insulin Dependent | P Valuea (Between Groups) |
|---|---|---|---|---|
| n, % | 34 | 10 (29.4) | 24 (70.6) | |
| Preoperative clinical characteristics | ||||
| Age, mean, SD | 39.3 (13.7) | 35.2 (14.4) | 41.0 (13.4) | 0.27 |
| Age, y; n (%) | 0.58 | |||
| <20 | 1 (2.9) | 0 (0) | 1 (4.2) | |
| 20–39 | 18 (52.9) | 7 (70.0) | 11 (45.8) | |
| 40–59 | 12 (35.3) | 2 (20.0) | 10 (41.7) | |
| ≥60 | 3 (8.8) | 1 (10.0) | 2 (8.3) | |
| Sex, n (%) | 0.05 | |||
| Female | 21 (61.8) | 9 (90.0) | 12 (50.0) | |
| Male | 13 (38.2) | 1 (10.0) | 12 (50.0) | |
| Race, n (%) | 0.51 | |||
| White | 32 (94.1) | 9 (90.0) | 23 (95.8) | |
| Hispanic | 1 (2.9) | 0 (0) | 1 (4.2) | |
| Non-Hispanic black | 1 (2.9) | 1 (10.0) | 0 (0) | |
| Baseline BMI, kg/m2; mean (SD) | 24.0 (3.5) | 23.0 (1.9) | 24.3 (3.9) | 0.32 |
| Baseline BMI, kg/m2; n (%) | 0.31 | |||
| <18.5 | 1 (2.9) | 0 (0) | 1 (4.2) | |
| 18.5–24.9 | 22 (64.7) | 9 (90.0) | 13 (54.2) | |
| 25–29.9 | 9 (26.5) | 1 (10.0) | 8 (33.3) | |
| ≥30 | 2 (5.9) | 0 (0) | 2 (8.3) | |
| Baseline glycemic status, n (%) | <0.01 | |||
| Normal | 17 (50.0) | 9 (90.0) | 8 (33.3) | |
| Prediabetesb | 17 (50.0) | 1 (10.0) | 16 (66.7) | |
| IFGc | 7 (20.6) | 0 (0) | 7 (29.2) | |
| IGTd | 12 (35.3) | 0 (0) | 12 (50.0) | |
| Category of increased riske | 7 (20.6) | 1 (10.0) | 6 (25.0) | |
| Pancreatitis pain duration ≥ 3 years | 21 (61.7) | 6 (60.0) | 15 (62.5) | 1.00 |
| Pancreatitis etiology, n (%) | 0.06 | |||
| Alcoholic | 5 (14.7) | 0 (0) | 5 (20.8) | |
| Genetic | 10 (29.4) | 1 (10.0) | 9 (37.5) | |
| Divisum | 3 (8.8) | 1 (10.0) | 2 (8.3) | |
| Traumatic | 1 (2.9) | 0 (0) | 1 (4.2) | |
| Necrotizing | 0 (0) | 0 (0) | 0 (0) | |
| Idiopathic | 15 (44.1) | 8 (80.0) | 7 (29.2) | |
| Perioperative characteristics | ||||
| Islet yield (IEq), median (IQR) | 437,030 | 727,137 | 293,990 | <0.01 |
| (213,190; 775,970) | (446,572; 1,257,323) | (200,074; 587,060) | ||
Abbreviations: IQR, interquartile range; SD, standard deviation.
P values represent the difference between insulin-independent and insulin-dependent groups.
Participants can have >1 criterion for diagnosis in this category.
Defined as fasting glucose 100–125 mg/dL.
Defined as 2-hour glucose on OGTT 140–199 mg/dL.
Defined as HbA1c 5.7%–6.4%.
Mean 1-year follow-up occurred at 11.8 ± 2.1 months after TPIAT. For 1 patient, the last follow-up visit was at 8 months, at which time the patient was insulin-dependent. For 1 other patient, the only follow-up visit was at 19 months, at which time the patient was insulin-independent with HbA1c <6.5%. In the insulin-independent group, mean HbA1c was 5.5% ± 0.33% (37 ± 4 mmol/mol), and 2 of the 10 were on oral hypoglycemic agents for prediabetes. In the insulin-dependent group, mean HbA1c was 8.0% ± 2.62% (64 ± 28 mmol/mol) at follow-up. The 24 patients in the insulin-dependent group were presumed to have diabetes based on HbA1c and/or continued requirement for insulin therapy. In the insulin-dependent group, 21% were on basal insulin only, 4% on bolus insulin only, and the remaining 75% were on both basal and bolus insulin. Of those using basal insulin, the mean daily dose was 12.0 ± 6.0 U. Of patients requiring bolus insulin, 32% required sliding scale only, whereas the remaining also required mealtime nutritional insulin. Twenty-one percent of the insulin-dependent patients were also on oral hypoglycemic agents. All of the insulin-independent (100%; 10/10) and all but 1 of the insulin-dependent patients (97%; 23/24) had a detectable C-peptide at 1 year (mean, 12.6 ± 6.4 months).
Supplemental Tables 1 (16.9KB, docx) and 2 (16.9KB, docx) list each patient categorized by their 1-year insulin requirement, as well as by their specific preoperative American Diabetes Association criteria for prediabetes. All patients with preoperative IFG and/or IGT had 1-year insulin dependence. Additionally, all 5 patients with preoperative HbA1c > 5.7% (39 mmol/mol) were insulin-dependent. Only 1 patient in the insulin-independent group met criteria for prediabetes, solely based on HbA1c testing. Among the insulin-dependent patients, of the 12 with preoperative IGT, 5 met the prediabetes definition exclusively by this categorization alone.
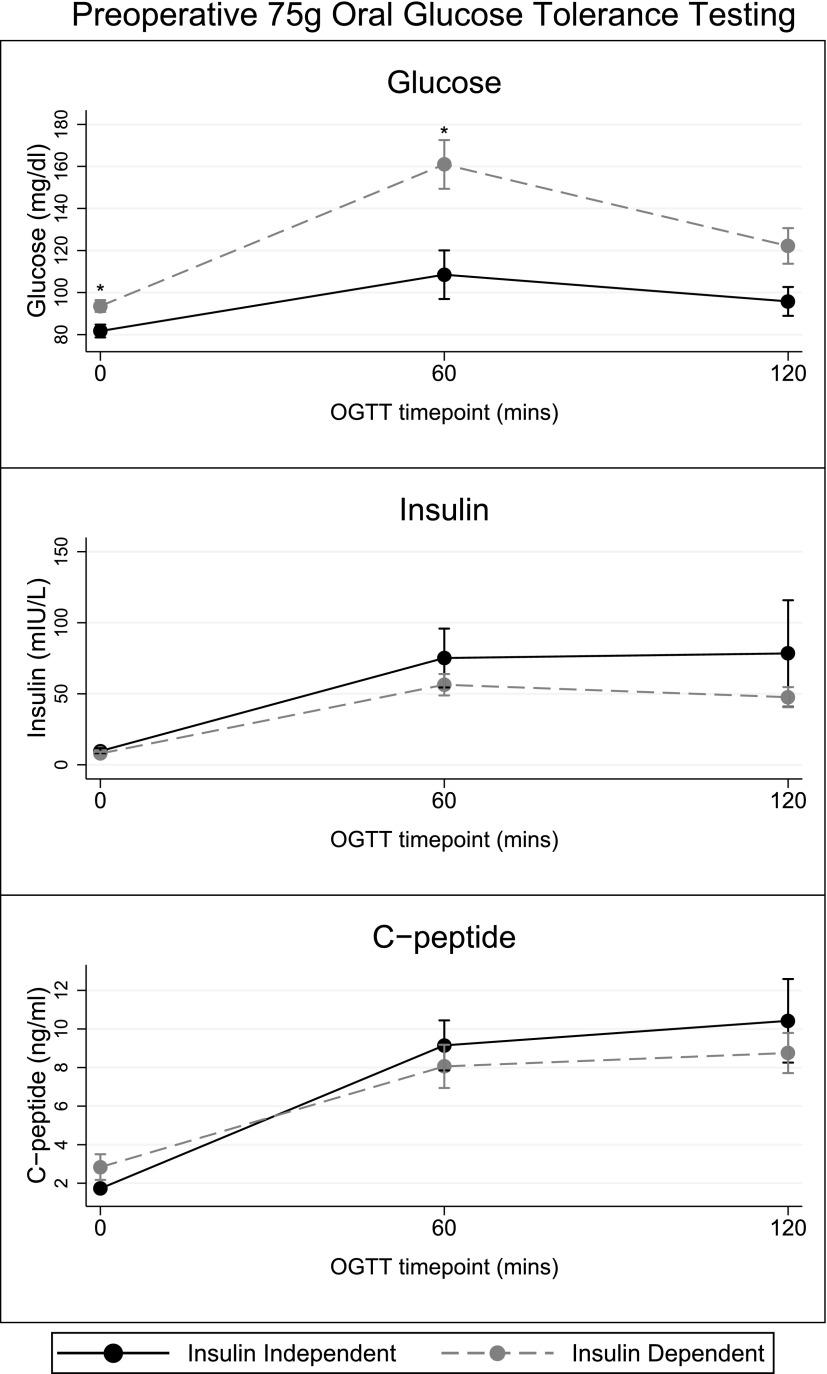
Regarding 75-g OGTT testing, as summarized in Fig. 1, the mean ± standard error glucose levels at times 0, 60, and 120 minutes for the insulin-independent group vs the insulin-dependent group were 82 ± 3 mg/dL vs 94 ± 3 mg/dL (P = 0.02), 109 ± 12 mg/dL vs 161 ± 12 mg/dL (P = 0.01), and 96 ± 7 vs 122 ± 9 mg/dL (P = 0.06). As summarized in Fig. 1, mean insulin levels during OGTT did not differ significantly between the 2 groups, although they were generally higher in the insulin-independent group. There was no significant difference in C-peptide values at any time point by group. HOMA-β values (n = 26) significantly differed between the insulin-independent vs insulin-dependent group (262% ± 87% vs 90% ± 13%, P < 0.01). Homeostatic model assessment of insulin resistance was similar in both groups.
Figure 1.
Glucose, insulin, and C-peptide levels (mean ± standard error) during a 75-g OGTT comparing those that are insulin-independent vs insulin-dependent at 1-year follow-up. For the insulin-independent vs insulin-dependent groups, levels for the fasting, 60-minute, and 120-minute glucose values were 82 ± 3 mg/dL vs 94 ± 3 mg/dL (P = 0.02), 109 ± 12 mg/dL vs 161 ± 12 mg/dL (P = 0.01), and 96 ± 7 vs 122 ± 9 mg/dL (P = 0.06). Similarly, levels for the fasting, 60-minute, and 120-minute insulin values were 10 ± 2 mIU/L vs 8 ± 1 mIU/L (P = 0.51), 75 ± 21 mIU/L vs 56 ± 8 mIU/L (P = 0.30), and 78 ± 38 mIU/L vs 48 ± 7 mIU/L (P = 0.19). Lastly, levels for the fasting, 60-minute, and 120-minute C-peptide values were 1.7 ± 0.2 ng/ml vs 2.8 ± 0.7 ng/ml (P = 0.31), 9.1 ± 1.3 ng/ml vs 8.1 ± 1.1 ng/ml (P = 0.61), and 10 ± 2.2 ng/ml vs 8.8 ± 1.0ng/ml (P = 0.46).
Supplemental Table 3 (16.9KB, docx) shows mean differences in glycemic measures dichotomized by islet yield of <500,000 islet equivalents (IEq) vs ≥500,000 IEq. Two-hour glucose on OGTT was the only measure that significantly differed between groups (P = 0.04), although several others had a trend toward significance.
In regression analyses, a normal (compared with prediabetes) preoperative glycemic status was a significant predictor of 1-year insulin independence in both unadjusted model 1 [odds ratio (OR) = 18.00, 1.93 to 167.99; P = 0.01), and when adjusting for age in model 2 (OR = 16.42, 1.73 to 156.37; P = 0.02). Table 2 lists the regression analysis for other glycemic predictors of 1-year insulin independence. All OGTT measures were dichotomized around the median. After age adjustment (Table 2, model 2), fasting glucose ≤ 90 mg/dL (OR = 6.56, 1.11 to 38.91; P = 0.04), 1-hour OGTT glucose ≤ 143 mg/dL (OR = 6.65, 1.11 to 39.91; P = 0.04), and 2-hour OGTT glucose ≤ 106 mg/dL (OR = 11.74, 1.46 to 94.14; P = 0.02) were all significant predictors of insulin independence. Fasting insulin and C-peptide levels at any time point on OGTT and preoperative HbA1c were not predictive of insulin independence.
Table 2.
Logistic Regression Models (OR, 95% Confidence Interval) Investigating the Relationship of Glycemic Predictors to 1-Year Insulin Independence After TPIATa
| Preoperative Glycemic Measureb | Model 1 | Model 2 |
|---|---|---|
| Fasting glucose | ||
| ≤90 mg/dL | 6.67 (1.15–38.60)c | 6.56 (1.11–38.91)c |
| >90 mg/dL | Reference | Reference |
| One-hour glucose | ||
| ≤143 mg/dL | 7.00 (1.18–41.36)c | 6.65 (1.11–39.91)c |
| >143 mg/dL | Reference | Reference |
| Two-hour glucose | ||
| ≤106 mg/dL | 6.22 (1.07–36.21)c | 11.74 (1.46–94.14)c |
| >106 mg/dL | Reference | Reference |
| Fasting C-peptide | ||
| <1.71 ng/mL | Reference | Reference |
| ≥1.71 ng/mL | 2.04 (0.38–10.94) | 2.47 (0.40–15.18) |
| Fasting insulin | ||
| ≤7.4 mIU/L | Reference | Reference |
| >7.4 mIU/L | 3.25 (0.48–22.00) | 1.71 (0.18–16.10) |
| HbA1c | ||
| <5.4% | 3.27 (0.67–15.82) | 2.74 (0.53–14.25) |
| ≥5.4% | Reference | Reference |
The n for each laboratory is as follows: fasting glucose (n = 34); 1-hour glucose (n = 32); 2-hour glucose (n = 33); fasting C-peptide (n = 28); fasting insulin (n = 27); HbA1c (n = 34).
Model 1 is unadjusted; model 2 is adjusted for age.
Preoperative glycemic measures are dichotomized at their median values.
P < 0.05.
In other regression models, HOMA-β (>88.9% vs ≤88.9%) was not a significant predictor of insulin independence. However, all of the 11 patients in the lowest tertile of HOMA-β levels (<72%) were insulin-dependent at 1 year.
Tables 3 and 4 demonstrate the ROC curve area under the curve (AUC) values for glycemic measures in our cohort. Regarding predictors of insulin independence, HOMA-β had the highest AUC of 0.88 (0.73 to 1.00; P < 0.01), followed by islet yield (AUC = 0.77, 0.58 to 0.96; P < 0.01). Regarding predictors of insulin dependence, 1-hour glucose on OGTT had the highest AUC of 0.80 (0.63 to 0.96; P < 0.01), followed by fasting glucose (AUC = 0.75, 0.57 to 0.94; P < 0.01), HbA1c (AUC = 0.74, 0.55 to 0.92; P = 0.01), and 2-hour glucose on OGTT (ROC = 0.71, 0.53 to 0.88; P = 0.02) for prediction of insulin dependence. The Youden index, which provides the optimal cutoff with the optimal sensitivity and specificity, is listed for each glycemic predictor in Tables 3 and 4. Notably, the optimal cutoffs are 140.8% for HOMA-β and 119 mg/dL for 1-hour glucose, the strongest predictors of insulin independence and dependence, respectively.
Table 3.
ROC Statistics for Predictors of 1-Year Insulin Independence
| Predictors of Insulin Independence | ROC AUC | 95% CI | P Value | 100% Sensitivity Cutoff | 100% Specificity Cutoff | Youden Cutoff Pointa | Youden Sensitivity (%) | Youden Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| Preoperative HOMA-β | 0.88 | 0.73–1.00 | <0.01 | <72% | ≥242.1% | 140.8% | 83.3 | 85.0 |
| Islet yield | 0.77 | 0.58–0.96 | <0.01 | <148,200 IEq | ≥1,164,722 IEq | 446,572 IEq | 80.0 | 70.82 |
The cutoff offering optimal sensitivity and specificity.
Table 4.
ROC Statistics for Predictors of 1-Year Insulin Dependence
| Predictors of Insulin Dependence | ROC AUC | 95% CI | P Value | 100% Sensitivity Cutoff | 100% Specificity Cutoff | Youden Cutoff Pointa | Youden Sensitivity (%) | Youden Specificity (%) |
|---|---|---|---|---|---|---|---|---|
| Preoperative 1-hour glucose | 0.80 | 0.63–0.96 | <0.01 | <64 mg/dL | ≥189 mg/dL | 119 mg/dL | 81.82 | 80.0 |
| Preoperative fasting glucose | 0.75 | 0.57–0.94 | <0.01 | <63 mg/dL | ≥104 mg/dL | 78 mg/dL | 91.67 | 50.0 |
| Preoperative HbA1c | 0.74 | 0.55–0.92 | 0.01 | <4.5% | ≥5.8% | 5.6% | 45.83 | 90.0 |
| Preoperative 2-hour glucose | 0.71 | 0.53–0.88 | 0.02 | <38 mg/dL | ≥141 mg/dL | 141 mg/dL | 52.17 | 100 |
The cutoff offering optimal sensitivity and specificity.
In a sensitivity regression analysis adjusting for islet yield, normal glycemic status at baseline remained a significant predictor of insulin independence (OR = 17.76, 1.46 to 216.61; P = 0.03). However, the relationship of OGTT glucose measures with insulin independence was attenuated such that they were no longer significant (all P > 0.05). In secondary analyses, normal preoperative glycemic status remained a significant predictor of well-controlled glycemic status at 1 year (n = 12 out of 34 using this definition) after adjustment for age and islet yield (OR = 9.22, 1.22 to 69.74; P = 0.03). Fasting glucose became a stronger predictor in adjusted analysis (OR = 9.68, 1.35 to 69.26; P = 0.02). All other glycemic predictors had similar findings to the primary analysis.
Discussion
Overall, our TPIAT insulin independence rate of 29% is similar to those reported in other analyses of 1-year outcomes (9). Preoperative β-cell function, as measured by HOMA-β, was the most robust predictor of insulin independence in ROC analysis, with an AUC even greater than islet yield. In regression analyses, lower preoperative OGTT fasting, 1- hour, and 2-hour glucose levels were significantly related to 1-year insulin independence. This relationship may be mediated in part by islet yield, but islet yield can only be measured perioperatively. Normal (compared with prediabetes) glycemic status was a significant predictor of 1-year insulin independence. Furthermore, all patients with prediabetes, when defined as IFG and/or IGT, had 1-year insulin dependence. Importantly, approximately one-third of patients diagnosed with prediabetes met this criterion exclusively by IGT alone and would have been missed without preoperative OGTT testing.
Although few prior studies have reported that preoperative glycemic measures are related to islet yields during TPIAT (20), we found that this association was only significant for preoperative 2-hour glucose and islet yield in our study. In reviewing previously reported studies, the 75-g OGTT is not currently a part of the standard evaluation process prior to TPIAT at most other centers. As a result, OGTT parameters may not have been previously studied as potential predictors of insulin independence. Because OGTT is a part of the standard preoperative evaluation at our center, we were able to more comprehensively investigate the relationship of glycemic predictors to 1-year insulin independence. Consistent with our findings, a cohort study at the Cleveland Clinic noted that peak glucose during a mixed meal tolerance test preoperatively was inversely correlated with islet yield (8), a previously reported predictor of insulin independence. In our study, the 1-hour OGTT glucose level represented the “peak” glucose during the OGTT evaluation and was markedly higher in the insulin-independent group, suggesting a similar relationship, although note that OGTT time points during the early phase of OGTT were not available in our study. Another study used preoperative continuous glucose monitoring in TPIAT patients and found that a higher ratio of time spent above a glycemic threshold of 120 mg/dL, particularly after meals, was predictive of postprocedure insulin requirement (21). We similarly found that 1-hour glucose levels ≤120 mg/dL on OGTT were significantly related to 1-year insulin independence, even when adjusted for islet yield (data not shown).
In prior studies, the prevalence of overt diabetes in those with chronic pancreatitis has been reported to be 30% to 40%. Additionally, up to 70% have abnormal glucose tolerance, with progressive worsening of glucose tolerance as duration of pancreatitis increases (22). Studies using OGTT in those with cystic fibrosis similarly demonstrate that elevations of glucose at 1 hour are associated with a higher risk of developing diabetes (23). Although the interpretation of 1-hour glucose on OGTT may not be as standardized, many experts still recommend obtaining this value as a marker of early β-cell dysfunction in chronic pancreatitis populations (24). Predicting who will progress to overt diabetes is challenging, but there is evidence that OGTT improves the predictive strength of models for future risk of diabetes in the general population (25), although results are admittedly mixed (16, 26). Reduced HOMA-β, a measure of β-cell reserve, has been shown to be lower in individuals from the general population who eventually progress to either IGT or prediabetes (15). In our TPIAT study, patients with a prediabetes state, higher 1-hour glucose on OGTT, or lower HOMA-β preoperatively were much more likely to require insulin therapy at 1 year. All of these measures reflect impaired β-cell function at baseline. These high-risk individuals may be at greater long-term risk of developing diabetes even without any surgical intervention. After potential islet disruption and partial loss of viability during transplantation, it is perhaps not surprising they are at increased risk of requiring insulin after TPIAT.
Although islet yield has been a consistent predictor of insulin independence in other studies, and the relationship of some of our preoperative glycemic predictors to 1-year insulin independence may ultimately relate to the recovered islet yield during surgery, many of the glycemic predictors we examined did not significantly differ by high vs low islet yield. Additionally, the association of normal (vs prediabetes) status with insulin independence remained significant when accounting for islet yield in regression analyses, providing further evidence that islet yield alone is not sufficient for prediction of insulin independence. Also, importantly, information on islet yield is not available until the time of surgery.
Strengths of our study include the comprehensive characterization of diabetes status preoperatively, including performance of a 2-hour OGTT for glucose, insulin, and C-peptide which also included a 1-hour level. This allowed us to classify glucose status using standard clinical diagnostic criteria and identify novel glycemic predictors of insulin independence after TPIAT. The performance of OGTT testing in our study, including 1-hour glucose levels, is consistent with recent recommendations for screening of patients with chronic pancreatitis for the presence of diabetes (24). Although prior studies had relatively more ambiguous definitions of insulin requirement, including partial graft function (3), our strict definition of insulin independence for our outcome allowed us to draw more clinically meaningful conclusions. We did not exclude patients because of demographic information or pancreatitis etiology. Additionally, as there are currently less than a dozen TPIAT centers nationally, accrual of patients may take a few years and our patients had a relatively wide and diverse catchment area, making our results more generalizable.
Limitations of our study include the sample size of our cohort. Although similar when compared with studies reported by other centers in the United States (2, 4, 5, 8, 12, 21, 27, 28), the relatively small population limits statistical power, particularly for regression analyses. Some patients did not have complete data for preoperative OGTT testing, particularly for postchallenge C-peptide and insulin. Also, our OGTT testing did not include time points earlier than 1 hour, making it possible that an earlier glycemic spike was missed. We did not require laboratories to be drawn at our institution for patient convenience, which could have impacted measurements. Reliability of islet counts should also be considered, as automatic counting was used for most patients in our study, which may overestimate islet yield as compared with manual counting. Although the method of islet counting does not impact the glycemic outcomes of our study, it should be considered in interpretation, particularly when comparing the results of our study to those from other centers. The potential imprecision of automated islet counting may have impacted the results of sensitivity regression analyses, yet we were still able to detect significant associations. A formal validation study is needed in the future to compare differences in the estimates of islet yield obtained from automatic vs manual counting. Future studies are also needed to explore whether specific genetic mutations may be related to insulin independence after TPIAT.
In this study, we report our experience with routine 2-hour OGTT testing as part of the standard preoperative evaluation prior to TPIAT. As a result, we were able to identify several glycemic predictors of insulin independence 1 year after TPIAT. In our cohort, normal glucose status, lower preoperative fasting, 1-hour and 2-hour glucose levels on OGTT, and higher β-cell function (as estimated by HOMA-β) were shown to be strong predictors of insulin independence. All patients with prediabetes preoperatively, when defined as IFG and/or IGT, had insulin dependence at 1 year, with many patients being identified solely on the basis of having impaired glucose tolerance. To date, islet yield has been the only consistent predictor of insulin independence reported in other studies but can only be ascertained during transplantation. Preoperative glycemic predictors offer the promise to identify those most likely to have insulin independence long-term. Although further studies are needed, our results support the potential utility of OGTT in presurgical evaluation as a low-cost and potentially very useful preoperative diagnostic assessment to improve selection and counseling of TPIAT candidates regarding postoperative insulin independence.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants K23-DK093583 (to R.R.K.) and T32-DK062707 (to M.Q.).
Disclosure Summary: V.S. has been a consultant to Novo Nordisk. The remaining authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- HbA1c
- hemoglobin A1c
- HOMA-β
- homeostatic model assessment of β-cell function
- IEq
- islet equivalent
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- ROC
- receiver operating characteristic
- TPIAT
- total pancreatectomy with islet autotransplantation
References
- 1.Bellin MD, Gelrud A, Arreaza-Rubin G, Dunn TB, Humar A, Morgan KA, Naziruddin B, Rastellini C, Rickels MR, Schwarzenberg SJ, Andersen DK. Total pancreatectomy with islet autotransplantation: summary of an NIDDK workshop. Ann Surg. 2015;261(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savari O, Golab K, Wang LJ, Schenck L, Grose R, Tibudan M, Ramachandran S, Chon WJ, Posner MC, Millis JM, Matthews JB, Gelrud A, Witkowski P. Preservation of beta cell function after pancreatic islet autotransplantation: University of Chicago experience. Am Surg. 2015;81(4):421–427. [PubMed] [Google Scholar]
- 3.Sutherland DE, Radosevich DM, Bellin MD, Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B, Balamurugan AN, Freeman ML, Pruett TL. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb MA, Illouz SC, Pollard CA, Gregory R, Mayberry JF, Tordoff SG, Bone M, Cordle CJ, Berry DP, Nicholson ML, Musto PP, Dennison AR. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37(3):282–287. [DOI] [PubMed] [Google Scholar]
- 5.Tai DS, Shen N, Szot GL, Posselt A, Feduska NJ, Habashy A, Clerkin B, Core E, Busuttil RW, Hines OJ, Reber HA, Lipshutz GS. Autologous islet transplantation with remote islet isolation after pancreas resection for chronic pancreatitis. JAMA Surg. 2015;150(2):118–124. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Desai KD, Dong H, Owzarski S, Romagnuolo J, Morgan KA, Adams DB. Prior surgery determines islet yield and insulin requirement in patients with chronic pancreatitis. Transplantation. 2013;95(8):1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argo JL, Contreras JL, Wesley MM, Christein JD. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74(6):530–536, discussion 536–537. [PubMed] [Google Scholar]
- 8.Johnston PC, Lin YK, Walsh RM, Bottino R, Stevens TK, Trucco M, Bena J, Faiman C, Hatipoglu BA. Factors associated with islet yield and insulin independence after total pancreatectomy and islet cell autotransplantation in patients with chronic pancreatitis utilizing off-site islet isolation: Cleveland Clinic experience. J Clin Endocrinol Metab. 2015;100(5):1765–1770. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Zhang M, Qin Y, Jiang R, Chen H, Xu X, Yang T, Jiang K, Miao Y. Systematic review and meta-analysis of islet autotransplantation after total pancreatectomy in chronic pancreatitis patients. Endocr J. 2015;62(3):227–234. [DOI] [PubMed] [Google Scholar]
- 10.Bhayani NH, Enomoto LM, Miller JL, Ortenzi G, Kaifi JT, Kimchi ET, Staveley-O’Carroll KF, Gusani NJ. Morbidity of total pancreatectomy with islet cell auto-transplantation compared to total pancreatectomy alone. HPB (Oxford). 2014;16(6):522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58(2):365–382. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad SA, Lowy AM, Wray CJ, D’Alessio D, Choe KA, James LE, Gelrud A, Matthews JB, Rilo HL. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201(5):680–687. [DOI] [PubMed] [Google Scholar]
- 13.Chinnakotla S, Beilman GJ, Dunn TB, Bellin MD, Freeman ML, Radosevich DM, Arain M, Amateau SK, Mallery JS, Schwarzenberg SJ, Clavel A, Wilhelm J, Robertson RP, Berry L, Cook M, Hering BJ, Sutherland DE, Pruett TL. Factors predicting outcomes after a total pancreatectomy and islet autotransplantation lessons learned from over 500 cases. Ann Surg. 2015;262(4):610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young MC, Theis JR, Hodges JS, Dunn TB, Pruett TL, Chinnakotla S, Walker SP, Freeman ML, Trikudanathan G, Arain M, Robertson PR, Wilhelm JJ, Schwarzenberg SJ, Bland B, Beilman GJ, Bellin MD. Preoperative computerized tomography and magnetic resonance imaging of the pancreas predicts pancreatic mass and functional outcomes after total pancreatectomy and islet autotransplant. Pancreas. 2016;45(7):961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care. 2004;27(6):1439–1446. [DOI] [PubMed] [Google Scholar]
- 16.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–S22. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 19.Chinnakotla S, Radosevich DM, Dunn TB, Bellin MD, Freeman ML, Schwarzenberg SJ, Balamurugan AN, Wilhelm J, Bland B, Vickers SM, Beilman GJ, Sutherland DE, Pruett TL. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg. 2014;218(4):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg R, Beilman GJ, Dunn TB, Pruett TL, Chinnakotla SC, Radosevich DM, Robertson RP, Ptacek P, Balamurugan AN, Wilhelm JJ, Hering BJ, Sutherland DE, Moran A, Bellin MD. Metabolic assessment prior to total pancreatectomy and islet autotransplant: utility, limitations and potential. Am J Transplant. 2013;13(10):2664–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltrán del Río M, Georgiev GI, Cercone R, Tiwari M, Rilo HL. Continuous glucose monitoring analysis as predictor of islet yield and insulin requirements in autologous islet transplantation after complete pancreatectomy. J Diabetes Sci Technol. 2014;8(6):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjoberg RJ, Kidd GS. Pancreatic diabetes mellitus. Diabetes Care. 1989;12(10):715–724. [DOI] [PubMed] [Google Scholar]
- 23.Schmid K, Fink K, Holl RW, Hebestreit H, Ballmann M. Predictors for future cystic fibrosis-related diabetes by oral glucose tolerance test. J Cyst Fibros. 2014;13(1):80–85. [DOI] [PubMed] [Google Scholar]
- 24.Rickels MR, Bellin M, Toledo FG, Robertson RP, Andersen DK, Chari ST, Brand R, Frulloni L, Anderson MA, Whitcomb DC; PancreasFest Recommendation Conference Participants . Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology. 2013;13(4):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathmann W, Kowall B, Heier M, Herder C, Holle R, Thorand B, Strassburger K, Peters A, Wichmann HE, Giani G, Meisinger C. Prediction models for incident type 2 diabetes mellitus in the older population: KORA S4/F4 cohort study. Diabet Med. 2010;27(10):1116–1123. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D’Agostino RBS Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167(10):1068–1074. [DOI] [PubMed] [Google Scholar]
- 27.Takita M, Naziruddin B, Matsumoto S, Noguchi H, Shimoda M, Chujo D, Itoh T, Sugimoto K, Onaca N, Lamont JP, Lara LF, Levy MF. Variables associated with islet yield in autologous islet cell transplantation for chronic pancreatitis. Proc Bayl Univ Med Cent. 2010;23(2):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan KA, Theruvath T, Owczarski S, Adams DB. Total pancreatectomy with islet autotransplantation for chronic pancreatitis: do patients with prior pancreatic surgery have different outcomes? Am Surg. 2012;78(8):893–896. [PubMed] [Google Scholar]