Abstract
Context:
Medullary thyroid cancer (MTC) is an aggressive tumor that harbors activating mutations of the RET proto-oncogene. We previously reported that RET inhibits transcriptional activity of ATF4, the master regulator of the stress response pathway, to prevent cell death.
Objective:
We hypothesized that loss of function of ATF4 plays a role in initiation of MTC.
Design:
Targeted deletion of Atf4 in mice was used to assess ATF4 function in the thyroid gland. ATF4 overexpression was achieved by adenoviral and lentiviral vectors. We used immunohistochemical analysis and western blotting of MTC tumors to determine protein levels of RET and ATF4 and the Kaplan-Meier method to determine their association with clinical outcome.
Results:
Targeted deletion of Atf4 in mice causes C-cell hyperplasia, a precancerous lesion for MTC. Forced ATF4 expression decreased survival of MTC cells and blocked the activation of RET downstream signaling pathways (phosphorylated ERK, phosphorylated AKT, and p70S6K). ATF4 knockdown decreased sensitivity to tyrosine kinase inhibitor–induced apoptosis. Moreover, ATF4 expression decreased RET protein levels by promoting RET ubiquitination. We found decreased or loss of ATF4 in 52% of MTC tumors (n = 39) compared with normal thyroid follicle cells. A negative correlation was observed between RET and ATF4 protein levels in MTC tumors, and low ATF4 expression was associated with poor overall survival in patients with MTC.
Conclusions:
ATF4 was identified as a negative regulator of RET, a candidate tumor suppressor gene, and may be a molecular marker that distinguishes patients at high risk of MTC from those with a longer survival prognosis.
ATF4 targets RET for degradation, act as a candidate tumor suppressor gene, and may be a molecular marker that distinguishes patients at high risk of MTC from those with a longer survival prognosis.
The RET proto-oncogene, a cell surface tyrosine kinase receptor, is widely known for its essential role in cell survival. RET germline missense and somatic mutations cause medullary thyroid cancer (MTC) and neuroendocrine tumors, whereas RET fusion proteins, overexpression, and copy number gains are present in a broad spectrum of additional cancers (papillary thyroid cancer, pancreatic cancer, melanoma, leukemia, lung adenocarcinomas, and breast cancer) (1–7). The most frequent RET somatic alterations in sporadic MTC are point mutations. Although RET somatic mutations have been found at different codons, 634 in exon 11 and 918 in exon 16 are the most frequently reported change (8). Tyrosine kinase inhibitors (TKIs) such as vandetanib and cabozantinib have shown promise in the treatment of MTC and have been approved by the US Food and Drug Administration (FDA), but they are not curative and patients develop resistance to therapy (9, 10).
We recently reported that RET directly interacts with ATF4, the master regulator of the stress response pathway, to inhibit stress-induced apoptosis (11). Furthermore, in MTC cell lines, we have shown that RET acts on ATF4 in both cytoplasm and the nucleus. This raises the possibility that RET has a direct nuclear function in the regulation of transcription, causing the repression of ATF4-dependent proapoptotic gene expression (11). Moreover, we have shown that RET phosphorylation of ATF4 enhances ubiquitination-dependent degradation of ATF4 in MTC (11). However, the significance of ATF4 in C-cell transformation, development of MTC, and ATF4-mediated regulation of RET expression remains unstudied.
Here, we report that ATF4 activates a negative-feedback loop, leading to the downregulation of RET expression while upregulating expression of proapoptotic genes. This mechanistic link between RET signaling and ATF4 in MTC may explain the incomplete responses observed in patients treated with FDA-approved TKIs (vandetanib and cabozantinib), in whom reduced ATF4 levels prevent induction of apoptosis. Consistent with this hypothesis, we found that ATF4 depletion causes resistance to TKI-mediated cell death. Moreover, we observed loss of ATF4 expression in 52% of MTC tumors and a correlation between this loss and poor overall survival. Finally, our studies have shown that targeted deletion of Atf4 causes C-cell hyperplasia in mice. These studies provide important insights into the mechanisms by which ATF4 exerts its tumor suppressor function in MTC.
Material and Methods
Reagents and antibodies
Sunitinib and cabozantinib were purchased from Tocris Bioscience and Selleckchem, respectively. Our sources for antibodies were as follows: ATF4 (C-20), ATF4 (D4B8), ERK, pERK, AKT, AKT pS473, p38 MAPK, pS6–S235-236, PUMA (Cell Signaling Technology, Danvers, MA), RET (C-19), RET (C-20), RET (H300; Santa Cruz), ubiquitin (P4D1; Santa Cruz Biotechnology, Dallas, TX), NOXA (Calbiochem, San Diego, CA), calcitonin (DAKO, Santa Clara, CA), and ATF4 (Ab23760; Abcam, Cambridge, MA).
Cell lines
Cells from the TT and HEK293T cell lines were purchased from ATTC (Manassas, VA). The TT cell line carries a RET C634W activating mutation in exon 11 that was verified by sequencing.
Plasmid construction and lentiviral transduction
ATF4 adenovirus was constructed according to the manufacturer’s protocols using the Ad Easy XL adenovirus vector system (Stratagene; Agilent Technologies, Santa Clara, CA). Viral particles were produced by the Vector Development Laboratory (Baylor College of Medicine). Lentiviral ATF4 short hairpin RNA (shRNA) plasmids were purchased from Sigma-Aldrich (St. Louis, MO). The lentiviral ATF4 construct was previously described (11) and was cotransfected into HEK 293T cells along with packaging (VPR8.9) and envelope (VSV-G) plasmids by using X-tremeGENE (Roche, Indianapolis, IN) for 2 days. The virus particles containing ATF4 or control vector were used to infect TT cells. Transfected cells were selected in media containing 2 μg/mL puromycin (Clontech, Mountain View, CA).
Cell viability and cell cycle
Cell viability was measured by using an MTT assay. Cells were plated in 96-well plates (40,000 cells per well) incubated with adenovirus-ATF4 at various multiplicity of infection for 16 hours followed by MTT assay at the indicated times. TT-shRNA-ATF4 cells were treated with TKIs for 48 hours. Cells were incubated in 200 µL of 0.6 mg/mL MTT for 4 hours; cells were then solubilized in dimethyl sulfoxide for 30 minutes after quantification with a spectrophotometer (Synergy HT; BioTek, Winooski, VT) at 595 nm. For cell cycle analysis, cells were collected and fixed in ice-cold 70% ethanol and stained with propidium iodide for flow cytometry analysis. Apoptotic cell death was determined using the BD ApoAlert annexin V-FITC apoptosis kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions, and cells were analyzed on the FACScan system (BD Biosciences).
Real-time polymerase chain reaction
Total RNA and complementary DNA were prepared with use of a Cell-to-CT Kit, and quantitative real-time polymerase chain reaction (PCR) was performed with use of TaqMan primer probes (Thermo Fisher Scientific, Waltham, MA) and hypoxanthine phosphoribosyltransferase as the control.
Biochemical assays
Cell extracts assays, western blot analysis, and immunoprecipitation were performed as described previously (11, 12).
In vivo ubiquitination assay
Cells were treated with MG132 (10 μM) for 4 hours and lysed. The ubiquitinated ATF4 was immunoprecipitated by ATF4 (c-20), and then western blotting with ubiquitin antibody was performed (13).
Animal studies
In Atf4 knockout mice, a neomycin phosphotransferase resistance (neo) cassette replaces the entire coding region of the endogenous mouse activating transcription factor 4 (Atf4) gene, abolishing gene function as previously described (14). Mice homozygous for the Atf4 allele exhibit low viability. All animal experiments were approved by the Animal Care and Use Committee at the University of Texas MD Anderson Cancer Center. Age-matched littermates (Atf4+/+ and Atf4+/−) 3 to 12 months of age and Atf4−/− (3 months of age) were used.
Tissue processing and histopathologic analysis
Sequential sections from formalin-fixed, paraffin-embedded tissue blocks were fixed to charged slides and immunostained with RET (H300) or ATF4 (Ab23760) antibodies. The study group consisted of 39 patients with a diagnosis of MTC who underwent surgery at MD Anderson Cancer Center. The fraction of RET- or ATF4-positive tumor cells and the staining intensity were assessed. An ATF4 score < 20% positive nuclei was considered low ATF4, regardless of intensity. An RET score was calculated by multiplying the percentage of stained tumor cells by the staining intensity (H score). Clinical and histopathologic characteristics of the study population are shown in Table 1.
Table 1.
Clinical and Pathologic Characteristics of MTC and Corresponding ATF4 Tumor Expression Level
| Characteristic | No. of Patients (N = 39) | ATF4 Low (N = 20) | ATF4 High (N = 19) |
|---|---|---|---|
| Age, y | |||
| Median age at diagnosis (range) | 58 (15–87) | 58.5 (30–87) | 62 (15–87) |
| Primary tumor (T), n (%) | P = 0.08 | ||
| T1 | 7 (18) | 3 (7.7) | 4 (10.2) |
| T2 | 12 (30.8) | 3 (7.7) | 9 (23) |
| T3 | 11 (28.2) | 7 (18) | 4 (10.2) |
| T4 | 9 (23) | 7 (18) | 2 (5.1) |
| Lymph node metastasis, n (%) | P = 0.16 | ||
| N0 | 10 (25.6) | 3 (7.7) | 7 (18) |
| N1 | 29 (74.3) | 17 (43.6) | 12 (30.7) |
| Distant metastasis, n (%) | P = 0.4 | ||
| M0 | 28 (71.8) | 13 (33.3) | 15 (38.5) |
| M1 | 7 (18) | 5 (12.8) | 2 (5.1) |
| MX | 4 (10.2) | 2 (5.1) | 2 (5.1) |
| RET mutation status, n (%) | |||
| WT | 19 (48.7) | 9 (23) | 10 (25.6) |
| C618S | 1 (2.6) | 0 (0) | 1 (2.6) |
| C620S | 2 (5.1) | 1 (2.6) | 1 (2.6) |
| C634R | 4 (10.3) | 1 (2.6) | 3 (7.7) |
| M918T | 13 (33.3) | 9 (23) | 4 (10.2) |
| Clinical tumor stage, n (%) | P = 0.17 | ||
| I | 3 (7.7) | 2 (5.1) | 1 (2.6) |
| II | 7 (18) | 1 (2.6) | 6 (15.4) |
| III | 20 (51.3) | 11 (28.2) | 9 (23) |
| IV | 9 (23) | 6 (15.4) | 3 (7.7) |
| Treatment, n (%) | |||
| No adjuvant therapy | 12 (30.8) | 4 (10.2) | 8 (20.5) |
| Unknown | 14 (35.9) | 8 (20.5) | 6 (15.4) |
| Chemotherapy | 7 (18) | 6 (15.4) | 1 (2.6) |
| Radiation | 2 (5.1) | 1 (2.6) | 1 (2.6) |
| TKIs | 4 (10.2) | 1 (2.6) | 3 (7.7) |
The thyroid glands from Atf4 heterozygote and homozygote mice that were euthanized at various ages were fixed in neutral buffered formalin (10% formaldehyde in phosphate buffer, pH 7.5), processed through ethanol and xylene, and embedded in paraffin. Serial sections (4 μM) were cut through the entire thyroid, mounted on poly-l-lysine microscope slides, and processed for immunohistochemical analysis and stained with rabbit polyclonal antibody to calcitonin or RET (H300). The calcitonin-positive cells from both thyroid lobes were counted in every other section by a blinded observer. The quantification showed the average number of C cells per lobe for 3 consecutive slides.
Statistical analysis
All data were expressed as means ± standard deviations. Data were compared by t test or by analysis of variance using GraphPad Prism 6 software (GraphPad, La Jolla, CA). Statistical significance was defined as P < 0.05. The survival curve was estimated by the Kaplan-Meier method with use of the log-rank test to assess significant differences for disease-free patient survival (in the figures: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Results
Atf4 heterozygote mice develop C-cell hyperplasia
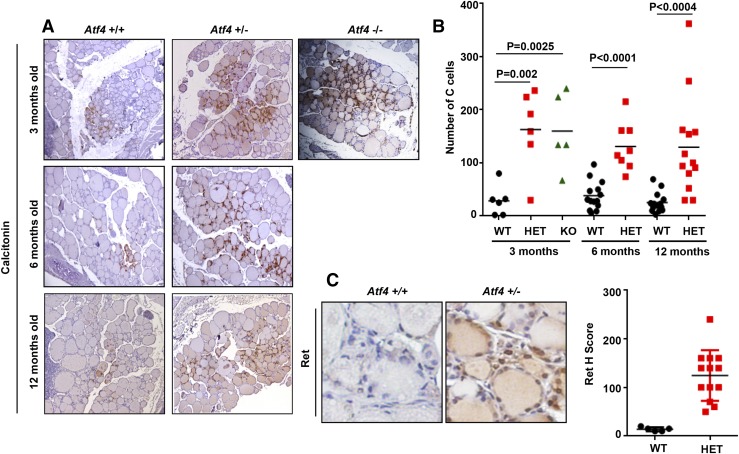
The thyroid gland is composed of follicular and C or parafollicular cells. Follicular cells secrete thyroxine and triiodothyronine and constitute 95% of thyroid cells; C cells, scattered among follicular cells, secrete calcitonin and make up 5% of the cells in rodents (15). To determine whether Atf4 affects C-cell proliferation and could be acting as a tumor suppressor gene in MTC, we quantified C-cell numbers in the thyroid from 3- to 12-month-old wild-type and Atf4 heterozygote and knockout littermates. With use of calcitonin staining, we found that the thyroid glands from Atf4 heterozygote and knockout mice had 2 to 3 times more C cells than their wild-type littermates [Fig. 1(A) and 1(B)]. Homozygous pups exhibit low viability, Atf4 knockout pups exhibit a reduction in oxidative stress-induced gene expression, resistance to oxidative death, and decreased insulin sensitivity compared with control mice (14). Adults are severely microphthalmic, with no recognizable lens, anterior chamber, iris, or vitreous body (14). The reduced viability of Atf4 knockout mice precluded further analysis after 3 months of age. RET has been shown to be involved in C-cell transformation and MTC development (15). To examine whether C-cell hyperplasia caused by Atf4 deletion is associated with Ret protein upregulation, we performed immunohistochemistry using an Ret-specific antibody on the thyroid glands of the wild-type and Atf4 heterozygote mice. We observed that Ret expression was increased in the thyroid gland of Atf4 heterozygote compared with wild-type mice, suggesting that Atf4 loss upregulates Ret protein levels [Fig. 1(C)].
Figure 1.
C-cell hyperplasia in ATF4 heterozygote (HET) and knockout (KO) mice. (A) Representative picture shows calcitonin staining of thyroids from wild-type (WT) and ATF4 heterozygote and knockout mice at indicated ages. (B) Quantification of the number of C cells in the thyroid of indicated genotypes (Student t test). Results are from the indicated numbers of animals with ages ranging from 3 to 12 mo. (C) Representative picture shows RET staining of thyroid tissue in an area of C cells from 1-y-old WT and Atf4 heterozygote mice. Quantification of RET staining (H score) is shown.
These findings suggest that increased numbers of C cells found in Atf4 heterozygote and knockout mice may have been because of increased proliferation of C cells: one copy loss of the Atf4 gene was enough to cause C-cell hyperplasia, a precursor lesion linked to the development of hereditary MTC.
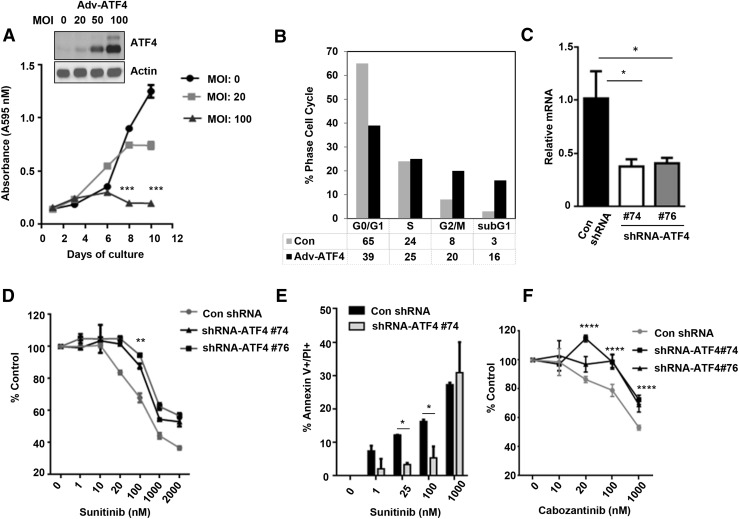
ATF4 overexpression induces cell death and ATF4 depletion causes resistance to TKIs
The observed effects on the postnatal increase in the number of C cells in ATF4 null mice prompted us to determine whether ATF4 expression inhibits the growth of MTC cells. To address this question, human TT cells derived from a patient with MTC carrying a frequent RET mutation (C634W) were infected with control or adenoviral vector encoding for ATF4, and cell viability was determined by an MTT assay for > 1 week. Western blot analysis showed efficient dose-dependent overexpression of ATF4 by adenoviral infection in TT cells [Fig. 2(A), inset]. We observed that prolonged exposure of cells to ATF4 decreased cell survival [Fig. 2(A)]. Fluorescence-activated cell-sorting analysis of cell-cycle distribution showed that a higher percentage of ATF4 overexpressing cells accumulated in subG1 populations compared with control cells [Fig. 2(B)]. TT cell lines stably expressing ATF4-specific shRNA were generated by lentiviral shRNA transduction. Real-time reverse transcription PCR confirmed that ATF4 messenger RNA (mRNA) was 50% to 60% lower in the ATF4 shRNA cells than in the controls [Fig. 2(C)]. Because TKI increases ATF4 occupancy to the promoter of proapoptotic genes (11), we hypothesize that the ATF4 knockdown may decrease the efficacy of TKI-induced cell death. We found that ATF4-depleted cells were less sensitive to sunitinib-induced cell death than control shRNA cells treated with sunitinib [Fig. 2(D)]. The quantification of apoptotic cells by annexin V/propidium iodide staining showed decreased cell death in sunitinib-treated ATF4-shRNA cells compared with control shRNA cells [Fig. 2(E)]. The same results were obtained when using another TKI, FDA approved for therapy of MTC, cabozantinib (XL184) [Fig. 2(F)].
Figure 2.
Forced ATF4 expression induces cell death. (A) Western blot shows ectopic expression of ATF4 in TT cells by adenoviral infection (Adv-ATF4) at indicated multiplicity of infection (MOI) for 24 h. TT cells were plated in 96-well plates (20,000 cells per well) and infected with control (Con) adenovirus or ATF4 adenovirus with various MOI for 16 h. The medium was replaced with fresh medium, and the cells were analyzed at the indicated times by MTT assay. (B) Fluorescence-activated cell-sorting analysis of cell-cycle distribution of ATF4 overexpressing cells (Adv-ATF4) using propidium iodide (PI) staining. (C) Quantitative real-time PCR showing mRNA levels of ATF4 in control shRNA and ATF4-shRNA TT cells. (D) MTT assay for cell viability in ATF4 shRNA and control shRNA cells treated with sunitinib at the indicated dose for 48 h. (E) TT con shRNA and ShRNA-ATF4 are treated with sunitinib at the indicated dose for 48 h and stained with annexin V-PI and analyzed by flow cytometry. (F) MTT assay for cell viability in ATF4 shRNA as a percent of the control shRNA cells treated with cabozantinib (XL184) at the indicated dose for 48 h. Shown are mean and standard deviation of 6 replicates in a representative experiment. Two-way analyses of variance and Bonferroni multiple comparison tests were used. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
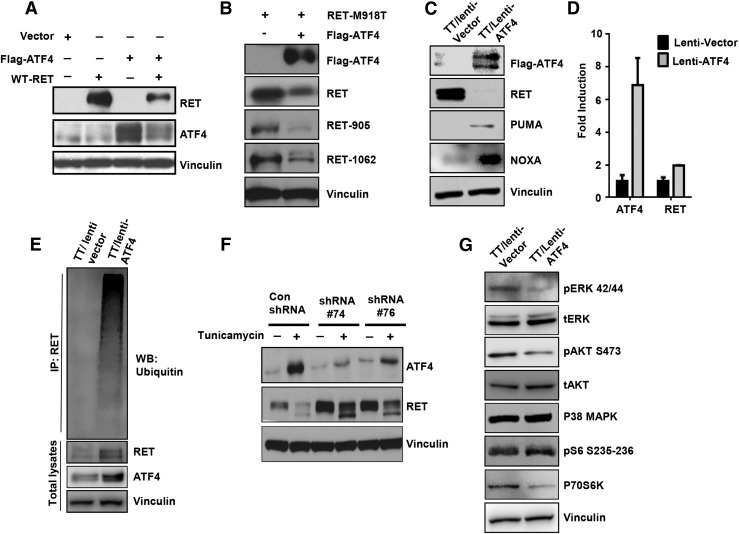
ATF4 promotes RET degradation and inhibits RET downstream signaling pathways
Because RET inhibition reduces cell viability and stimulates apoptosis in MTC, we determined whether forced expression of ATF4 in TT cells decreases RET protein levels and inhibits RET signaling. HEK 293T cells were cotransfected with RET-WT or RET-M918T and ATF4 and showed decreased RET protein levels along with reduced RET phosphorylation at tyrosine residues 905 and 1062 [Fig. 3(A) and 3(B)]. Similar results were found by forced ATF4 expression in TT cells by using lentiviral constructs, which showed downregulation of RET expression and increased expression of ATF4 proapoptotic target genes NOXA and PUMA [Fig. 3(C)]. To further elucidate the mechanism by which ATF4 regulates RET expression, we tested the RET mRNA levels in TT cells overexpressing ATF4 by using real-time reverse transcription PCR. We observed that ATF4 did not affect RET mRNA levels, suggesting posttranscriptional regulation [Fig. 3(D)]. To determine the effect of ATF4 on RET stability, we performed a ubiquitination assay in control and TT cells overexpressing ATF4 treated with the proteasome inhibitor MG132. In the presence of MG132, a high molecular mass species caused by polyubiquitination of ATF4 was observed in the TT overexpressing cells [Fig. 3(E)]. These data suggest that ATF4 promotes RET ubiquitination and degradation, inducing cell death.
Figure 3.
ATF4 expression promotes RET ubiquitination. (A and B) HEK 293T cells were transfected with the indicated plasmids, and western blot (WB) analysis was performed with the indicated antibodies. (C) Western blot analysis of TT cells infected with the lentivirus harboring ATF4 with the indicated antibodies. (D) RNA was isolated from cells treated as in (C) and subjected to real-time PCR using ATF4 or RET Taqman primers probes. (E) MTC cells expressing lentiviral-ATF4 were treated with MG132 (4 h), and denatured extracts were immunoprecipitated with an anti-RET antibody followed by western blot analysis with antiubiquitin antibody. (F) ATF4-shRNA (clones of 74 and 76) and nontargeting shRNA (Con) are treated with tunicamycin (2 μg/mL) for 24 h, and western blot analysis was performed with the indicated antibodies. (G) ATF4 expression inhibits RET signaling. MTC cells expressing lentiviral-ATF4 were immunoblotted with the indicated antibodies. IP, immunoprecipitation.
To investigate the protein levels of RET in ATF4-depleted cells, and because the basal level of ATF4 protein levels are low in TT cells, we treated the shRNA-ATF4 and nontargeting shRNA cells with the endoplasmic reticulum (ER) stress-inducing agent tunicamycin to upregulate ATF4 mRNA. Both ATF4 shRNA clones showed no ATF4 induction after treatment with tunicamycin, showing efficient depletion of ATF4 by shRNA [Fig. 3(F)]. We observed that RET expression was upregulated in ATF4-depleted cells [Fig. 3(F)].
Analysis of RET downstream signaling showed that decreased RET protein levels were associated with inhibition of phospho-ERK and phospho–AKT S473 but that the level of total ERK or AKT was not affected. Because RET-dependent activation of mammalian target of rapamycin signaling was demonstrated in MTC (16), we examined the phosphorylation status of the mammalian target of rapamycin downstream target, p70S6K. Forced ATF4 expression in TT cells decreased the level of phosphorylated p70S6K in MTC cells [Fig. 3(G)]. In contrast, levels of p38MAPK and pS6 S235-236 were not affected. These results support the role of ATF4 as a negative regulator of RET signaling.
Low ATF4 expression correlates with poor overall survival of patients with MTC
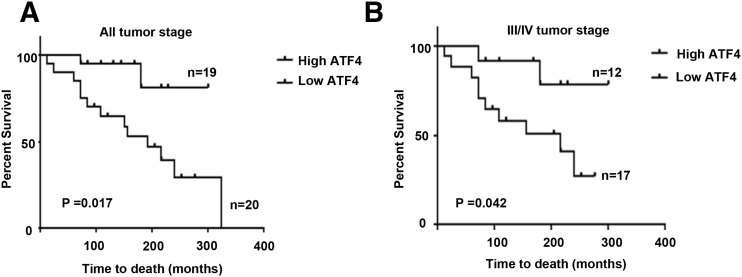
To evaluate the role of ATF4 in the pathogenesis of MTC, we examined the protein levels of ATF4 and RET in normal thyroid and MTC tumor tissues by immunohistochemical analysis [Fig. 4(A–C)]. The clinical features of these patients, including age, clinical disease stage and node stage, distant metastasis, RET mutation status, and treatment strategies, are summarized in Table 1. We observed that ~52% of analyzed MTC tumors (n = 39) had low or loss of ATF4 protein (staining ≤ 20%) [Fig. 4(A) and 4(C)]. The quantification of RET staining is shown by calculation of the h score considering the intensity of staining [Fig. 4(B)]. A subset of tumor samples (n = 26) was analyzed by western blotting for ATF4 and RET protein levels. We found a negative correlation between the expression of ATF4 and RET, independent of RET mutational status, which indicated that higher levels of RET were correlated with lower levels of ATF4 (r = −0.89; R2 = 0.799; P < 0.0001) [Fig. 4(D) and 4(E)]. Furthermore, patients with MTC, including all tumor grades, who had tissue samples with decreased or absent ATF4 expression, had significantly shorter overall survival [P = 0.017; hazard ratio (log-rank) = 0.2 to 4.83; 95% confidence interval, 0.095 to 0.74) than patients whose tumors expressed high levels of ATF4 [Fig. 5(A)]. When overall survival was examined between ATF4 expression level and tumors with stage III/IV, median survival was not reached with high ATF4–expressing tumors, whereas the median survival of patients with low ATF4–expressing tumors was 216 months [P = 0.04; hazard ratio (log-rank) = 0.024 to 4.09; 95% confidence interval, 0.097 to 0.94). These data suggest that decreased ATF4 expression is associated with a more aggressive form of MTC.
Figure 4.
Loss of ATF4 was associated with poor overall survival in patients with MTC. (A) Representative images from immunohistochemical analyses in normal thyroid and 39 archived MTC tissues using RET and ATF4 antibodies. (B) Scatterplot shows the quantification of RET protein levels in MTC tumors by immunohistochemical analysis. An RET score was calculated by multiplying the percentage of stained tumor cells by the staining intensity (H score) (n = 39). (C) Scatterplot shows the percent of nuclear ATF4 staining. An ATF4 score < 20% positive nuclei was considered low ATF4, regardless of intensity (n = 39). (D) RET and ATF4 protein levels in the representative tumor tissues (n = 26) with sporadic and hereditary MTC by western blot analysis. (E) Densitometric values for RET, ATF4, and vinculin were used to standardize for equal protein loading among the samples assayed. Negative correlation is shown between RET and ATF4 protein levels.
Figure 5.
Loss of expression of ATF4 protein correlates with higher rates of death. Kaplan-Meier plots of overall survival according to ATF4 expression evaluated by immunohistochemical analysis. Overall survival was defined from the date of diagnosis or surgery to the date of death of all causes or last follow-up. P values were calculated by log-rank (Mantel-Cox). (A) Kaplan-Meier curves for 39 patients with MTC classified on the basis of their tumoral expression of ATF4. (B) Kaplan-Meier curves for 29 patients with MTC (stage III/IV) classified on the basis of their tumoral expression of ATF4.
Discussion
In this report, we showed that 1) Atf4 heterozygote and knockout mice develop C-cell hyperplasia, suggesting that Atf4 may regulate C-cell proliferation and/or survival; 2) ATF4 reduces RET protein levels by ubiquitination and inhibits the RET signaling pathway to induce cell death; 3) ATF4 depletion decreases sensitivity to TKIs; 4) a negative correlation exists between RET and ATF4 expression in MTC tumors; and 5) decreased or loss of ATF4 protein levels in MTC tumors correlates with poor overall survival in patients with MTC.
We recently reported that the full-length activated RET receptor can translocate to the nucleus and inhibit expression of proapoptotic genes through binding and inactivation of ATF4 (11). The enhanced nuclear localization of RET seen in MTC is associated with downregulation of ATF4, which is blocked by tyrosine kinase inhibition, or in models using a kinase-dead RET (11). The overexpression of ATF4 causes upregulation of several genes that promote apoptosis, including NOXA and PUMA (11). In this study, we showed that ATF4 activates a negative-feedback loop, leading to the downregulation of RET expression while upregulating expression of proapoptotic genes. The mechanism underlying this negative-feedback loop is through RET ubiquitination and degradation. We speculate that ATF4 is able to regulate the transcription of E3 ubiquitin ligases, which in turn are able to promote degradation of RET. This may explain resistance to TKIs in ATF4-depleted cells, suggesting that ATF4 could be the mediator of TKI-induced cell death.
C cells (or parafollicular cells) in the thyroid gland are derived from the ultimobranchial body part of the fourth and fifth parapharyngeal pouches and neural crest. These cells produce the hormone calcitonin. Aberrant RET expression mediates the development of C-cell hyperplasia and, subsequently, MTC (15). In mice expressing the Ret-MEN2A (Ret-C634W) gene under the control of the rat calcitonin gene-related peptide/calcitonin promoter, only C-cell hyperplasia was induced, whereas use of Moloney murine leukemia virus long terminal repeat resulted in very late onset of MTC (17, 18). Use of an Ret-MEN2B (Ret-M918T) transgene produced MTC at a higher frequency (19). We found that young adult Atf4 knockout mice failed to develop MTC but exhibited C-cell hyperplasia, which is an accumulation of C cells within the thyroid gland. This abnormality precedes the development of MTC and may stay indolent for years before its transformation to MTC. This enhancement of C-cell numbers in heterozygote mice could be caused by increased Ret protein levels leading to enhanced C-cell proliferation. Our data suggest that one copy loss of Atf4 is sufficient to cause C-cell hyperplasia in young mice. The fact that Atf4 null mice did not develop MTC suggests that either our analyses were conducted too early because of premature death of the mice or that genetic ablation of Atf4 per se is not sufficient for MTC development and might require additional genetic insults. Recognition that activation of RET or inactivation of ATF4 can inhibit C-cell death through its effects on NOXA and PUMA provides a reasonable mechanism for the accumulation of C cells seen in children with hereditary MTC.
Histopathologic studies further revealed that ATF4 expression levels were decreased or lost in MTC tumors and were significantly correlated with poor overall survival in patients with MTC, suggesting that the nuclear function of ATF4 is a crucial factor in a patient's response to therapy. We observed a complete absence of ATF4 protein in a subset of MTC tumors, suggesting that several mechanisms could be involved in ATF4 loss, including loss of heterozygosity. Of note, chromosome 22q13.1, the locus of ATF4, is frequently deleted in MTC and in other types of cancer (20–23). Moreover, RET expression is negatively correlated with ATF4 expression in MTC tumors, which is consistent with reciprocal regulation of RET and ATF4.
ATF4-mediated apoptosis has been shown to occur in response to ER stress through activation of proapoptotic targets. Chemotherapeutic agents, such as fenretinide, bortezomib, and cannabinoid, induce ER stress-mediated apoptosis through upregulation of ATF4 mRNA translation (24–26). In addition, knockdown of ATF4 reduced vemurafenib-induced ER stress-mediated apoptosis (27). ATF4 is a primary mediator of the cellular response to ER stress, which induces activation of the apoptotic pathway; therefore, restoration of the ATF4 function by small molecule induction could be beneficial to sensitize tumors to apoptotic cell death. Understanding the early steps in the pathway that leads to malignant transformation of C cells will allow us to develop new strategies for MTC prevention and treatment and may be a promising mechanism to force transformed C cells to enter into apoptotic pathways.
A clear understanding of the feedback loops shared by RET and ATF4 may help explain the variable aggressiveness patterns seen in patients with MTC. Although TKIs have shown promise in the treatment of MTC, they are cytostatic and not curative, and their adverse effects limit their use in high dosages. Identification of additional targets, particularly ones that play a prominent role in regulation of both proliferation and apoptosis, would lead to novel and much-needed therapeutic options for MTC malignancy. In conclusion, our study uncovers the potential use of ATF4 as a prognostic and predictive marker of response to therapy and a candidate tumor suppressor gene in MTC.
Acknowledgments
We thank the flow cytometry and cellular imaging core facility, supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672 and Vector Development Laboratory (Baylor College of Medicine). We also thank Tamara K. Lock in the department of scientific publications for editing the manuscript.
Acknowledgments
This work was supported by NIH Grant P50CA168505 (to R.B.-Y.) and grants from the American Thyroid Association (to R.B.-Y.) and Kosberg Foundation (to R.F.G.).
Disclosure Summary: R.F.G. has received from Astra Zeneca funding for vandetanib phase II/III clinical trial studies for the treatment of MTC. The remaining authors have nothing to disclose.
Footnotes
- ER
- endoplasmic reticulum
- FDA
- US Food and Drug Administration
- mRNA
- messenger RNA
- MTC
- medullary thyroid cancer
- PCR
- polymerase chain reaction
- shRNA
- short hairpin RNA
- TKI
- tyrosine kinase inhibitor
References
- 1.Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14(3):173–186. [DOI] [PubMed] [Google Scholar]
- 2.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, Iwakawa R, Ogiwara H, Oike T, Enari M, Schetter AJ, Okayama H, Haugen A, Skaug V, Chiku S, Yamanaka I, Arai Y, Watanabe S, Sekine I, Ogawa S, Harris CC, Tsuda H, Yoshida T, Yokota J, Shibata T. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18(3):375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattelli A, Nalvarte I, Boulay A, Roloff TC, Schreiber M, Carragher N, Macleod KK, Schlederer M, Lienhard S, Kenner L, Torres-Arzayus MI, Hynes NE. Ret inhibition decreases growth and metastatic potential of estrogen receptor positive breast cancer cells. EMBO Mol Med. 2013;5(9):1335–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohshima Y, Yajima I, Takeda K, Iida M, Kumasaka M, Matsumoto Y, Kato M. c-RET molecule in malignant melanoma from oncogenic RET-carrying transgenic mice and human cell lines. PLoS One. 2010;5(4):e10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, Zhou M, Han S, Zhang Q. The relationship between overexpression of glial cell-derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int Med Res. 2008;36(4):656–664. [DOI] [PubMed] [Google Scholar]
- 6.Ballerini P, Struski S, Cresson C, Prade N, Toujani S, Deswarte C, Dobbelstein S, Petit A, Lapillonne H, Gautier EF, Demur C, Lippert E, Pages P, Mansat-De Mas V, Donadieu J, Huguet F, Dastugue N, Broccardo C, Perot C, Delabesse E. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia. 2012;26(11):2384–2389. [DOI] [PubMed] [Google Scholar]
- 7.Grieco M, Santoro M, Berlingieri MT, Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G, Fusco A, Vecchio G. PTC is a novel rearranged form of the ret proto-oncogene and is frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60(4):557–563. [DOI] [PubMed] [Google Scholar]
- 8.Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, Agate L, Vivaldi A, Faviana P, Basolo F, Miccoli P, Berti P, Pacini F, Pinchera A. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93(3):682–687. [DOI] [PubMed] [Google Scholar]
- 9.Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagheri-Yarmand R, Sinha KM, Gururaj AE, Ahmed Z, Rizvi YQ, Huang SC, Ladbury JE, Bogler O, Williams MD, Cote GJ, Gagel RF. A novel dual kinase function of the RET proto-oncogene negatively regulates activating transcription factor 4-mediated apoptosis. J Biol Chem. 2015;290(18):11749–11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J Biol Chem. 2007;282(14):10432–10440. [DOI] [PubMed] [Google Scholar]
- 13.Bloom J, Pagano M. Experimental tests to definitively determine ubiquitylation of a substrate. Methods Enzymol. 2005;399:249–266. [DOI] [PubMed] [Google Scholar]
- 14.Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99(3):736–745. [DOI] [PubMed] [Google Scholar]
- 15.Cote GJ, Grubbs EG, Hofmann MC. Thyroid C-cell biology and oncogenic transformation. Recent Results Cancer Res. 2015;204:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gild ML, Landa I, Ryder M, Ghossein RA, Knauf JA, Fagin JA. Targeting mTOR in RET mutant medullary and differentiated thyroid cancer cells. Endocr Relat Cancer. 2013;20(5):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michiels FM, Chappuis S, Caillou B, Pasini A, Talbot M, Monier R, Lenoir GM, Feunteun J, Billaud M. Development of medullary thyroid carcinoma in transgenic mice expressing the RET protooncogene altered by a multiple endocrine neoplasia type 2A mutation. Proc Natl Acad Sci USA. 1997;94(7):3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai K, Iwashita T, Murakami H, Hiraiwa N, Yoshiki A, Kusakabe M, Ono K, Iida K, Nakayama A, Takahashi M. Tissue-specific carcinogenesis in transgenic mice expressing the RET proto-oncogene with a multiple endocrine neoplasia type 2A mutation. Cancer Res. 2000;60(18):5254–5260. [PubMed] [Google Scholar]
- 19.Acton DS, Velthuyzen D, Lips CJ, Höppener JW. Multiple endocrine neoplasia type 2B mutation in human RET oncogene induces medullary thyroid carcinoma in transgenic mice. Oncogene. 2000;19(27):3121–3125. [DOI] [PubMed] [Google Scholar]
- 20.Ye L, Santarpia L, Cote GJ, El-Naggar AK, Gagel RF. High resolution array-comparative genomic hybridization profiling reveals deoxyribonucleic acid copy number alterations associated with medullary thyroid carcinoma. J Clin Endocrinol Metab. 2008;93(11):4367–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flicker K, Ulz P, Höger H, Zeitlhofer P, Haas OA, Behmel A, Buchinger W, Scheuba C, Niederle B, Pfragner R, Speicher MR. High-resolution analysis of alterations in medullary thyroid carcinoma genomes. Int J Cancer. 2012;131(2):E66–E73. [DOI] [PubMed] [Google Scholar]
- 22.Marsh DJ, Theodosopoulos G, Martin-Schulte K, Richardson AL, Philips J, Röher HD, Delbridge L, Robinson BG. Genome-wide copy number imbalances identified in familial and sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88(4):1866–1872. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka N, Nishisho I, Yamamoto M, Miya A, Shin E, Karakawa K, Fujita S, Kobayashi T, Rouleau GA, Mori T, Takai SI. Loss of heterozygosity on the long arm of chromosome 22 in pheochromocytoma. Genes Chromosomes Cancer. 1992;5(4):399–403. [DOI] [PubMed] [Google Scholar]
- 24.Carracedo A, Lorente M, Egia A, Blázquez C, García S, Giroux V, Malicet C, Villuendas R, Gironella M, González-Feria L, Piris MA, Iovanna JL, Guzmán M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9(4):301–312. [DOI] [PubMed] [Google Scholar]
- 25.Qing G, Li B, Vu A, Skuli N, Walton ZE, Liu X, Mayes PA, Wise DR, Thompson CB, Maris JM, Hogarty MD, Simon MC. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22(5):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2010;285(9):6091–6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck D, Niessner H, Smalley KS, Flaherty K, Paraiso KH, Busch C, Sinnberg T, Vasseur S, Iovanna JL, Drießen S, Stork B, Wesselborg S, Schaller M, Biedermann T, Bauer J, Lasithiotakis K, Weide B, Eberle J, Schittek B, Schadendorf D, Garbe C, Kulms D, Meier F. Vemurafenib potently induces endoplasmic reticulum stress-mediated apoptosis in BRAFV600E melanoma cells. Sci Signal. 2013;6(260):ra7. [DOI] [PMC free article] [PubMed] [Google Scholar]