Abstract
Context:
The rarest genetic form of congenital hyperinsulinism (HI) has been associated with dominant inactivating mutations in uncoupling protein 2 (UCP2), a mitochondrial inner membrane carrier that modulates oxidation of glucose vs amino acids.
Objective:
To evaluate the frequency of UCP2 mutations in children with HI and phenotypic features of this form of HI.
Design:
We examined 211 children with diazoxide-responsive HI seen at The Children’s Hospital of Philadelphia (CHOP) between 1997 and October 2016.
Setting:
CHOP Clinical and Translational Research Center.
Results:
Of 211 cases of diazoxide-responsive HI, we identified 5 unrelated children with UCP2 mutations (5 of 211; 2.4%). All 5 were diagnosed with HI before 6 months of age; diazoxide treatment was only partly effective in 3 of the 5. Among the 5 cases, 4 unique mutations (3 missense and 1 splicing) were identified. Three mutations were novel; 1 was previously reported. In vitro functional assays showed 30% to 75% decrease in UCP2 activity. Two of the children, when not taking diazoxide, developed hypoketotic-hypoglycemia after fasting 15 to 20 hours; a similar trend toward hypoglycemia after fasting 24 hours occurred in 4 adult carriers. In contrast, both children and 2 of the 4 carriers developed symptomatic hypoglycemia 4 hours following oral glucose. Unusual oscillating glucose and insulin responses to oral glucose were seen in both cases and carriers.
Conclusions:
These data indicate that dominant UCP2 mutations are a more important cause of HI than has been recognized and that affected individuals are markedly hypersensitive to glucose-induced hypoglycemia.
We studied 5 children with UCP2-inactivating mutations that cause diazoxide-responsive congenital HI. We found that UCP2 mutation carriers have unusual hypersensitivity to glucose-induced hypoglycemia.
Congenital hyperinsulinism (HI) is the most common cause of persistent hypoglycemia in infants and children and has been associated with 9 genetic loci (1, 2). The least common genetic type of HI is associated with inactivating mutations in mitochondrial uncoupling protein 2 (UCP2), which has only been reported in 2 infants (3). These 2 unrelated cases, described in 2008 by Gonzalez-Barroso et al.(3), were discovered to be heterozygous for missense mutations in UCP2 through screening of a group of 10 cases of HI with no detectable mutations. Both infants were responsive to treatment with diazoxide and were reported to be able to discontinue therapy at 1 to 2 years of age. In the ensuing 8 years, there have been no reports of any additional cases to confirm the possibility of an association between HI and UCP2.
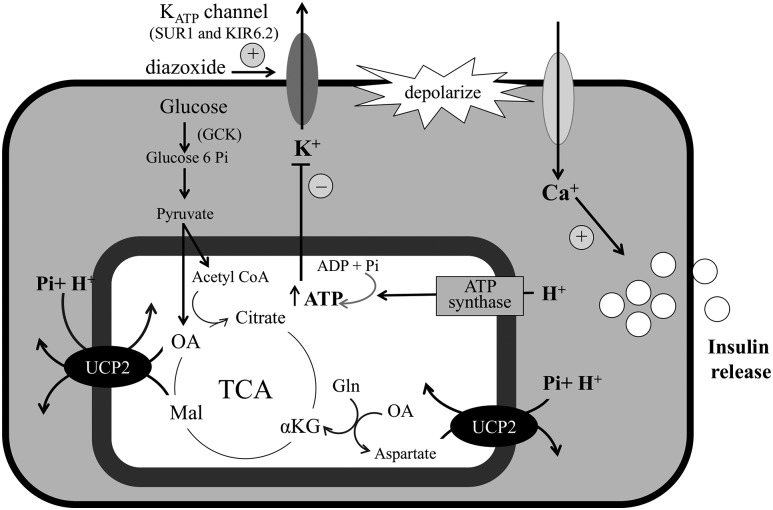
UCP2 is a mitochondrial inner membrane transporter related to UCP1, which is a mitochondrial membrane transporter that is known to leak protons into the mitochondrial matrix and uncouple oxidative phosphorylation for heat production in brown adipose tissue. A recent report by Vozza et al.(4) from the Palmieri laboratory showed that UCP2 is not an uncoupling protein, but instead transports protons and phosphate from the cytosol across the inner mitochondrial membrane in exchange for oxaloacetate and malate, intermediates of the tricarboxylic acid cycle, and aspartate (Fig. 1). By reducing the availability of mitochondrial oxaloacetate, UCP2 acts to limit oxidation of glucose in favor of amino acids. This novel role for UCP2 is supported by data in isolated rat pancreatic islets showing that UCP2 overexpression decreases adenosine triphosphate content and inhibits glucose-stimulated insulin secretion (GSIS) (5, 6). Furthermore, RNA interference knockdown of UCP2 expression in pancreatic β-cells increases GSIS (7).
Figure 1.
UCP2 modulates β-cell insulin secretion. UCP2 activity increases oxaloacetate to enhance glucose oxidation and restrain the oxidation of amino acids via glutamate metabolism to α-ketoglutarate. αKG: αketoglutarate; acetyl CoA, acetyl coenzyme A; ADP, adenosine 5′-diphosphate; ATP, adenosine triphosphate; GCK, glucokinase; Gln, glutamine; KIR6.2/SUR1, adenosine triphosphate–dependent potassium channel; Mal, malate; OA, oxaloacetate; Pi, phosphate; TCA, tricarboxylic acid.
This new information on the biochemical function of UCP2 in pancreatic β-cell insulin regulation suggests that UCP2 might be a more important cause of congenital HI than has been recognized, and that there might be specific phenotypic features in patients with HI due to UCP2 mutations. To test these hypotheses, we screened a large panel of children with diazoxide-responsive HI for mutations in UCP2 and characterized the glucose and insulin responses to fasting and oral glucose in a group of patients and carriers found to have inactivating mutations of UCP2. The results suggest that HI due to UCP2 mutations is more frequent and more persistent than currently appreciated and that the dysregulation of insulin secretion may make these patients highly sensitive to glucose-induced hypoglycemia.
Methods
Cases
Because previously reported children with HI due to UCP2 mutations were responsive to treatment with diazoxide, we included probands who were referred to The Children’s Hospital of Philadelphia (CHOP) between 1997 and October 2016 and diagnosed with congenital HI that responded to treatment with diazoxide. No UCP2 mutations have been identified among our cases of diazoxide unresponsive HI. We excluded cases with transient perinatal-stress HI. The diagnosis of HI was based on previously described criteria: hypoglycemia associated with inadequate suppression of plasma insulin concentrations and evidence of excessive insulin action, including inappropriately suppressed plasma β-hydroxybutyrate and free fatty acids and an inappropriate glycemic response to glucagon stimulation (8). Patients were considered diazoxide-responsive if fasting hypoglycemia was controlled by diazoxide at doses <15 mg/kg/d, as evidenced by maintaining plasma glucose concentrations >70 mg/dL for 18 hours of fasting or appropriate hyperketonemia (β-hydroxybutyrate >2 mmol/L) before plasma glucose decreased to <50 mg/dL (2).
Biochemical studies
Fasting tests to diagnose or evaluate control of HI by diazoxide were performed as previously described (9). Plasma glucose concentrations were measured using a Nova point-of-care glucose meter (Nova Biomedical, Waltham, MA), and plasma β-hydroxybutyrate was measured with a Precision Xtra meter (Abbott Laboratories, Abbott Park, IL). Fasting tests were terminated for plasma glucose <50 mg/dL or plasma β-hydroxybutyrate >2.5 mmol/L. At the time of hypoglycemia (plasma glucose <50 mg/dL), blood samples were obtained to measure plasma insulin, β-hydroxybutyrate, and free fatty acids. Glucagon stimulation tests were performed by administering 1 mg of glucagon intravenously or intramuscularly and then measuring plasma glucose every 10 minutes for 40 minutes. Tests performed in mutation carrier relatives of probands included a 24-hour fasting test and an oral glucose tolerance test (oGTT) using 75 g of glucose, with measurement of plasma glucose and insulin every 30 minutes for 5 hours.
Mutation analysis
Genomic DNA was isolated from peripheral blood (5 PRIME, Gaithersburg, MD) or from saliva (Oragene DNA self-collection kit; DNA Genotek, Kanata, ON, Canada). Coding sequences and intron/exon splice junctions were amplified and directly sequenced on an ABI 3730 capillary DNA analyzer (Applied Biosystems, Carlsbad, CA). Sequences were analyzed and compared with the published sequence for UCP2 (NM_003355). Genetic variants were searched against the Genome Aggregation Database, which includes 141,352 unrelated individuals, including 12,942 individuals of African descent, to determine population frequency (10). The functional consequences of novel missense mutations were predicted with bioinformatics software, SIFT (J. Craig Venter Institute, La Jolla, CA) (11), PolyPhen2 (http://genetics.bwh.harvard.edu/pph2/) (12), and Mutation Taster (mutationtaster.org) (13). Human Splicing Finder (http://www.umd.be/HSF/) was used to predict splicing alterations resulting from intronic mutations (14).
Functional analysis of UCP2 mutations
To assess the pathogenic potential of the identified UCP2 variants, functional assays were performed using phosphate/aspartate transporter assays as described by Vozza et al. (4). Wild-type and mutant UCP2 proteins were expressed in E. coli and purified. The same amount of each recombinant protein was used for in vitro reconstitution of the carrier into liposomes. Liposomes were preloaded internally with 20 mM aspartate. Transport was started by addition of 0.5 mM [33P]-phosphate and terminated after 2 minutes. The activity of the [33P]-phosphate/aspartate exchange (i.e., the distinctive transport reaction catalyzed by UCP2) was measured in reconstituted liposomes in 4 independent experiments. Significance was defined as P < 0.05 in difference in activity of mutant vs wild-type protein by unpaired t test.
Consent
The study was reviewed and approved by the CHOP Institutional Review Board. Written informed consent was obtained from all adult subjects or from the parents of child subjects.
Results
Mutation analysis
A total of 211 children with diazoxide-responsive HI seen at CHOP between 1997 and October 2016 were screened for mutations in the known HI loci. Mutations in ABCC8, KCNJ11, GCK, GLUD1, HADH, HNF4A, or HNF1A were identified in 100 of these children (47%). Mutations in UCP2 were found in 5 of the remaining children (5 of 211; 2.4%).
As shown in Table 1, among these 5 unrelated cases, 4 unique UCP2 mutations were identified. Three were coding sequence missense mutations, and 1 was an intronic splicing mutation. Case 1 had a missense mutation (p.Ala268Gly) that had been previously reported by Gonzalez-Barroso et al. (3); the remaining 3 mutations were novel. Two of the 5 unrelated probands shared a novel p.Gly61Ser mutation. In silico prediction software suggested that 3 of the 4 mutations would be damaging, whereas the p.Ser47Asn mutation was predicted to be tolerated (11–13). The population frequency of all 4 mutations was low in the total population (0.01% to 0.16%). However, for the 3 missense mutations, the frequency was slightly increased in the subgroup with African descent, most notably, the p.Ala268Gly mutation with a frequency of 1.5% compared with 0.16% in the total population. Conversely, the c.816-2 a>g splicing mutation occurs at a slightly higher frequency in a subgroup with non-Finnish European compared with African descent (0.02% vs 0.00%) (10).
Table 1.
UCP2 Mutations Found in 5 Children With Diazoxide-Responsive HI
| Case | Nucleotide | Amino Acid | Population Frequency (%) |
In Silico Prediction | Pi/Asp Exchange Rate (mmol/min/g protein) (n = 4; mean ± SD) | |
|---|---|---|---|---|---|---|
| Total | African | |||||
| 1 | c.803 C>G | p.Ala268Glya | 0.16 | 1.5 | Damaging | 0.85 ± 0.11 (P = 0.0001) |
| 2, 5 | c.181 G>A | p.Gly61Ser | 0.01 | 0.10 | Damaging | 1.57 ± 0.25 (P = 0.0012) |
| 3 | c.140 G>A | p.Ser47Asn | 0.03 | 0.31 | Tolerated | 2.27 ± 0.41 (P = 0.0295) |
| 4 | c.816-2 A>G | N/A | 0.0 | 0.00 | Damaging | N/A |
| Wild-type: 3.2 ± 0.51 | ||||||
Abbreviations: N/A, not applicable; SD, standard deviation.
Previously reported in a child with HI (3).
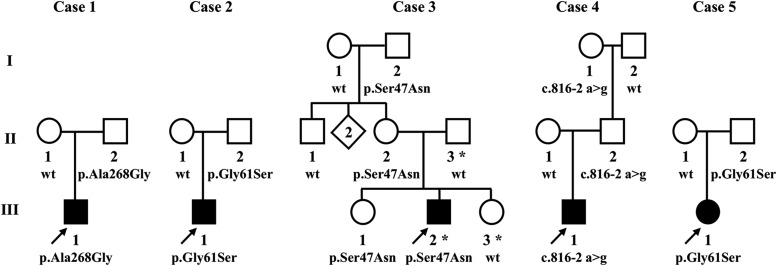
As shown in Fig. 2, none of the probands’ family members who carried a UCP2 mutation had been diagnosed with hypoglycemia. However, the fathers of cases 1, 4, and 5, as well as the paternal grandmother of case 4, gave histories of frequent episodes of symptoms compatible with hypoglycemia that were relieved by food. Two of these fathers developed symptomatic hypoglycemia during glucose tolerance testing (see later).
Figure 2.
Pedigrees of 5 children with HI associated with mutations in UCP2. Arrows indicate probands diagnosed with HI. DNA was unavailable for testing in individuals without a number. ▪,●, hypoglycemia diagnosed; ⋄, multiple individuals not tested. *Case 3 also carried a paternally inherited novel variant of unknown significance in HNF1A (p.Gly574Asp) (see text). wt, wild-type.
In addition to the maternally inherited UCP2 mutation, case 3 was found to carry a paternally transmitted novel variant of unknown significance in HNF1A that is predicted to be damaging and is not present in online frequency databases. Mutations in HNF1A are associated with monogenic maturity-onset diabetes of the young, type 3, in early adulthood and transient neonatal HI. Despite high-risk factors for diabetes (obesity and metabolic syndrome), the father of case 3 with the HNF1A variant had not developed diabetes at the age of 38 years; the younger sister of case 3, who carried the HNF1A but not the UCP2 variant, had no evidence of neonatal hypoglycemia, despite having been closely monitored. These findings suggest that the HNF1A variant may not be pathogenic, but its possible contribution to HI in case 3 cannot be ruled out.
The effects of the 3 missense UCP2 mutations on [33P]-phosphate/aspartate exchange in vitro were assessed in liposomes reconstituted with purified wild-type or mutant UCP2 proteins. As shown in Table 1, liposomes reconstituted with wild-type UCP2 protein catalyzed efficient uptake of [33P]-phosphate similar to that reported previously (4). In contrast, uptake of [33P]-phosphate was decreased in liposomes reconstituted with the UCP2 proteins harboring the 3 missense mutations (P values, 0.0001 to 0.03). Similar results were found using phosphate, malate, or oxaloacetate instead of aspartate as the intraliposomal counter-substrate (data not shown). These observations indicate that the 3 missense mutations are associated with reduced activity of UCP2, although the degree of reduction in vitro appeared to be only modest (30% to 75%).
Clinical characteristics of UCP2 HI
As shown in Table 2, 4 of the 5 patients with HI with UCP2 mutations were of African descent, and 1 was of Western European origin. Birth weight was high for gestational age in only 1 of the 5 cases (case 1); birth weight was appropriate for gestational age in 3 (cases 3, 4, and 5) and small for gestational age in 1 (case 2). The median age of presentation was 7 weeks (range, 2 days to 6 months). Hypoglycemia was recognized after a hypoglycemic seizure in all but 1 patient (case 5), who was a premature infant diagnosed at 3 weeks of age while still in the neonatal intensive care unit. Most of the children were responsive to treatment with diazoxide; however, case 2 showed only a partial response and required continuous intragastric dextrose for management of hypoglycemia until 11 months of age. This patient had experienced intrauterine growth restriction that can be associated with a perinatal-stress form of HI (2), and this may have contributed to the incomplete response to diazoxide. Based on the suggestion that hypoglycemia in UCP2 HI resolves in early childhood (3), diazoxide therapy was discontinued for cases 1 and 3 at 9.5 and 6.5 years of age. However, as described later, both children exhibited evidence of ongoing hyperinsulinemic hypoglycemia during fasting and glucose stimulation tests, and both continue to require treatment with diazoxide. Cases 4 and 5 are 2.5 years and 1 year of age, respectively, and continue to require diazoxide to control hypoglycemia. (More complete descriptions of the 5 cases are available in the Supplemental Appendix (22.3KB, docx) .)
Table 2.
Clinical Phenotype of 5 Children With UCP2 Mutations
| Case | Ethnicity | Birth Weight | Age at Presentation | Diazoxide Responsiveness | Age Trialed Without Diazoxide | Current Age (y) | Current Treatment |
|---|---|---|---|---|---|---|---|
| 1 | African American | 4.7 kg (>97th percentile) | 6 mo | Yes | 9.5 y | 11 | Diazoxide |
| 2 | African American | 2.6 kg (<3rd percentile) | 2 d | Partial | 11 mo | 6.5 | Unknown |
| 3 | African American | 3.7 kg (65th percentile) | 7 wk | Yes | 6.5 y | 8 | Diazoxide |
| 4 | Western European | 4.0 kg (70th percentile) | 4 mo | Yes | N/A | 2.5 | Diazoxide |
| 5 | African American | 1.2 kg (31st percentile) | 3 d | Yes | N/A | 1 | Diazoxide |
Abbreviation: N/A, not available.
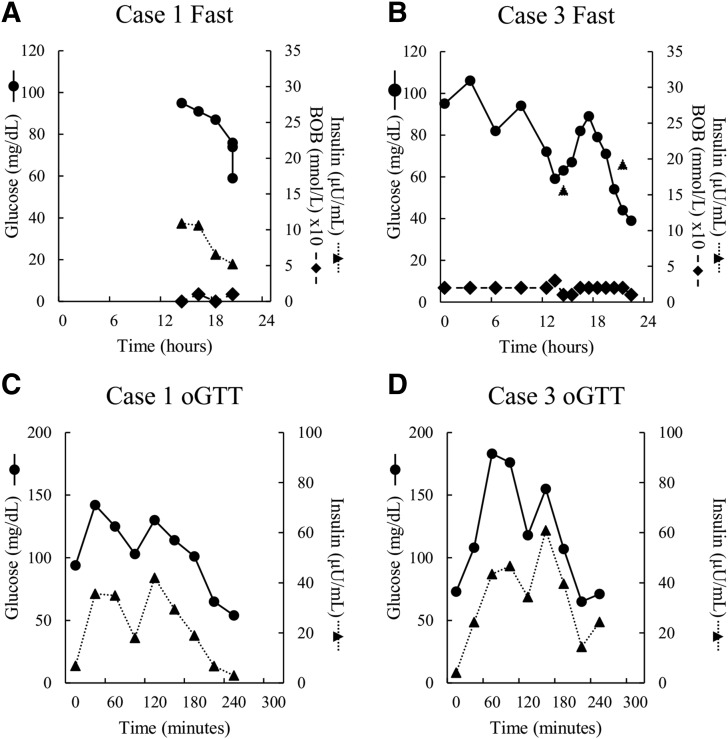
To evaluate the phenotype associated with UCP2 mutations, responses to oGTT and 24-hour fasting tests were assessed in cases 1 and 3 at 6.5 and 9.5 years of age, respectively, while the patients were not receiving diazoxide therapy, and in 4 of the carriers of UCP2 mutations, who ranged in age from 14 to 45 years, from 3 families. As shown in Supplemental Table 1 (16.3KB, docx) , none of the carriers had diabetes mellitus, although all were overweight and had biochemical evidence of insulin resistance. These tests demonstrated evidence of ongoing HI in both children. As shown in Fig. 3, case 1 was able to fast for 20 hours, during which time his plasma glucose levels were maintained within the normal range, but failed to show an elevation of plasma β-hydroxybutyrate before his plasma glucose decreased quickly to 56 mg/dL, and thus developed symptomatic hypoglycemia [Fig. 3(A)]. During a 24-hour fasting test [Fig. 3(B)], case 3 also developed symptomatic hypoketotic hypoglycemia and had an abnormal positive glycemic response to glucagon at the time of hypoglycemia, indicating persistence of HI.
Figure 3.
(A–D) Twenty-four–hour fasting and oGTTs in 2 children with UCP2 HI. Cases 1 and 3 underwent testing at ages 6.5 and 9.5 years, respectively, after discontinuation of diazoxide treatment. Symbols show plasma glucose (solid line, circle), insulin (dotted line, triangle), and β-hydroxybutyrate (BOB; dashed line, diamond).
As shown in Fig. 3, an unusual pattern of oscillating plasma glucose values was observed during the fasting test in case 3: at 13 hours, the glucose decreased to 59 mg/dL before increasing to 89 mg/dL at 17.5 hours and subsequently falling to 39 mg/dL at 22 hours. This oscillating pattern of plasma glucose concentrations was also observed during oGTT in both cases 1 and 3 [Fig. 3(C) and 3(D)], at the end of which both children became symptomatically hypoglycemic. Plasma insulin levels in cases 1 and 3 showed an oscillatory pattern during oGTTs that closely matched the oscillations in plasma glucose concentrations. Peak plasma insulin responses to oral glucose challenge in both cases 1 and 3 were at the upper end of the range normally seen in young children.
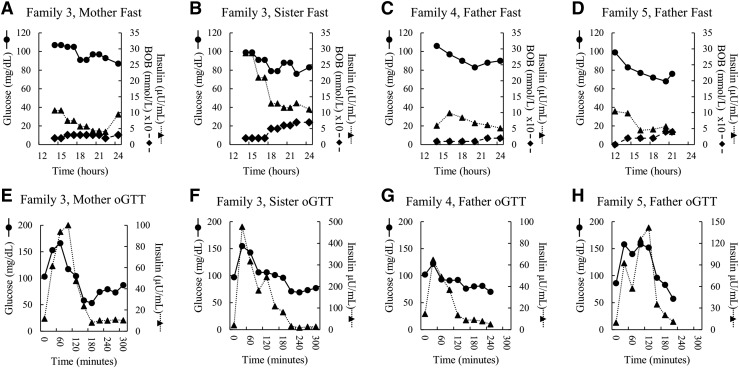
Following oral glucose loading, 2 of the 4 carriers developed symptomatic hypoglycemia (53 mg/dL in subject 3-II-2 and 57 mg/dL in subject 5-II-2). During the 24-hour fast, none of the 4 carriers developed hypoglycemia; however, plasma glucose values were declining toward 70 mg/dL in all 4 individuals, and the father of case 5 developed symptoms of hypoglycemia at the end of the fast. All 4 of the carrier relatives exhibited somewhat elevated peak insulin responses to oral glucose, which were most notable in the sister of case 3, who had a peak insulin value of nearly 500 µU/mL [Fig. 4(F)]; reference normal mean peak insulin, 115 µU/mL in lean and 187 µU/mL in obese adolescents (15)]. As shown in Fig. 4, all of the 4 carriers of UCP2 mutations showed an oscillatory pattern of plasma glucose concentrations similar to that observed in cases 1 and 3. This was most apparent in the responses to oral glucose [shown in Fig. 4(E), 4(F), and 4(H)] but was also seen in the responses to fasting.
Figure 4.
(A–H) Twenty-four–hour fasting and oGTTs in 4 UCP2 mutation carriers. Carrier family members of cases 3, 4, and 5 (ages 36, 14, 37, and 45 years; 3-II-2, 3-III-1, 4-II-2, 5-II-2 in Fig. 2) underwent testing (details shown in Supplemental Table 1 (16.3KB, docx) ). Symbols show plasma glucose (solid line, circle), insulin (dotted line, triangle), and β-hydroxybutyrate (BOB; dashed line, diamond).
Discussion
The results of these studies indicate that inactivating mutations of UCP2 may be a more common cause of congenital HI than has been previously appreciated. Although no cases have been reported since the original report by Gonzalez-Barroso et al. (3) in 2008, the 5 children identified in the CHOP series indicate that the frequency of UCP2 mutations among diazoxide-responsive HI cases (2.4%) is similar to that of other rare forms of HI due to mutations in HNF4A (2%), HNF1A (3%), or HADH (2%) (16). In contrast to the original report, the experience with our series of 5 cases shows that the HI in patients with UCP2 mutations does not quickly nor completely resolve but can persist through childhood or longer. A distinctive feature found in our cases and their carrier relatives was that, in addition to fasting hypoglycemia, oral glucose loading could also provoke profound hypoglycemia.
The function of UCP2 has recently been clarified in studies by Vozza et al. (4) to differ from that of UCP1, which uncouples oxidative phosphorylation by leaking hydrogen ions across the inner mitochondrial membrane. Instead, as shown in Fig. 1, UCP2 acts to transport oxaloacetate, malate, and aspartate out of the mitochondrial matrix and moves phosphate and hydrogen ions from the cytosol into mitochondria. By depleting the pool of mitochondrial tricarboxylic acid cycle intermediates, increases in UCP2 activity tend to restrict glucose oxidation in favor of the oxidation of glutamine and amino acids. Complete ablation of UCP2 activity in UCP2 knockout mice leads to hypoglycemia resulting from increased GSIS in response to higher adenosine triphosphate production (17). Thus, the mutations in UCP2 found in our 5 cases with HI may enhance the oxidation of glucose in pancreatic β-cells, leading to amplification of the insulin response to glucose. This mechanism is consistent with the observation of large insulin responses to oral glucose, which we observed in 2 cases and in 3 of the 4 carriers who were tested. Heightened sensitivity of the β-cell to glucose loading may explain the rather severe postprandial hypoglycemia observed 3 to 4 hours after oral glucose challenge in cases 1 and 3.
The oscillations in the plasma concentrations of insulin and glucose during the oGTT in cases 1 and 3 and in most of the adult UCP2 carriers, as well as the similar oscillatory pattern of insulin and glucose during fasting in case 3, are worth noting (Figs. 3 and 4). Biphasic or triphasic patterns of glucose response to oral glucose occur in a minority of normal individuals and appear to be associated with better insulin sensitivity and β-cell function and lower risk of developing type 2 diabetes mellitus in obese adults and adolescents (18–20). The relationship of oscillatory insulin and glucose responses and postglucose symptomatic hypoglycemia in patients with UCP2 mutations remains speculative. Although mild reactive hypoglycemia after oral glucose is not uncommon in adults, the severe symptomatic postprandial hypoglycemia seen in our cases with UCP2 mutations is extremely rare in children who have not had gastric surgery and appears to be a distinguishing feature of HI due to UCP2 mutations.
In contrast to several of the other forms of congenital HI, fetal overgrowth (birth weight high for gestational age) was not a common finding in our 5 cases with UCP2 mutations. This is consistent with the concept that the defect primarily produces an exaggeration of the insulin response to a glucose load, rather than persistent hyperinsulinemia in the basal state. Heightened responsiveness to glucose-stimulated insulin release may explain why 3 of the 5 children with UCP2 HI in our series had difficulty achieving adequate glycemic control even when taking high doses of diazoxide. In retrospect, it is possible that their apparent poor response to diazoxide may have been due to episodes of postprandial rather than fasting hypoglycemia. It is noteworthy that, while not taking diazoxide (Fig. 3), both cases 1 and 3 became hypoglycemic only after prolonged fasting but had quite dramatic hypoglycemia following oral glucose. This suggests that evaluation of the efficacy of diazoxide therapy in patients with UCP2 deficiency requires assessment of responses to carbohydrate loading as well as the usual testing of fasting tolerance.
An unexplained feature of our UCP2 cases is that 4 of the 5 were of African descent. Although the number of cases is small, this is striking, given that individuals of African descent account for only 15% of all diazoxide-responsive cases seen at CHOP. It is interesting that the UCP2 mutations in these 4 cases, particularly the p.Ala268Gly variant, are reported to occur at increased frequency in African populations; whether this reflects a founder effect or some potential advantage in carriers of the variants is unknown.
Several pieces of evidence supporting the interpretation that the UCP2 mutations found in our cases are disease causing include: (1) identification of identical mutations in 2 pairs of affected children (p.Ala268Gly and p.Gly61Ser); (2) histories of hypoglycemic symptoms in 3 adult mutation carriers and documented hypoglycemia during fasting in 1 of these; (3) similarities in responses to oral glucose and fasting among affected and “unaffected” mutation carriers; (4) evidence that the mutations are associated with decreased UCP2 function, albeit modest, from expression studies in vitro; and (5) new information clarifying the potential pathophysiologic role of UCP2 impairment. Limitations of evidence that the UCP2 mutations found in our cases are disease causing include the surprisingly modest reductions in UCP2 activity found in expression studies of the 3 missense mutations and the fact that 2 of the mutations, especially p.Ala268Gly, occur in as high as 1.5% of asymptomatic individuals of African descent. Although the 30% to 75% reduction found in UCP2 transport activity seems unusually modest for a disease-causing dominant defect, the degree of functional compromise necessary to cause insulin dysregulation in human islets in vivo is not known; it is also possible that the mutations in UCP2 alter insulin regulation via mechanisms not reflected by the in vitro assay of activity. Whereas it seems unlikely for a disease-causing defect to affect 1.5% of the population, HI associated with UCP2 mutations, similar to other dominant forms of HI such as that associated with mutations of GLUD1 and ABCC8 (21, 22), may cause mild enough disease to escape recognition and have no noticeable impact on reproductive capacities.
Conclusions
The results of these studies confirm that inactivating mutations of UCP2 are associated with a dominant, diazoxide-responsive form of congenital HI. UCP2 HI is at least as common as other better-known rare causes of the disorder and should be considered in genetic mutation screening for children with HI. Children with UCP2 HI may require diazoxide treatment for long periods, perhaps indefinitely. The results of these studies suggest that UCP2 plays an important role in coordinating glucose-stimulated insulin secretion that may be relevant to diabetes and postprandial hypoglycemia. Children with HI due to UCP2 mutations appear to be highly susceptible to developing hypoglycemia after a glucose load. Therefore, evaluation of treatment should include monitoring the response to oral carbohydrate.
Acknowledgments
We thank the nurses and research staff of The Hyperinsulinism Center and the Clinical Translational Research Center Core Laboratory. We also thank the Genome Aggregation Database and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at http://gnomad.broadinstitute.org/about.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R37 DK056268 (to C.A.S.), UL1RR024134, and UL1TR000003, and by Italian Association for Cancer Research Grant IG 15404), as well as by The Clifford and Katherine Goldsmith Philanthropic Fund.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHOP
- The Children’s Hospital of Philadelphia
- GSIS
- glucose-stimulated insulin secretion
- HI
- hyperinsulinism
- oGTT
- oral glucose tolerance test
- UCP2
- uncoupling protein 2
References
- 1.Stanley CA, and DeLeon DD, eds. Monogenic Hyperinsulinemic Hypoglycemia Disorders. Basel, Switzerland: Karger; 2012. [Google Scholar]
- 2.Stanley CA. Perspective on the genetics and diagnosis of congenital hyperinsulinism disorders. J Clin Endocrinol Metab. 2016;101(3):815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González-Barroso MM, Giurgea I, Bouillaud F, Anedda A, Bellanné-Chantelot C, Hubert L, de Keyzer Y, de Lonlay P, Ricquier D. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One. 2008;3(12):e3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D, Paradies E, Scarcia P, Palmieri F, Bouillaud F, Fiermonte G. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA. 2014;111(3):960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50(6):1302–1310. [DOI] [PubMed] [Google Scholar]
- 6.Chan CB, Harper ME. Uncoupling proteins: role in insulin resistance and insulin insufficiency. Curr Diabetes Rev. 2006;2(3):271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Affourtit C, Brand MD. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem J. 2008;409(1):199–204. [DOI] [PubMed] [Google Scholar]
- 8.Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. J Pediatr. 1980;96(2):257–259. [DOI] [PubMed] [Google Scholar]
- 9.Lord K, De León DD. Monogenic hyperinsulinemic hypoglycemia: current insights into the pathogenesis and management. Int J Pediatr Endocrinol. 2013;2013(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Roberto E, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616)285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- 12.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–362. [DOI] [PubMed] [Google Scholar]
- 14.Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D’Alessio DA. Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab. 2006;91(1):185–191. [DOI] [PubMed] [Google Scholar]
- 16.Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, Ganapathy K, Bhatti T, Stanley CA, Ganguly A. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98(2):E355–E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–755. [DOI] [PubMed] [Google Scholar]
- 18.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care. 2003;26(4):1026–1033. [DOI] [PubMed] [Google Scholar]
- 19.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol. 2011;300(4):R941–R948. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Michaliszyn SF, Nasr A, Lee S, Tfayli H, Hannon T, Hughan KS, Bacha F, Arslanian S. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care. 2016;39(8):1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, Ganguly A, Shyng SL, Stanley CA. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118(8):2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMullen C, Fang J, Hsu BY, Kelly A, de Lonlay-Debeney P, Saudubray JM, Ganguly A, Smith TJ, Stanley CA; Hyperinsulinism/Hyperammonemia Contributing Investigators . Hyperinsulinism/hyperammonemia syndrome in children with regulatory mutations in the inhibitory guanosine triphosphate-binding domain of glutamate dehydrogenase. J Clin Endocrinol Metab. 2001;86(4):1782–1787. [DOI] [PubMed] [Google Scholar]