Abstract
Context:
Pioglitazone reduces cardiovascular risk in nondiabetic patients after an ischemic stroke or transient ischemic attack (TIA) but is associated with increased risk for bone fracture.
Objective:
To characterize fractures associated with pioglitazone by location, mechanism, severity, timing, and sex.
Design, Setting, and Patients:
Patients were 3876 nondiabetic participants in the Insulin Resistance Intervention after Stroke trial randomized to pioglitazone or placebo and followed for a median of 4.8 years. Fractures were identified through quarterly interviews.
Results:
At 5 years, the increment in fracture risk between pioglitazone and placebo groups was 4.9% [13.6% vs 8.8%; hazard ratio (HR), 1.53; 95% confidence interval (CI), 1.24 to 1.89). In each group, ∼80% of fractures were low energy (i.e., resulted from fall) and 45% were serious (i.e., required surgery or hospitalization). For serious fractures most likely to be related to pioglitazone (low energy, nonpathological), the risk increment was 1.6% (4.7% vs 3.1%; HR, 1.47; 95% CI, 1.03 to 2.09). Increased risk for any fracture was observed in men (9.4% vs 5.2%; HR, 1.83; 95% CI, 1.36 to 2.48) and women (14.9% vs 11.6%; HR, 1.32; 95% CI, 0.98 to 1.78; interaction P = 0.13).
Conclusions:
Fractures affected 8.8% of placebo-treated patients within 5 years after an ischemic stroke or TIA. Pioglitazone increased the absolute fracture risk by 1.6% to 4.9% and the relative risk by 47% to 60%, depending on fracture classification. Our analysis suggests that treatments to improve bone health and prevent falls may help optimize the risk/benefit ratio for pioglitazone.
We present an analysis of bone fractures observed in a placebo-controlled randomized clinical trial of pioglitazone for secondary prevention in 3876 nondiabetic patients with cerebrovascular disease.
Pioglitazone, an insulin sensitizer of the thiazolidinedione (TZD) class of peroxisome proliferator–activated receptor (PPAR)γ agonists, has been shown to significantly reduce the risk of stroke and myocardial infarction (MI) in nondiabetic patients with cerebrovascular disease enrolled in the Insulin Resistance Intervention After Stroke (IRIS) trial (relative risk reduction of 24%) (1). This risk reduction occurred in the setting of background therapy that included statins, antihypertensive agents, and antiplatelet therapy. However, among the monitored adverse events in IRIS, the incidence rates for weight gain, peripheral edema, and bone fracture were found to be greater among participants assigned to pioglitazone compared with placebo. Of these adverse effects, bone fracture represents a particular concern for patients and clinicians.
The association of TZDs with an increased risk for fracture has been reported previously in clinical trials involving patients with diabetes (2–4), and mechanistic studies have provided additional evidence linking this drug class to adverse bone effects (5). The present study was conducted to provide detailed information on the incidence, severity, mechanism, skeletal location, and timing of fractures observed in the IRIS trial of patients with insulin resistance but without diabetes.
Methods
Study design
The methods, protocol, and main results for the IRIS trial have been previously published (1, 6). IRIS was an investigator-initiated, randomized, double-blind, placebo-controlled study in 3876 insulin-resistant, nondiabetic patients with a recent stroke or transient ischemic attack (TIA) designed to test if pioglitazone would reduce the incidence of stroke and MI. Participants were recruited at 179 research sites in Australia, Canada, Germany, Israel, Italy, the United Kingdom, and the United States. The study was funded by the US National Institute of Neurologic Disorders and Stroke. Active drug and placebo tablets were provided by Takeda Pharmaceuticals International, Inc.
Study procedures
Eligible patients were randomly assigned in a 1:1 ratio to receive pioglitazone or placebo within 6 months of a qualifying neurologic event. Patients were excluded when they had taken antihyperglycemic therapy for a diagnosis of diabetes within 90 days of the screening blood test or when they met 2005 American Diabetes Association criteria for diabetes (i.e., fasting plasma glucose ≥7.0 mmol/L, repeated and confirmed) or when hemoglobin A1c ≥ 7.0% (the threshold for starting antidiabetic therapy). Insulin resistance was defined as homeostasis model assessment of insulin resistance score >3.0 on the screening test. Other major exclusions included heart failure, dependent edema, predicted survival <4 years, oral corticosteroid use, and history of bladder cancer.
Participants were interviewed every 2 weeks in the first 3 months following randomization and then quarterly from month 4 through 60 or the last scheduled contact before August 2015, whichever came first. At each contact, participants were asked about potential adverse effects (weight gain, edema, shortness of breath, or muscle aches), health events, and hospitalizations.
If no new or worsening potential adverse effects were reported, the study drug dose was titrated from 15 mg of pioglitazone daily or matching placebo to 45 mg daily over 2 months. Dose reduction was permitted to manage adverse effects and help participants remain on the drug. The study drug was permanently stopped for heart failure, bladder cancer, or a second low-energy fracture.
Bone fracture surveillance and adjudication
Bone fracture as a potential safety concern of TZD therapy was identified shortly after the IRIS trial began recruitment in 2005. Following the report of a higher rate of fractures in women receiving another TZD, rosiglitazone (7), the manufacturer of pioglitazone alerted health care providers to a similar finding based on an analysis of its clinical trial database (8). In response to these reports, bone fracture was added as a safety outcome for the IRIS trial in 2007 and the informed consent was modified to include language on fracture risk (6). Current participants were asked about any fractures after trial entry, and a fracture query was added to the quarterly interview. Additionally, participants were advised to follow standard strategies to preserve bone health, including daily calcium and vitamin D intake and weight-bearing exercise. Men and women who had reached 70 and 65 years of age, respectively, were advised to obtain a bone density study if they had not had one within 5 years.
For any fracture reported by a participant or noted in a hospital discharge diagnosis, medical records, including radiograph reports, were obtained and submitted for adjudication to an independent committee composed of an orthopedist, a radiologist, and a metabolic bone specialist who were blinded to treatment assignment. Two committee members had to agree on fracture occurrence, date, bone involved, and whether the fracture was pathologic or nonpathologic, stress or nonstress, low or high energy, and serious or nonserious. A pathologic fracture was defined as fracture caused by weakening in the bone due to underlying disease process (e.g., neoplasia, Paget’s disease of bone, or osteomyelitis). A stress fracture was characterized as one with imaging evidence of hairline defect and localized pain in bone subject to repetitive stress or minimal trauma. A low-energy fracture was defined as one occurring after a fall from sitting or standing height, including tripping on a flat surface, or after a fall from a low platform or bed. A serious fracture was defined as one that required surgery (i.e., pinnings, vertebroplasties), caused or prolonged hospitalization, or was considered serious by the investigator.
Statistical analysis
Analyses of fracture events were performed according to the intention-to-treat principle. Kaplan–Meier analysis was used to estimate cumulative fracture-free survival rates (9) and the log-rank statistic with type I error of 0.05 (2-sided) was used to assess the difference over time between treatment groups. The effect of pioglitazone relative to placebo was quantified as a hazard ratio (HR) with 95% confidence interval (CI) from the Cox model (10). The effect of pioglitazone was assessed overall, by bone involved, and by fracture type, including an analysis of the subset of fractures that were low energy and not related to pathology to isolate fractures that were more likely to be medication related. Because initial reports of fracture risk with TZDs were restricted to women with diabetes (7, 8), analyses were also conducted for men and women separately.
The association between study drug use during the trial and fracture was examined by comparing fracture rates after 12 months within strata defined by mean daily study drug dose during the first year. Mean dose was calculated as the cumulative self-reported daily dose (0, 15, 30, or 45 mg) as a proportion of number of days.
Except for overall fracture rate by treatment group, these analyses were not prespecified in the IRIS protocol. Results have not been adjusted for multiple comparisons. SAS version 9.3 was used for all analyses (SAS Institute Inc., Cary, NC).
Role of the funding source
The National Institute of Neurological Disorders and Stroke, the funding agency, and Takeda Pharmaceuticals International, Inc., which provided pioglitazone and matching placebo tablets for the trial, had no role in data collection, data analysis or interpretation, or writing of this report. The corresponding author and coauthors had full access to the study data and had final responsibility for the decision to submit for publication.
Results
Study population
The study cohort comprised 3876 participants randomized between February 2005 and January 2013. Median years of follow-up were comparable for treatment groups (pioglitazone vs placebo) overall and by sex (4.8 vs 4.7 for men and 4.9 vs 5.0 for women). Total participant-years of follow-up was 7951 for pioglitazone (5343 in men, 2607 in women) and 7952 (5110 in men and 2841 in women) for placebo.
Baseline features
Mean age at randomization was 63 years and 65% of participants were male. Treatment groups, overall and by sex, were comparable at baseline for most features potentially related to risk for bone fracture (Table 1; Supplemental Table 1 (283.1KB, docx) ), although there were a few differences: men assigned to pioglitazone were more likely to be taking antiepilepsy medication and thiazide diuretics compared with men assigned to placebo, whereas women in the pioglitazone group were more likely to be taking proton pump inhibitors, less likely to be taking drugs for osteoporosis, and more likely to report >1 alcohol drinks per day compared with women in the placebo group.
Table 1.
Baseline Features by Treatment Group
| Baseline Feature | Pioglitazone (n = 1939) |
Placebo (n = 1937) |
Pa | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Demographics | |||||
| Sex, female | 646 | 33 | 692 | 36 | 0.11 |
| Age, y | 63 ± 11 | 63 ± 11 | 0.99 | ||
| Race, black | 218 | 11 | 225 | 12 | 0.71 |
| Hispanic ethnicity | 75 | 4 | 72 | 4 | 0.80 |
| Lived alone | 564 | 29 | 574 | 30 | 0.71 |
| Medical history | |||||
| Stroke at entry | 1693 | 88 | 1682 | 87 | 0.53 |
| National Institutes of Health stroke scale | 0 (0, 2) | 0 (0, 1) | 0.32 | ||
| Modified Rankin score | 1 (0, 2) | 1 (0, 1) | 0.38 | ||
| Coronary artery disease | 241 | 12 | 221 | 11 | 0.33 |
| Health practices | |||||
| Current smoker | 323 | 17 | 299 | 15 | 0.30 |
| >2(1) drinks/d if male(female) | 153 | 8 | 121 | 6 | 0.05 |
| Aerobic exercise | 936 | 48 | 929 | 48 | 0.98 |
| Cognitive and physical examination | |||||
| 3MS scoreb | 96 (92, 99) | 97 (92, 99) | 0.63 | ||
| Body mass index | 30 ± 6 | 30 ± 5 | 0.65 | ||
| Hemoglobin A1c, % | 5.8 (5.6, 6.1) | 5.8 (5.5, 6.1) | 0.89 | ||
| Medications | |||||
| Antidepressant | 364 | 19 | 401 | 21 | 0.13 |
| Proton pump inhibitor | 392 | 20 | 371 | 19 | 0.40 |
| Thiazide diuretic | 521 | 27 | 499 | 26 | 0.42 |
| Benzodiazepine or BzRAc | 170 | 9 | 169 | 9 | 0.95 |
| Epilepsy drug | 65 | 3 | 51 | 2 | 0.19 |
| Osteoporosis drug | 46 | 2 | 62 | 3 | 0.12 |
| Vitamin Dd | 275 | 19 | 289 | 20 | 0.44 |
The ± values are means ± standard deviation. Continuous variables with skewed distribution are shown as median (25th percentile, 75th percentile). Participants with missing data by treatment group (pioglitazone, placebo) are: race (33, 33); Hispanic (12, 8); National Institutes of Health stroke scale (1, 1); modified Rankin score (0, 1); smoking status (3, 2); alcohol use (23, 27); aerobic exercise (8, 19); modified Mini-Mental State score (67, 69); body mass index (6, 6); hemoglobin A1c (1, 0); medications (7, 6); vitamin D (495, 506).
P from χ2 test for proportions or Wilcoxon rank sum test for continuous variables.
Modified Mini-Mental State examination score.
Benzodiazepine receptor agonist.
Query regarding use of vitamin D supplements was added in 2008.
Bone fractures during follow-up
Of the 634 bone fractures submitted for adjudication, 25 were ruled out (4.0% in pioglitazone group and 3.8% in placebo group) and 8 were classified as having insufficient information to make a diagnosis (1.3% in both groups). Most fractures were low energy, not related to stress or pathology, and not serious (i.e., did not require surgery or hospitalization) (Table 2). Of the 277 fractures classified as serious, all but 39 involved hospitalization. Three patients died within 30 days of a fracture (1 woman in the pioglitazone group due to infection, 1 woman and 1 man in placebo group due to MI and infection, respectively).
Table 2.
Risk of Fracture, Overall and by Type, by Treatment Group
| Pioglitazone (n = 1939) |
Placebo (n = 1937) |
Risk ∆ (%)c | HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Fractures | Ptsa | Risk (%)b | Fractures | Ptsa | Risk (%)b | |||
| Any fracture | 376 | 218 | 13.6 | 225 | 145 | 8.8 | 4.9 | 1.53 (1.24–1.89) |
| Energy | ||||||||
| Low energy | 295 | 178 | 11.2 | 184 | 119 | 7.2 | 4.0 | 1.51 (1.20–1.91) |
| High energy | 67 | 35 | 2.1 | 31 | 22 | 1.3 | 0.8 | 1.60 (0.94–2.73) |
| Unknown | 14 | 14 | 0.9 | 10 | 8 | 0.5 | 0.5 | 1.86 (0.74–4.66) |
| Stress | ||||||||
| Nonstress | 353 | 204 | 12.8 | 204 | 137 | 8.3 | 4.5 | 1.51 (1.22–1.88) |
| Stress | 15 | 10 | 0.6 | 14 | 8 | 0.6 | 0.0 | 1.25 (0.49–3.17) |
| Unknown | 8 | 8 | 0.5 | 7 | 6 | 0.4 | 0.1 | 1.40 (0.44–4.41) |
| Pathology | ||||||||
| Nonpathologic | 371 | 215 | 13.4 | 214 | 141 | 8.6 | 4.9 | 1.55 (1.26–1.92) |
| Pathologic | 3 | 3 | 0.2 | 6 | 3 | 0.2 | 0.0 | 1.00 (0.20–4.96) |
| Unknown | 2 | 2 | 0.1 | 5 | 4 | 0.2 | −0.1 | 0.50 (0.09–2.72) |
| Serious/nonseriousd | ||||||||
| Serious | 178 | 99 | 6.2 | 99 | 62 | 3.7 | 2.4 | 1.61 (1.17–2.21) |
| Nonserious | 190 | 128 | 8.2 | 123 | 92 | 5.6 | 2.6 | 1.41 (1.07–1.84) |
| Unknown | 8 | 6 | 0.4 | 3 | 2 | 0.1 | 0.3 | 3.00 (0.61–14.89) |
| Low energy, nonpathologice | 292 | 176 | 11.1 | 178 | 118 | 7.2 | 3.9 | 1.51 (1.19–1.90) |
| Serious, low energy, nonpathologicd,e | 127 | 76 | 4.7 | 83 | 52 | 3.1 | 1.6 | 1.47 (1.03–2.09) |
Number of participants with fracture.
Fracture risk at 5 years from Kaplan–Meier life table.
RD at 5 years from Kaplan–Meier life table.
Fracture considered serious if surgery, procedure, or hospitalization required.
Restricted fracture is defined as low-energy and nonpathologic fracture.
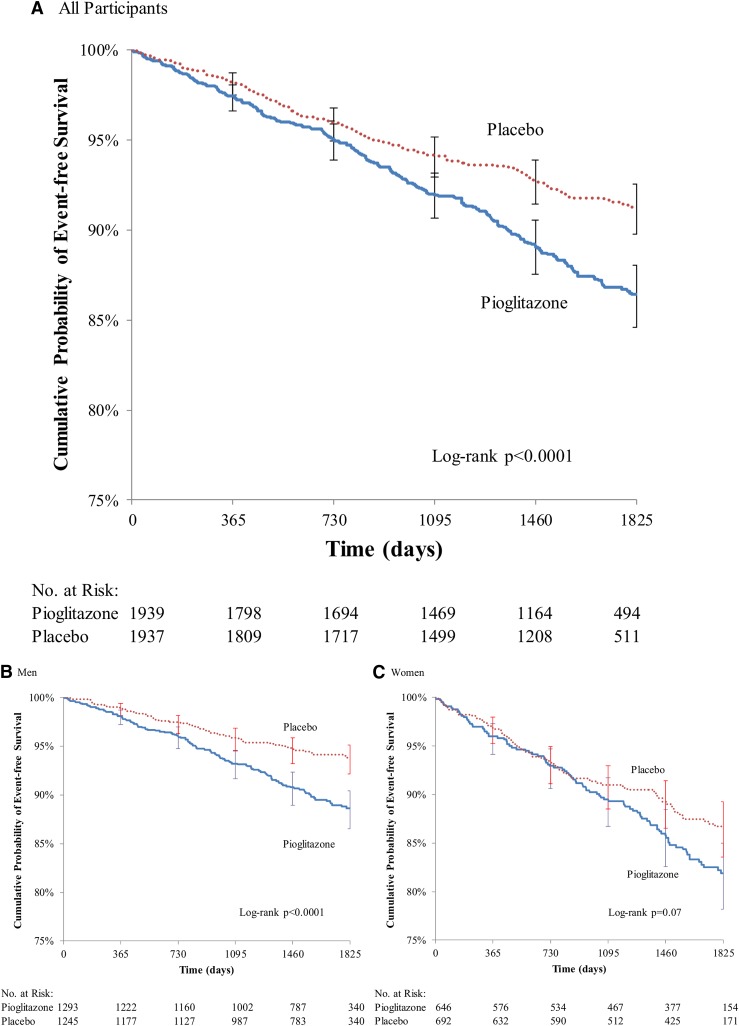
Overall, a total of 376 fractures occurred in 218 participants assigned to pioglitazone compared with 225 fractures in 145 participants assigned to placebo [5-year risk of first fracture, 13.6% vs 8.8%; risk difference (RD), 4.9%; HR, 1.53; 95% CI, 1.24 to 1.89] (Table 2). An increase in risk in the pioglitazone group compared with placebo group was observed for both nonserious and serious fractures. Risks were low and similar across treatment groups for fractures that were high energy, stress type, or associated with pathology. In an analysis restricted to the 470/601 (68%) of fractures that were low energy and nonpathologic (and therefore more likely to reflect differential risk due to the study drug), the RD at 5 years was 3.9% (11.1% vs 7.2%; HR, 1.51; 95% CI, 1.19 to 1.90). A further attenuation of the RD was observed for low energy, nonpathologic fractures that required surgery or hospitalization (4.7% vs 3.1%; RD, 1.6%; HR, 1.47; 95% CI, 1.03 to 2.09). Adjustment for fracture risk features that differed at baseline (i.e., alcohol use > 2/1 drinks per day for men/women, proton pump inhibitor, thiazide diuretic, epilepsy drug, and osteoporosis drug use at baseline) did not change any of these findings (Supplemental Table 2 (283.1KB, docx) ). The curves for fracture-free survival appear to diverge after 2 years for any fracture [Fig. 1(A)] and low-energy, nonpathologic fractures [Supplemental Fig. 1(A) (283.1KB, docx) ].
Figure 1.
Time to first fracture. Overall fractures (A) and those by sex (B, men; C, women) are shown.
Risk of fracture by location and sex
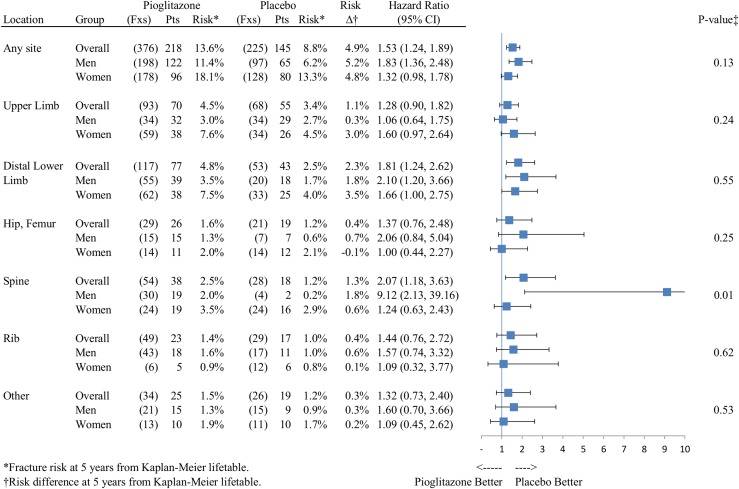
The most common bones fractured were (in descending order) the spine, rib, foot (tarsal, metatarsal, phalanges), fibula, radius, tibia, humerus, and hip (Supplemental Table 3 (283.1KB, docx) ). In the overall cohort, we observed no selective effect of pioglitazone on any specific bone or skeletal area. HRs ranged from 1.28 (95% CI, 0.90 to 1.82) for upper limb fractures to 2.07 for the spine (95% CI, 1.18 to 3.63) (Fig. 2).
Figure 2.
Risk of fracture by location, overall, and by sex.
The risk of any fracture in the pioglitazone group compared with placebo was increased in both men and women (HR for men, 1.83; 95% CI, 1.36 to 2.48; HR for women, 1.32; 95% CI, 0.98 to 1.78) (Fig. 2; Supplemental Table 2 (283.1KB, docx) ). A statistical test of the difference in HRs between sexes was not significant (P for interaction of treatment with sex = 0.13).
Risk for serious fracture in the pioglitazone group compared with placebo was significantly elevated in men (5.4% vs 2.2%; HR, 2.34; 95% CI, 1.45 to 3.76) but not women (7.8% vs 6.4%; HR, 1.18; 95% CI, 0.76 to 1.83) (Supplemental Table 2 (283.1KB, docx) ). When serious fractures were restricted to low-energy, nonpathologic events, the risk remained elevated in men (3.8% vs 1.4%; HR, 2.64; 95% CI, 1.46 to 4.77) and was further reduced in women (6.6% vs 6.2%; HR, 1.03; 95% CI, 0.65 to 1.63). For both any serious fractures and low-energy, nonpathologic serious fractures, HRs for men were significantly higher than for women (P for interaction of treatment with sex = 0.04 and 0.01, respectively).
In men, a total of 198 fractures occurred in 122 participants in the pioglitazone group compared with 97 fractures in 65 participants in the placebo group (11.4% vs 6.2%; RD, 5.2%). The risk increment for low-energy, nonpathologic fractures in men was 4.0% (8.3% vs 4.3%; HR, 1.90; 95% CI, 1.33 to 2.72). Divergence of fracture-free survival curves for men appeared to occur in the first year for any fracture (log-rank P < 0.0001) [Fig. 1(B)] and after year 1 for low-energy, nonpathologic fractures (log-rank P = 0.0004) [Supplemental Fig. 1(B (283.1KB, docx) )]. The HR for fracture in the pioglitazone group, compared with the placebo group, was elevated for all anatomic sites and reached statistical significance for the distal lower limb and spine (Fig. 2; Supplemental Tables 3 (283.1KB, docx) and 4 (283.1KB, docx) ).
In women, there were a total of 178 fractures in 96 participants in the pioglitazone group and 128 fractures in 80 participants in the placebo group (5-year risk, 18.1% vs 13.3%; RD, 4.8%). For low-energy, nonpathologic fractures the risk increment in women was 4.5% (16.7% vs 12.3%; HR, 1.32; 95% CI, 0.97 to 1.80). Fracture-free survival curves for women did not appear to diverge until after year 2 for any fracture (log-rank P = 0.07) or low-energy, nonpathologic fractures (log-rank P = 0.08) [Fig. 1(C); Supplemental Fig. 1(C (283.1KB, docx) )]. For women, the HR for fracture in the pioglitazone compared with placebo group was nonsignificantly elevated for all anatomic sites (except for the hip/femur, where risk did not differ). The increased risk in women appeared to be primarily for more common fractures of the upper extremity and distal lower limbs (Fig. 2; Supplemental Tables 3 (283.1KB, docx) and 4 (283.1KB, docx) ). Adjustment for fracture risk features that differed at baseline did not change any of the sex-specific findings (Supplemental Tables 2 (283.1KB, docx) and 4 (283.1KB, docx) ).
Study drug use and fracture risk
Among participants in the trial at year 1, women in both treatment groups reported lower adherence to the study drug during the first year compared with men (Supplemental Table 5 (283.1KB, docx) ). Only 64% of women in the pioglitazone group reported taking at least 30 mg/d of the study drug during year 1 compared with 76% of men. The rates were 77% and 88% for women and men, respectively, in the placebo group. Among men in the pioglitazone group, those who reported taking at least 30 mg/day in year 1 were at higher risk of subsequent fracture compared with men with lower adherence, but this trend was not observed in women.
Discussion
In the IRIS trial of nondiabetic patients with insulin resistance, pioglitazone reduced the risk for the primary study outcome of stroke and myocardial infarction but was associated with an increased incidence of bone fracture. After 5 years, the relative increase in risk for any fracture was 53% and the absolute risk increment was 4.9%. Not all fractures, however, were serious (i.e., required hospitalization or surgery) or likely to be related to pioglitazone therapy (i.e., those not resulting from high-energy trauma or malignancy). In an effort to help clinicians and patients with personal decision-making, we conducted an ancillary analysis that was restricted to serious fractures most likely attributable to pioglitazone (i.e., low energy, nonpathologic, requiring surgery or hospitalization). In this analysis, the relative risk increase was essentially unchanged (47%) but the absolute risk increment was reduced to 1.6%. In IRIS, the effect of pioglitazone on bone fracture appeared to emerge after 2 years of therapy. No one bone or skeletal region was more affected than another.
The first signal that TZD therapy may increase fracture risk emerged in 2006 as an unexpected finding from nonadjudicated spontaneous adverse event reports in the A Diabetes Outcome Progression Trial (ADOPT) involving patients with type 2 diabetes (7). For women, fracture rates per 100 patient years were 2.7, 1.5, and 1.3 for rosiglitazone, metformin, and glyburide groups, respectively, whereas for men, fracture rates did not differ significantly by treatment (1.2, 1.0, and 1.1, respectively). Divergence in time-to-event curves for women occurred after 1 year (2). A retrospective review of adverse events in the Prospective Pioglitazone Clinical Trial in Macrovascular Events trial found higher fracture rates for women assigned to pioglitazone compared with placebo (1.0 vs 0.5 per 100 patient-years) but not for men (0.6 vs 0.7) (3, 11). As in ADOPT, risk curves began to diverge after 1 year of follow-up. In the last large trial of TZD therapy to report prior to IRIS, the Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes (RECORD) trial of rosiglitazone reported a significantly higher risk for fractures for rosiglitazone compared with active control in women (2.1 vs 1.1 per 100 patient-years) but not in men (1.0 vs 0.8) (4).
Despite enrolling nondiabetic patients in IRIS, we observed higher fracture rates compared with the trials in diabetic patients (in IRIS, rates per 100 patient-years in pioglitazone vs placebo: 2.3 vs 1.3 for men, 3.7 vs 2.8 for women). These higher rates may reflect our protocol for active fracture surveillance (RECORD was the only prior trial to include direct questioning for bone fracture), the older average age of our participants (63 years vs 56 to 57 years in ADOPT and RECORD), longer follow-up (4.8 years vs 3 years in the Prospective Pioglitazone Clinical Trial in Macrovascular Events), or the known increased risk for fracture after stroke (12–15). Notwithstanding these differences in fracture ascertainment and baseline risk, our findings are consistent with the increased relative risk for any fracture for TZD-treated patients, ranging from 27% to 56%, reported in earlier trials.
Although, the higher HR for men compared with women in IRIS differs from previous reports, neither IRIS nor the earlier trials were powered to rule out chance as an explanation for the observed differences between sexes. Our finding of a greater relative risk for serious bone fractures (requiring surgery or hospitalization) in men assigned to pioglitazone vs placebo, compared with women (P for interaction of treatment and sex = 0.04), may reflect better study drug adherence among male participants, or a chance finding due to multiple statistical comparisons. Indeed, our data do not exclude the possibility of increased risk for women assigned to pioglitazone compared with placebo for serious fracture (or serious, low-energy, nonpathologic fractures), because the CIs are wide.
In contrast to prior reports, we found that the risk for fracture was not confined to any specific group of bones or skeletal area. In previous trials, differences between placebo and active treatment groups were reported mainly for the more common appendicular fracture sites, such as the feet, hands, arms, tibia, and fibula. In IRIS, hip/femur and spinal fractures were also increased in the pioglitazone group, statistically so in the latter. The HR for spine fracture for men in IRIS was 9.12 (95% CI, 2.13 to 39.16), compared with 1.24 (95% CI, 0.63 to 2.43) for women, although this finding was striking, it was based on only 21 men with spine fractures.
The mechanisms by which TZDs may affect bone health are likely complex (16, 17) and not fully understood. Drugs in this class activate PPARγ, and both isoforms of PPARγ are expressed in bone. Activation of PPARγ2 affects lineage allocation of mesenchymal stem cells, suppressing osteoblast differentiation and inducing marrow adipocyte proliferation (18). Activation of PPARγ1 enhances the formation and activity of osteoclasts (19). Most clinical trials that have examined markers of bone turnover suggest a diminution in bone formation and an increase in bone resorption (5, 20–26), which is consistent with experimental studies, although several studies failed to confirm these findings (27–29). Most (5), but not all (28), studies examining serial bone mineral density by dual x-ray absorptiometry have found modest bone loss. In the 5 studies that have examined bone mineral density after TZD cessation, there is some evidence for an attenuation of the adverse effect (20, 26–29).
A key cellular regulator of bone metabolism is the osteocyte, a type of long-lived cell buried within bone tissue that has the ability to sense mechanical strain on the skeleton and orchestrate bone remodeling by controlling the formation and activity of osteoclasts and osteoblasts. Interestingly, TZDs have also been reported to induce osteocyte apoptosis (30). Osteocyte apoptosis in experimental animals is associated with rapid changes in bone remodeling, with increased bone breakdown and suppressed bone formation, as well as the appearance of microcracks (31). These changes could, if they occur in humans, contribute to the risk for fractures, particularly in long bones.
Our findings include new information on the general problem of bone fracture after ischemic stroke or TIA. Most fractures were low energy, involving a fall from a sitting or standing position, or from bed. The most common bones fractured were the spine, followed by the rib, foot, fibula, radius, tibia, humerus, and hip. Fractures of the spine, radius, and hip are those most often observed in patients with osteoporosis. Taken together, these findings suggest that osteoporosis treatment and/or fall prevention might reduce fracture risk after ischemic stroke or TIA for all patients, including those treated with pioglitazone.
Our study had some limitations. First, we did not have baseline information on bone density or prior fractures, which are major risk factors for fracture in older adults. Second, we did not monitor the incidence of falls after baseline, another risk factor for fracture. Data on bone density and fall risk might have identified specific opportunities to mitigate fracture risk in our population. Finally, we did not measure the frequency or duration of fracture-related disability.
The net benefit of pioglitazone after an ischemic stroke or TIA is a function of the drug’s competing effects on preventing vascular disease and causing bone fracture. The effect on stroke or MI in IRIS has been well documented (i.e., 24% relative risk reduction and 3% absolute risk reduction over 5 years) (1). The effect on fracture is detailed in this study and will help patients make an informed, personal decision regarding pioglitazone therapy. It is conceivable that fracture risk mitigation, including fall prevention, and screening and treatment of osteoporosis would further and favorably affect the benefit/harm ratio for pioglitazone therapy.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant U01NS044876. Pioglitazone and placebo for the IRIS trial were provided by Takeda Pharmaceuticals International.
Author contributions: C.M.V., W.N.K., S.E.I., and L.H.Y. contributed to design of the data analyses and interpretation of the results and were involved in the writing and revision of the report. C.M.V. performed the statistical analyses and drafted the manuscript. All authors contributed to a critical review of the manuscript.
Acknowledgments
Clinical trial registry: ClinicalTrials.gov no. NCT00091949 (registered 20 September 2004), US Food and Drug Administration Investigational New Drug application no. 64,622, European Clinical Trials Database 2008-005546-23.
Disclosure Summary: C.M.V. was a consultant for Takeda Pharmaceuticals International regarding prostate cancer events in the IRIS trial. S.E.I. is a consultant to or has served on research steering committees for Astra Zeneca, Boehringer Ingelheim, Daichii Sankyo, Lexicon, Janssen, Merck, Poxel, Sanofi, and vTv Pharmaceuticals. He has also served on data monitoring committees for Novo Nordisk and Intarcia. L.H.Y. received research grant support unrelated to this study from Merck, Mifcor, and Novartis (to Yale University). The remaining authors have nothing to disclose.
Footnotes
- ADOPT
- A Diabetes Outcome Progression Trial
- CI
- confidence interval
- HR
- hazard ratio
- IRIS
- Insulin Resistance Intervention After Stroke
- MI
- myocardial infarction
- PPAR
- peroxisome proliferator–activated receptor
- RD
- risk difference
- RECORD
- Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes
- TIA
- transient ischemic attack
- TZD
- thiazolidinedione
References
- 1.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR; IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.2337/dc07-2270. Kahn S, Zinman B, Lachin J, Haffner S, Herman W, Holman R, Kravitz B, Yu D, Heise M, Aftring R, Viberti G; A Diabetes Outcome Progression Trial (ADOPT) Study Group. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care. 2008;31(5):845–851. [DOI] [PubMed] [Google Scholar]
- 3.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators . Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32(3):187–202. [DOI] [PubMed] [Google Scholar]
- 4.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV; RECORD Study Team . Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–2135. [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1007/s00125-015-3660-2. Billington E, Grey A, Bolland M. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015;58(10):2238–2246. doi:10.1007/s00125-015-3660-2. [DOI] [PubMed] [Google Scholar]
- 6. doi: 10.1016/j.ahj.2014.07.016. Viscoli CM, Brass LM, Carolei A, Conwit R, Ford GA, Furie KL, Gorman M, Guarino PD, Inzucchi SE, Lovejoy AM, Parsons MW, Peduzzi PN, Ringleb PA, Schwartz GG, Spence JD, Tanne D, Young LH, Kernan WN, for the Iris Trial investigators. Pioglitazone for secondary prevention after ischemic stroke and transient ischemic attack: rationale and design of the Insulin Resistance Intervention after Stroke Trial. Am Heart J. 2014;168(6):823–829.e6. doi:10.1016/j.ahj.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. [DOI] [PubMed] [Google Scholar]
- 8. Takeda Pharmaceuticals North America. Observation of an increased incidence of fractures in female patients who received long-term treatment with ACTOS tablets for type 2 diabetes mellitus. Dear Healthcare Provider Letter. Available at: http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM153896.pdf. Accessed 1 March 2007. [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Cox DR. Regression models and life-tables. J R Stat Soc (Ser A). 1972;34:187–202. [Google Scholar]
- 11.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokán M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J; PROactive Investigators . Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. [DOI] [PubMed] [Google Scholar]
- 12.Ramnemark A, Nyberg L, Borssén B, Olsson T, Gustafson Y. Fractures after stroke. Osteoporos Int. 1998;8(1):92–95. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1161/01.str.32.3.702. Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32(3):702–706. [DOI] [PubMed] [Google Scholar]
- 14.Dennis MS, Lo KM, McDowall M, West T. Fractures after stroke: frequency, types, and associations. Stroke. 2002;33(3):728–734. [DOI] [PubMed] [Google Scholar]
- 15.Benzinger P, Rapp K, König HH, Bleibler F, Globas C, Beyersmann J, Jaensch A, Becker C, Büchele G. Risk of osteoporotic fractures following stroke in older persons. Osteoporos Int. 2015;26(4):1341–1349. [DOI] [PubMed] [Google Scholar]
- 16.Meier C, Schwartz AV, Egger A, Lecka-Czernik B. Effects of diabetes drugs on the skeleton. Bone. 2016;82:93–100. [DOI] [PubMed] [Google Scholar]
- 17.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19(2):129–137. [DOI] [PubMed] [Google Scholar]
- 18.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-γ2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143(6):2376–2384. [DOI] [PubMed] [Google Scholar]
- 19.Wei W, Zeve D, Wang X, Du Y, Tang W, Dechow PC, Graff JM, Wan Y. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor γ-expressing bone marrow cell population. Mol Cell Biol. 2011;31(23):4692–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92(4):1305–1310. [DOI] [PubMed] [Google Scholar]
- 21. doi: 10.1210/jc.2007-0431. Berberoglu Z, Gursoy A, Bayraktar N, Yazici A, Bascil Tutuncu N, Guvener Demirag N. Rosiglitazone decreases serum bone-specific alkaline phosphatase activity in postmenopausal diabetic women. J Clin Endocrinol Metab. 2007;92(9):3523–3530. [DOI] [PubMed] [Google Scholar]
- 22.Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93(5):1696–1701. [DOI] [PubMed] [Google Scholar]
- 23.Berberoglu Z, Yazici AC, Demirag NG. Effects of rosiglitazone on bone mineral density and remodelling parameters in postmenopausal diabetic women: a 2-year follow-up study. Clin Endocrinol (Oxf). 2010;73(3):305–312. [DOI] [PubMed] [Google Scholar]
- 24.Borges J, Bilezikian J, Jones-Leone A, Acusta A, Ambery P, Nino A, Grosse M, Fitzpatrick L, Cobitz A. A randomized, parallel group, double-blind, multicentre study comparing the efficacy and safety of Avandamet (rosiglitazone/metformin) and metformin on long-term glycaemic control and bone mineral density after 80 weeks of treatment in drug-naive type 2 diabetes mellitus patients. DiabetesObes Metab. 2011;13(11):1036–1046. [DOI] [PubMed] [Google Scholar]
- 25. doi: 10.1530/EJE-11-1061. van Lierop A, Hamdy N, van der Meer R, Jonker J, Lamb H, Rijzewijk L, Diamant M, Romijn J, Smit J, Papapoulos S. Distinct effects of pioglitazone and metformin on circulating sclerostin and biochemical markers of bone turnover in men with type 2 diabetes mellitus. Eur J Endocrinol. 2012;166(4):711–716. [DOI] [PubMed] [Google Scholar]
- 26.Bilezikian JP, Josse RG, Eastell R, Lewiecki EM, Miller CG, Wooddell M, Northcutt AR, Kravitz BG, Paul G, Cobitz AR, Nino AJ, Fitzpatrick LA. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(4):1519–1528. [DOI] [PubMed] [Google Scholar]
- 27. doi: 10.1210/jc.2010-2077. Harsløf T, Wamberg L, Møller L, Stødkilde-Jørgensen H, Ringgaard S, Pedersen S, Langdahl B. Rosiglitazone decreases bone mass and bone marrow fat. J Clin Endocrinol Metab. 2011;96(5):1541–1548. [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1210/jc.2012-4096. Bone H, Lindsay R, McClung M, Perez A, Raanan M, Spanheimer R. Effects of pioglitazone on bone in postmenopausal women with impaired fasting glucose or impaired glucose tolerance: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2013;98(12):4691–4701. [DOI] [PubMed] [Google Scholar]
- 29. doi: 10.1530/EJE-13-0793. Grey A, Bolland M, Fenwick S, Horne A, Gamble G, Drury P, Reid I. The skeletal effects of pioglitazone in type 2 diabetes or impaired glucose tolerance: a randomized controlled trial. Eur J Endocrinol. 2013;170(2):255–262. [DOI] [PubMed] [Google Scholar]
- 30.Sorocéanu MA, Miao D, Bai X-Y, Su H, Goltzman D, Karaplis AC. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol. 2004;183(1):203–216. [DOI] [PubMed] [Google Scholar]
- 31.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–475. [DOI] [PubMed] [Google Scholar]