Abstract
Context:
The role of the extracellular matrix (ECM) in regulating adipocyte metabolism in the context of metabolic disease is poorly defined.
Objective:
The objective of this study was to define the metabolic phenotype of adipocytes associated with human diabetes (DM) and the role of the ECM in regulating adipocyte metabolism.
Design:
Adipose tissues from obese patients were studied in standard 2-dimensional (2D) cell culture and an in vitro model of decellularized adipose tissue ECM repopulated with human adipocytes, and results were correlated with DM status.
Setting:
This study was conducted at the Academic University Medical Center and Veteran’s Administration Hospital.
Patients:
Seventy patients with morbid obesity undergoing bariatric surgery were included in the study.
Interventions:
Visceral and subcutaneous adipose tissues were collected at the time of bariatric surgery.
Outcome measures:
This study used metabolic assays for glucose uptake, lipolysis, and lipogenesis in adipocytes in 2D cell culture and 3-dimensional ECM culture.
Results:
Adipocytes from subjects with DM manifest decreased glucose uptake and decreased lipolysis in 2D culture. ECM supports differentiation of mature adipocytes and recapitulates DM-specific differences in adipocyte metabolism observed in 2D culture. ECM from subjects without DM partially rescues glucose uptake and lipolytic defects in adipocytes from subjects with DM, whereas ECM from subjects with DM impairs glucose uptake in adipocytes from subjects without DM.
Conclusions:
DM is associated with adipocyte metabolic dysfunction. The ECM regulates adipocyte metabolism. Nondiabetic ECM rescues metabolic dysfunction in DM adipocytes, whereas DM ECM imparts features of metabolic dysfunction to nondiabetic adipocytes. These findings suggest the ECM as a target for manipulating adipose tissue metabolism.
An in vitro model of adipose tissue extracellular matrix repopulated with adipocytes was used to demonstrate that the extracellular matrix regulates adipocyte metabolism in a disease-specific manner.
Adipose tissue metabolic dysfunction contributes to systemic metabolic disease, including type II diabetes mellitus (DM), but precise cellular metabolic alterations and underlying mechanisms remain poorly defined. Quantitative and qualitative changes in the adipose tissue extracellular matrix (ECM) have been associated with metabolic disease and may contribute to adipocyte dysfunction (1–6). Nonetheless, the relationship between adipose tissue fibrosis and human diabetes is unclear, and robust in vitro models of adipocyte-ECM interactions are lacking. The goals of this study were to address these knowledge gaps and to examine the hypothesis that the ECM regulates adipocyte metabolism in a disease-specific manner.
METHODS
Human subjects
Human subjects undergoing bariatric surgery were enrolled with Institutional Review Board approval at the University of Michigan and Ann Arbor Veteran’s Administration Hospitals. Visceral adipose tissue (VAT) from the greater omentum and subcutaneous adipose tissue (SAT) from the abdominal wall was collected from 70 subjects. Subjects with diabetes were defined by clinical diagnosis of type 2 diabetes requiring treatment with medication and hemoglobinA1c (HbA1c) ≥6.5%. Subjects without DM were defined by no clinical history of diabetes and HbA1c <6.5% per American Diabetes Association criteria (7).
Preadipocyte isolation, adipocyte 2-dimensional culture
Adipose tissue was digested with Type II collagenase [2 mg/mL in phosphate-buffered saline (PBS)/2% BSA] (Life Technologies, Inc., Carlsbad, CA) at 37°C for 60 minutes and centrifuged at 250 relative centrifugal force. The stromal-vascular cell pellet was retrieved and plated overnight, and adherent cells were passaged three times to enrich for preadipocytes, which were frozen in Dulbecco’s modified Eagle medium (DMEM)/F12, 15% fetal calf serum (FCS), and 10% dimethyl sulfoxide in liquid nitrogen. To generate mature adipocytes, preadipocytes (60,000 cells/well in 24-well plates) were plated in DMEM/F12 and 15% FCS until confluent, cultured for 7 days in differentiation medium (DMEM/F12, 2.5 mM glutamine, 15 mM HEPES, 10 mg/mL transferrin, 33 μM biotin, 0.5 μM human insulin, 17 μM pantothenate, 0.1 μM dexamethasone, 2 nM T3, 540 μM IBMX, 1 μM ciglitazone), and cultured for 7 day in maintenance medium (DMEM/F12, 2.5 mM glutamine, 15 mM HEPES, 10 mg/ml transferrin, 33 μM biotin, 0.5 μM human insulin) until differentiated.
ECM preparation
ECM isolation was modified from published protocols for adipose and lung tissues (8–10). VAT explants (1 g) were freeze-thawed in 10 mM Tris, 5 mM EDTA, and 1% phenylmethanesulphonylfluoride (PMSF) (pH 8.0) from −80°C, 20 minutes to 37°C, 5 minutes 3 times. Explants were incubated at 37°C for 24 hours in 0.25% Trypsin/0.1% EDTA; washed in rinsing buffer (8 g/L NaCl, 200 mg/L KCl, 1 g/L Na2HPO4, 200 mg/L KH2PO4, 1% PMSF) at 37°C for 20 minutes three times; incubated at 37°C for 24 hours in 55 mM Na2HPO4, 17 mM KH2PO4, 4.9 mM MgSO4⋅7H2O, 160 U/mL DNase I type II, 100 μg/mL RNase type IIIA, 80 U/mL lipase type VI-S (Sigma-Aldrich, Inc., St. Louis MO, USA), and 1% PMSF; and washed sequentially in rinsing buffer at 37°C for 20 minutes three times; once in 99.9% isopropanol, 1% PMSF at 25°C for 24 hours; rinsing buffer at 37°C for 20 minutes three times; 70% EtOH at 37°C for 20 minutes three times; and storage solution (PBS, 1% PMSF) at 37°C for 20 minutes once. Explants were stored in storage solution 4°C.
ECM-adipocyte culture
Decellularized ECM tissue was rinsed in 70% ethanol and rehydrated in PBS, cut/weighed into 100-mg fragments, seeded with 60,000 preadipocytes in 20 μL of complete growth medium (DMEM, 10% FCS), and incubated at 37°C, 5% CO2, for 40 minutes to allow cells to adhere. After 0.5 mL of complete growth medium was added, the tissue was incubated at 37°C, 5% CO2, for 24 hours and transferred to a fresh culture plate, and 0.5 mL of complete growth medium was added. The solutions were cultured for 3 days and then cultured in 0.5 mL differentiation medium for 14 days to generate mature adipocytes in ECM.
Collagen I immunohistohemistry
Adipose tissue or ECM was fixed in 10% formalin, paraffin-embedded, sectioned onto slides, heat treated, deparaffinized, and rehydrated. Heat-induced epitope retrieval was performed with FLEX-TRS High pH Retrieval buffer (Dako-Agilent Technologies, Inc., Glostrup, Denmark), and cells were stained with rabbit polyclonal collagen I-α-1 antibody (1:1000) (Thermo Scientific, Kalamazoo, MI). The FLEX-HRP-EnVision System (Dako-Agilent Technologies) was used for detection. Slides were counterstained with hematoxylin and imaged on an Olympus IX 81 microscope (Olympus, Inc., Shinjuku, Tokyo, Japan).
Oil Red-O staining
Adipose tissue or ECM adipocytes frozen in liquid nitrogen and cut into 5-μm sections onto slides were stained with Oil Red-O Stain Kit (American Master Tech Scientific, Inc., Lodi, CA) and imaged on an Olympus IX-81 microscope. Adipocytes in 2-dimensional (2D) culture were washed with 1X PBS, fixed in 4% formalin and then 60% isopropanol, stained with 200 μL Oil Red-O Stain Kit, washed in PBS, air dried, and resuspended in 200 μL 100% isopropanol. Absorbance was read at 525 nM on an Epoch Microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT).
Scanning electron microscopy
Tissues were fixed in Sorensen’s phosphate buffer (2.5% glutaraldehyde) at 25°C for 12 hours, postfixed in Sorensen’s buffer (1% osmium tetroxide) at 4°C for 1 hour, serially dehydrated in ethanol, washed in hexamethyldisalizane, air-dried, mounted on scanning electron microscopy–stub with colloidal graphite, dried, and sputter-coated with gold. Images were captured on a scanning electron microscope (1910; Amray, Inc., Bedford, MA) using Solutions X-Stream Image-Capture software (SEMTech Solutions Inc., Billerica, MA).
Glucose uptake
Adipocytes in 2D culture or 3-dimensional (3D) ECM were cultured at 37°C for 72 hours in 0.5 mL maintenance medium and then in serum-free DMEM:F12 at 37°C for 12 hours and in PBS/1% BSA at 37°C for 2 hours. Cells were washed in PBS and then cultured in 0.5 mL PBS with or without 100 nM human insulin at 37°C for 40 minutes and in 0.5 mL PBS with or without 200 nM insulin, 0.1 mM 2-deoxy glucose (Sigma-Aldrich Inc.), and 2μ Ci/mL deoxy-d-glucose-2-[1,2-3H(N)] (PerkinElmer Inc., Waltham, MA) at 37°C for 40 minutes and washed with PBS. After 420 μL 1% SDS solution was added, cells were lysed with pipetting; 10 μL of cell lysate was used for Bradford protein assay, and 400 μL of lysate was transferred to 2 mL scintillation fluid. Counts per minute (cpm) were determined on a scintillation counter and normalized to cell lysate protein concentration.
Lipogenesis
Lipogenesis assay was modified from published protocols (11, 12). Adipocytes in 2D culture or 3D ECM were cultured at 37°C for 72 hours in 0.5 mL maintenance medium, washed with PBS, cultured at 37°C for 24 hours in 0.5 mL serum starvation medium (DMEM:F12, 100 nM insulin), cultured at 37°C for 24 hours in 0.5 mL lipogenesis medium [serum starvation medium + 10 μM sodium acetate and 0.5 μCi 3H-acetate) (PerkinElmer Inc.)], and washed with PBS. Then 120 μL 0.1 N HCl added, followed by pipetting to lyse cells. Lysate (10 μL) was used for Bradford protein assay; 100 μL of lysate was added to 500 μL of 2:1 chloroform/methanol (v/v) and incubated at 25°C for 5 minutes; 250 μL H2O added, incubated at 25°C for 5 minutes, and centrifuged at 3000 relative centrifugal force at 25°C for 10 minutes. Lipid phase was transferred to 2 mL scintillation fluid, and cpm was measured on a scintillation counter and normalized to cell lysate protein concentration. For the “no insulin” arm, cells were cultured in serum starvation medium lacking insulin; for the “insulin + C75” arm, cells were cultured in serum starvation medium with insulin and C75 (10 μg/mL).
Lipolysis
Adipocytes in 2D- or 3D-ECM culture were cultured at 37°C for 72 hours in 0.5 mL maintenance medium with or without 3 µM isoproterenol, and the glycerol concentration in supernatants was measured with a lipid metabolite assay kit (Sigma-Aldrich, Inc.) and normalized to either cell lysate protein concentration measured by Bradford assay or cell lysate DNA concentration measured by CyQuant assay (Thermo Scientific).
Quantitative real-time PCR
Adipocytes in 2D culture were lysed in Trizol, and ECM-adipocyte cultures were homogenized with a BeadBug Homogenizer (Benchmark Scientific, Inc., Edison, NJ). RNA was extracted with the RNAEasy Fibrous Tissue MiniKit (Qiagen, Inc., Hilden, Germany). Equal amounts of input RNA were reverse-transcribed, and quantitative real-time polymerase chain reaction (PCR) was performed using Taqman primer-probes (Life Technologies, Inc., Carlsbad, CA) with actin as an endogenous control on a StepOnePlus thermocycler (Applied Biosystems, Inc., Foster City, CA). The 2-dCT method was used to calculate fold differences in transcript levels and setting undetermined values at dCT = 40.
Statistical analysis
Paired or independent t test was used to compare continuous data between groups. Fisher’s exact test was used to compare dichotomous demographic variables. Delta CT values were compared for quantitative real-time PCR data from 2D culture, adjusting for age and sex. Linear regression was used to determine correlations. Analysis of covariance using general linear models was used to compare outcome measures while adjusting for age and sex as covariates. For ECM-adipocyte (ECM-AD) experiments, to account for possible correlation within subjects given that data involve repeated measures, a linear mixed model with random intercepts for subject matrix and subject cell was used to compare metabolic and quantitative real-time PCR (dCT values) data between arms while adjusting for age and sex of subjects from whom ECM and preadipocytes were derived. Correlations of HbA1c-ECM or HbA1c-AD as continuous variables with metabolic outcome measures in the ECM-AD model were studied using an identical linear mixed model analysis, adjusting for age and sex of the subjects from whom either ECM or adipocytes were derived. Means and standard errors of means (displayed in the figures as error bars) are estimated, adjusting for age and sex.
Results
Adipocyte metabolism is dysregulated in patients with diabetes
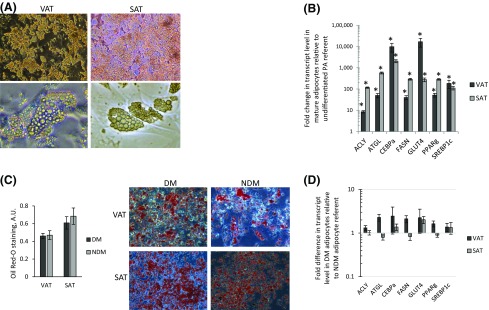
Human preadipocytes have been shown to retain depot- and patient-specific characteristics in culture (13–15). We generated adipocytes from preadipocytes isolated from VAT and SAT from obese patients undergoing bariatric surgery stratified by DM status. Patients with DM were older than patients without DM and included more men (Table 1). Light microscopy, Oil Red-O staining, and quantitative real-time PCR demonstrate that differentiated adipocytes in 2D culture accumulate cytoplasmic lipid and upregulate expression of adipogenic genes, with no differences between DM and non–diabetes mellitus (NDM) adipocytes with respect to these measures (Fig. 1).
Table 1.
Subject Demographics
| DM (n = 27) | NDM (n = 43) | P Valuea | |
|---|---|---|---|
| Clinical characteristics | |||
| Sex (% female) | 33% | 67% | 0.007 |
| Age, y [mean (SD)] | 53 (11) | 44 (10) | <0.001 |
| BMI, kg/m2 [mean (SD)] | 45 (5) | 44 (7) | 0.860 |
| HbA1c, % (mean) | 7.1 | 5.6 | <0.001 |
| Comorbid diseases, % | |||
| Sleep apnea | 89 | 63 | 0.026 |
| Hypertension | 85 | 51 | 0.005 |
| Dyslipidemia | 74 | 44 | 0.025 |
| Medications, % | |||
| ACE inhibitor | 26 | 16 | 0.368 |
| β-blocker | 30 | 5 | 0.010 |
| Insulin | 37 | 0 | <0.001 |
| Metformin | 89 | 5 | <0.001 |
| Statin | 67 | 16 | <0.001 |
| Sulfonylurea | 33 | 0 | <0.001 |
| Thiazolidinedione | 7 | 0 | 0.145 |
Abbreviations: ACE, angiotensin converting enzyme; BMI, body mass index; SD, standard deviation.
Independent t test was used to compare continuous variables between DM and NDM groups; Fisher’s exact test was used to compare dichotomous variables between DM and NDM groups.
Figure 1.
Adipocyte 2D culture. (A) Human adipocytes in in vitro 2D culture. Representative photomicrographs of in vitro differentiated human VAT and SAT adipocytes (top, magnification ×20; bottom, magnification ×40). (B) Adipogenic gene expression in human adipocytes. Quantitative real-time PCR data comparing adipogenic gene transcript levels in RNA from human adipocytes differentiated for 14 days in vitro with undifferentiated preadipocytes (PA) cultured for 72 hours in nonadipogenic media. Ordinate: fold difference in transcript level in mature adipocytes relative to undifferentiated preadipocyte referent = 1. *P < 0.001, paired t test. Preadipocytes/adipocytes are from 12 subjects. (C) Oil Red-O staining in human adipocytes. Left: Oil Red-O staining quantified by spectrophotometry in human adipocytes. No differences were observed between DM and NDM adipocytes (P > 0.520 for VAT and SAT) (n = 10 subjects with DM and n = 12 subjects without DM for VAT; n = 9 subjects with DM and n = 9 subjects without DM for SAT). Right: Representative photomicrographs of Oil Red-O–stained human adipocytes. (D) Adipogenic gene expression in human DM and NDM adipocytes. Quantitative real-time PCR data comparing adipogenic gene transcript levels in RNA from human DM and NDM adipocytes. Ordinate: Fold difference in transcript level in DM adipocytes relative to NDM adipocytes as referent = 1. P > 0.260 for all comparisons except ATGL (P = 0.120) in VAT and FASN (P = 0.140) in VAT (n = 8 subjects with DM and n = 8 subjects without DM for VAT; n = 6 subjects with DM and n = 6 subjects without DM for SAT). A.U., arbitrary units.
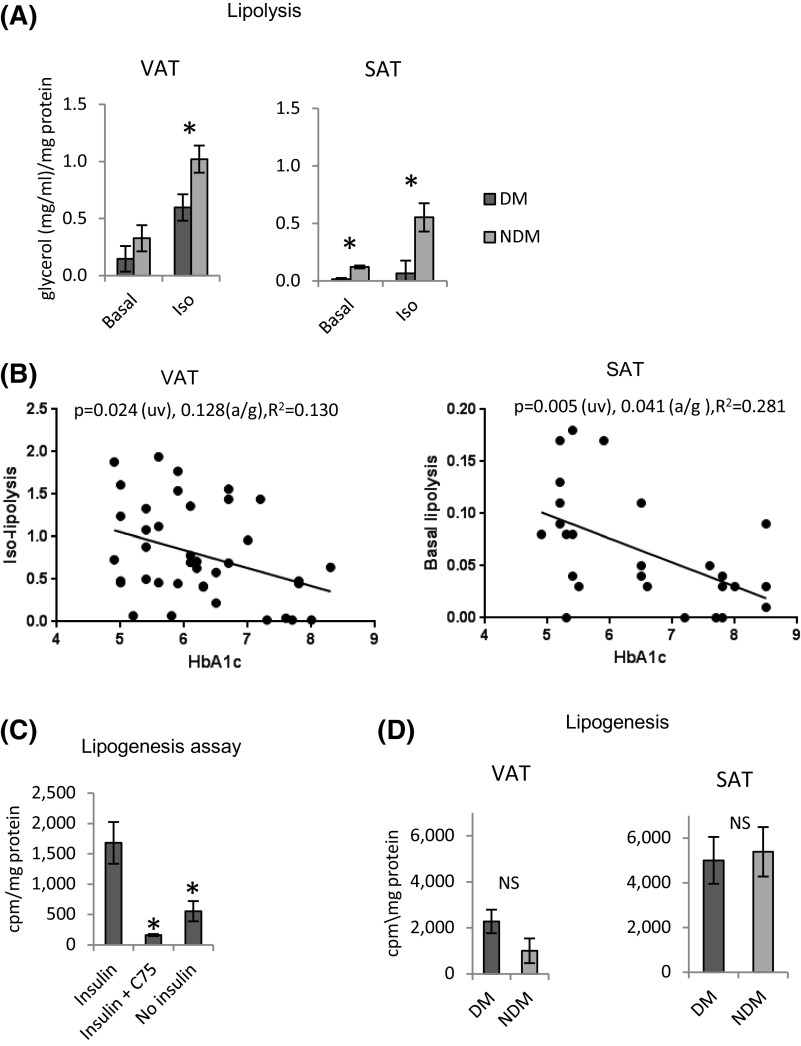
Basal and insulin-stimulated glucose uptake was decreased in VAT and SAT adipocytes from subjects with DM; these differences remained significant after adjusting for age and sex [Fig. 2(A)]. Linear regression analysis demonstrated a negative correlation between glucose uptake and HbA1c in VAT and SAT adipocytes; these correlations remained significant or approached significance after adjusting for age and sex [Fig. 2(B)]. No correlations were observed between age and basal or insulin-stimulated glucose uptake in VAT or SAT adipocytes (data not shown).
Figure 2.
Glucose uptake is impaired in DM adipocytes. (A) Glucose uptake is impaired in DM adipocytes. Adipocytes were differentiated from VAT or SAT preadipocytes from DM or NDM subjects cultured with or without insulin then studied with glucose uptake assay. Ordinate: Age- and sex-adjusted glucose uptake measured by 3H-2D glucose in cell lysates (cpm) normalized to cell lysate protein concentration (mg/mL). *P < 0.050, **P < 0.100 comparing NDM and DM groups, independent t test, age-adjusted (n = 18 subjects with DM and n = 18 subjects without DM for VAT; n = 12 subjects with DM and n = 14 subjects without DM for SAT). (B) Glucose uptake correlates inversely with HbA1c. Linear regression analysis correlating VAT adipocyte glucose uptake with HbA1c. Ordinate: HbA1c (%); abscissa: glucose uptake, cpm normalized to cell lysate protein concentration (mg/mL). P values shown are univariate/unadjusted (uv) and are age- and sex adjusted (a/g). HbA1c did not correlate with basal or insulin-stimulated glucose uptake in SAT adipocytes; age did not correlate with basal or insulin-stimulated glucose uptake in VAT or SAT adipocytes.
Isoproterenol-stimulated lipolysis, but not basal lipolysis, was decreased in VAT adipocytes from subjects with DM compared with subjects without DM; these differences remained significant after adjusting for age and sex. Age- and sex-adjusted basal and isoproterenol-stimulated lipolysis was decreased in SAT adipocytes from subjects with DM compared with subjects without DM [Fig. 3(A)]. Linear regression demonstrated indirect correlations between HbA1c levels and isoproterenol-stimulated lipolysis in VAT adipocytes and basal lipolysis in SAT adipocytes [Fig. 3(B)]. No correlations were observed between HbA1c levels and basal lipolysis in VAT adipocytes or isoproterenol-stimulated lipolysis in SAT adipocytes or between age and basal or isoproterenol-stimulated lipolysis in VAT or SAT adipocytes (data not shown).
Figure 3.
Lipolysis is decreased in DM adipocytes. (A) Lipolysis is impaired in DM adipocytes. Adipocytes differentiated from VAT and SAT preadipocytes from subjects with DM or subjects without DM were cultured for 72 hours with or without isoproterenol (3 mM) and then studied with glycerol release assay. Ordinate: Age- and sex-adjusted glycerol concentration in cell culture supernatant (mg/mL) normalized to protein concentration of cell lysate (mg/mL). *P < 0.050 comparing NDM and DM groups, independent t test, age- and sex-adjusted (n = 20 subjects with DM and n = 19 subjects without DM for VAT; n = 13 subjects with DM and n = 12 subjects without DM for SAT). (B) Lipolysis correlates inversely with HbA1c. Linear regression analysis correlating HbA1c with isoproterenol-stimulated lipolysis in VAT adipocytes (left) and basal lipolysis in SAT adipocytes (right). Ordinate: Glycerol release (mg/dL) normalized to cell lysate protein concentration (mg/mL). P values shown are univariate (uv) and age- and sex adjusted (a/g). HbA1c did not correlate with basal lipolysis in VAT adipocytes or with isoproterenol-stimulated lipolysis in SAT adipocytes (data not shown); age did not correlate with basal or isoproterenol-stimulated lipolysis in VAT or SAT adipocytes (data not shown). (C) 3H-acetate incorporation as a measure of lipogenesis. Human VAT adipocytes cultured 72 hours with 3H-acetate and insulin, insulin + C75 (FASN inhibitor), or no insulin, followed by cell lysis, extraction, and scintillation counting of lipid phase. Ordinate: Lipogenesis measured by 3H-acetate incorporation into lipid fraction of cell lysate (cpm) normalized to cell lysate protein concentration (mg/mL). *P < 0.050 comparing insulin arm with insulin + C75 or no insulin arms, paired t test, age-adjusted (n = 12 subjects with DM and n = 12 subjects without DM). (D) Lipogenesis is similar in DM and NDM adipocytes. Adipocytes differentiated from VAT and SAT preadipocytes from DM or NDM subjects cultured for 72 hours with 3H-acetate and insulin followed by cell lysis, extraction, and scintillation counting of lipid phase. Ordinate: cpm in lipid fraction normalized to cell lysate protein concentration (mg/mL). NS designates P > 0.100 comparing NDM and DM groups, independent t test, age- and sex adjusted (n = 12 subjects with DM and n = 11 subjects without DM for VAT; n = 11 subjects with DM and n = 10 subjects without DM for SAT).
Lipogenic capacity was evaluated using 3H-acetate incorporation, which was induced by insulin and reduced by C75, a small molecule inhibitor of fatty acid synthase [Fig. 3(C)]. Age- and sex-adjusted adipocyte lipogenesis did not differ between subjects with DM and subjects without DM in VAT or SAT [Fig. 3(D)]. Linear regression analysis demonstrated no correlations between HbA1c levels or age and lipogenesis in VAT or SAT adipocytes (data not shown).
Together these data demonstrate a DM-specific adipocyte metabolic phenotype characterized by defects in glucose uptake and lipolysis.
ECM regulates adipocyte metabolism
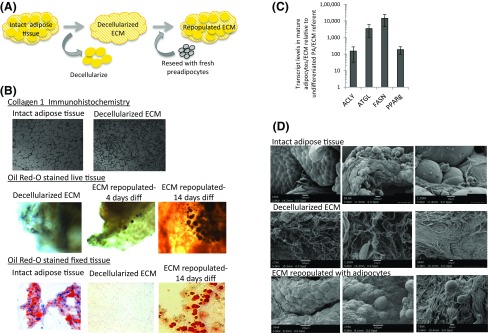
We next studied ECM regulation of adipocyte metabolism using an in vitro 3D culture model using decellularized human adipose tissue as a substrate for preadipocyte growth and adipocyte differentiation. We focused study on ECM and preadipocytes derived from VAT, given its disproportionate role in metabolic disease. Decellularized ECM retains collagen microarchitecture and supports differentiation of preadipocytes into mature adipocytes (Fig. 4).
Figure 4.
3D-ECM adipocyte culture. (A) ECM-AD model. Adipose tissue was decellularized and then repopulated with preadipocytes that are subsequently differentiated within the ECM. (B) Decellularized adipose tissue ECM maintains collagen 1 microarchitecture and supports adipocyte differentiation. Top: Collagen 1 immunohistochemistry of whole human VAT before and after decellularization demonstrating maintenance of microarchitecture. Middle: 3D confocal photomicrographs of live human adipocytes within ECM; intact decellularized human VAT stained with Oil Red-O before reseeding with preadipocytes and 4 days and 14 days after seeding with preadipocytes followed by adipogenic differentiation. Blue: DAPI staining of cell nuclei; red: Oil Red-O staining of intracellular lipid. Bottom: Formalin-fixed, paraffin-embedded, 5-μM–sectioned, Oil Red-O–stained human VAT prior to decellularization, immediately after decellularization, and after decellularization, preadipocyte-seeding, and 14 days of adipogenic differentiation, demonstrating cytoplasmic lipid accumulation in adipocytes within ECM. (C) Adipocytes in ECM upregulate adipogenic gene expression. Quantitative real-time PCR data comparing adipogenic gene transcript levels in RNA from human adipocytes differentiated in ECM for 14 days to undifferentiated preadipocytes (PA) in ECM cultured for 72 hours in nonadipogenic media. Ordinate: Fold difference in transcript level in mature adipocytes relative to undifferentiated preadipocyte referent = 1; all fold differences were significant (P < 0.001, paired t test). ECM from 10 subjects; preadipocytes/adipocytes from 11 subjects. (D) Adipose tissue ECM repopulated with adipocytes. Scanning electron micrographs of whole VAT, decellularized VAT, and decellularized VAT seeded with preadipocytes followed by 14 days of adipogenic differentiation.
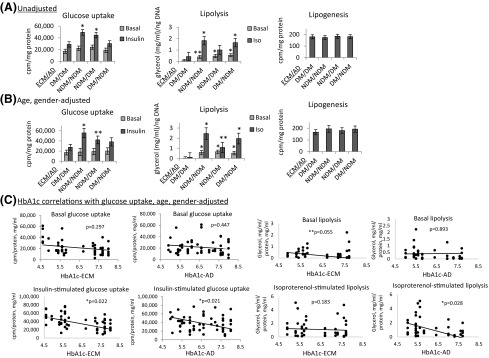
To examine the role of ECM-derived signals in regulating the adipocyte phenotype, we compared the effect of ECM from DM and NDM VAT on adipocyte metabolic functions. ECM from subjects with DM reconstituted with preadipocytes from subjects with DM (DM/DM ECM-AD), when compared with ECM from subjects without DM reconstituted with preadipocytes from subjects without DM (NDM/NDM ECM-AD), recapitulated observations in 2D culture of decreased basal and insulin-stimulated glucose uptake and decreased isoproterenol-stimulated lipolysis [Fig. 5(A) and 5(B)]. Discordance was observed between 2D and 3D-ECM culture with respect to basal lipolysis, in that DM/DM ECM-AD demonstrated reduced basal lipolysis compared with NDM/NDM ECM-AD [Fig. 5(A)]; in contrast, no difference was observed in basal lipolysis between DM and NDM VAT adipocytes in 2D culture. Controls of “empty” ECM not seeded with preadipocytes demonstrated negligible glucose uptake, lipolysis, and lipogenesis (data not shown).
Figure 5.
ECM regulates adipocyte metabolism. (A, B) 3D-ECM from subjects with DM or subjects without DM seeded with preadipocytes from subjects with DM or subjects without DM, differentiated into adipocytes, and studied with metabolic phenotyping. Data bars are labeled with patient source (NDM, DM) of ECM and adipocytes (ECM/AD) (e.g., NDM/NDM denotes both ECM and preadipocytes derived from patients without DM; NDM/DM denotes ECM from patients without DM combined with preadipocytes from patients with DM). Ordinates: Glucose uptake (cpm) normalized to ECM/cell lysate protein concentration (mg/mL). Lipolysis: culture supernatant glycerol concentration (mg/mL) normalized to ECM/cell lysate DNA concentration (ng/mL). Lipogenesis: 3H-acetate incorporation into lipid fraction of ECM/cell lysate (cpm) normalized to ECM/cell lysate protein concentration (mg/mL). *P < 0.050, **P < 0.100 comparing the indicated data point with the corresponding data point (basal or insulin-stimulated for glucose uptake, basal or isoproterenol-stimulated for lipolysis) in the DM/DM arm using mixed model analysis adjusting for repeated measures (n = 9 subjects without DM and 8 subjects with DM for ECM; n = 10 subjects without DM and 9 subjects with DM for preadipocytes for glucose uptake; n = 6 subjects without DM and n = 6 subjects with DM for ECM; n = 6 subjects without DM and n = 6 subjects with DM for preadipocytes for lipolysis; n = 6 subjects without DM and 6 subjects with DM for ECM; n = 6 subjects without DM and n = 6 subjects with DM for preadipocytes for lipogenesis. (A) Unadjusted data. (B) Data adjusted age and sex for both cell and ECM arms. (C) Correlations of glucose uptake and lipolysis, with HbA1c of subjects from whom ECM was derived (HbA1c-ECM) or HbA1c from whom adipocytes were derived (HbA1c-AD), adjusting for age and sex of subjects from whom ECM were derived for HbA1c-ECM analysis and adjusting for age and sex of subjects from whom adipocytes were derived for HbA1c-AD analysis.
We next examined the hypothesis that ECM can exert DM-specific effects on adipocyte metabolism by differentiating preadipocytes from DM and NDM VAT in different ECM environments (i.e., ECM from DM and NDM VAT). Differentiation of preadipocytes from subjects with DM in NDM ECM (NDM/DM ECM-AD) fully rescued insulin-stimulated glucose uptake and basal lipolysis to levels similar to those observed in NDM/NDM ECM-AD. The glucose uptake rescue effect was attenuated and approached significance after adjusting for age and sex of subjects from whom ECM and preadipocytes were derived, whereas rescue of basal lipolysis remained significant with adjustment of data for age and sex. Furthermore, adjustment for age and sex revealed a partial rescue effect of isoproterenol-stimulated lipolysis by NDM ECM on DM preadipocytes (NDM/DM ECM-AD) that was not statistically significant in unadjusted analysis [Fig. 5(A) and 5(B)].
Conversely, DM ECM inhibited insulin-stimulated glucose uptake in NDM adipocytes, evidenced by experiments in which preadipocytes from subjects without DM were differentiated in DM ECM (DM/NDM ECM-AD), in which insulin-stimulated glucose uptake was suppressed to levels similar to those observed in DM/DM ECM-AD; this effect remained significant after adjustment for age and sex. In contrast, differentiation of preadipocytes from subjects without DM in DM ECM (DM/NDM ECM-AD) did not decrease basal or isoproterenol-stimulated lipolysis. Finally, basal glucose uptake and lipogenesis were not different between DM/DM and NDM/NDM ECM-AD, and these functions were not regulated by any combination of ECM and adipocytes [Fig. 5(A) and 5(B)]. Taken together, these results demonstrate that the ECM regulates adipocyte metabolism in a disease-specific manner. NDM ECM rescues glucose uptake and basal lipolysis in DM adipocytes, and DM ECM impairs glucose uptake, but not lipolysis, in NDM adipocytes.
To further define the relationship of ECM-adipocyte interactions with DM status, we studied correlations of HbA1c as a continuous variable of subjects from whom ECM was derived (HbA1c-ECM) or subjects from whom adipocytes were derived (HbA1c-AD), with metabolic outcome measures, while controlling for age and sex. Both HbA1c-ECM and HbA1c-AD correlated inversely with insulin-stimulated glucose uptake, whereas HbA1c-ECM correlated inversely with basal lipolysis and HbA1c-AD correlated inversely with isoproterenol-stimulated lipolysis [Fig. 5(C)]. No correlations were observed between HbA1c-ECM or HbA1c-AD and basal glucose uptake or lipogenesis (data not shown). These data suggest that the diabetic status of the patients from whom ECM was derived predominantly dictates basal lipolysis, the diabetic status of the patients from whom adipocytes were derived predominantly dictates isoproterenol-stimulated lipolysis, and the diabetic status of both ECM and adipocytes regulates glucose uptake.
To determine if ECM influences adipocyte differentiation, transcript levels of adipogenic genes (ATGL, ACLY, FASN, PPAR-γ) were studied in differentiated ECM-AD tissues. No significant differences in any transcript levels were observed between DM/DM, NDM/NDM, NDM/DM, and DM/NDM ECM-AD groups (data not shown), suggesting that differences in differentiation are not a dominant mechanism underlying ECM-adipocyte interactions in the 3D ECM-AD model.
Discussion
Adipocyte metabolic phenotype in DM
The specific aberrations in adipocyte metabolism associated with human metabolic disease are poorly defined. We characterize the adipocyte metabolic phenotype associated with DM in obese humans. We confirm impaired glucose uptake as a feature of this phenotype, consistent with prior studies (16–18). The relationship between adipocyte lipogenic phenotype and systemic metabolic disease is controversial. Prior data demonstrate decreased adipose tissue lipogenic gene expression in patients with metabolic disease (19–21), although at least 1 study demonstrates the opposite (22). Many published reports study gene expression rather than functional assays. Using labeled acetate incorporation into fatty acids, we demonstrate no DM-specific differences in adipocyte lipogenesis in 2D or 3D ECM culture, suggesting that alterations in de novo lipogenesis do not significantly contribute to adipocyte metabolic dysfunction in DM. These results do not address other modes of lipid storage in adipocytes, such as triacylglycerol synthesis.
It is relatively well established that adipose tissue basal lipolysis is increased and that β-adrenergic–stimulated lipolysis is decreased in human obesity (23–25). The adipose tissue lipolytic phenotype specific to metabolic disease is less well defined, with some studies demonstrating increased adipocyte lipolysis in metabolic disease (26, 27) and others demonstrating the opposite (28–31). We demonstrate decreased basal lipolysis in SAT adipocytes and decreased β-adrenergic–stimulated lipolysis in VAT and SAT adipocytes in subjects with DM, suggesting that a lipolytic defect characterizes adipocytes in DM. Transgenic manipulations in mice that increase adipocyte lipolysis are associated with favorable metabolic phenotypes (32–34), and gene expression data suggest that decreased lipid processing capacity characterizes insulin-resistant adipose tissue (35, 36). Taken together, these observations, along with the established relationship between adipocyte hypertrophy and metabolic disease (4, 37, 38), support our data and suggest that DM is associated with a shift in adipocytes toward a phenotype predisposed to lipid storage (hypertrophy) rather than lipid catabolism (lipolysis).
ECM regulation of adipocyte metabolism
We and others have demonstrated aberrancies in adipose tissue fibrosis in obesity (1–6), prompting us to explore the role of the ECM in regulating adipocyte metabolism using an in vitro model of decellularized human adipose tissue ECM repopulated with preadipocytes that are differentiated into adipocytes within the ECM. Precedent for this model exists because decellularized tissue models demonstrate independent effects of pulmonary ECM on myofibroblast function (8).
Combining ECM and adipocytes from subjects with DM and subjects without DM (i.e., NDM/NDM or DM/DM ECM-AD combinations) recapitulates the metabolic phenotype of isolated adipocytes in standard 2D culture but with important differences. For example, we observed concordance between 2D culture and 3D-ECM culture with respect to DM-specific differences in glucose uptake and β-adrenergic–stimulated lipolysis but discordance with respect to basal lipolysis, which was similar between DM and NDM adipocytes in 2D culture but decreased in DM/DM ECM-AD compared with NDM/NDM ECM-AD in ECM culture. These data suggest that the ECM disproportionately contributes to DM-specific decreases in basal lipolysis, differences that are notably absent in adipocytes in 2D culture in the absence of ECM. In contrast, basal (noninsulin stimulated) glucose uptake was decreased in DM adipocytes in 2D culture but was similar between DM/DM ECM-AD and NDM/NDM ECM-AD in ECM culture, suggesting that the ECM attenuates defects in basal glucose uptake in the context of DM. Correlations of HbA1c of subjects from whom ECM or adipocytes were derived with glucose uptake and lipolysis reinforce these findings, revealing that diabetic status of ECM and adipocytes independently contribute to different features of ultimate adipose tissue metabolic phenotype. These observations demonstrate the utility of this model in dissecting distinct effects of ECM on adipocyte metabolism and speak to distinct roles for ECM and adipocytes in regulating different DM-specific features of cellular metabolism.
Most importantly, we demonstrate DM-specific effects of the ECM on adipocyte metabolism: NDM ECM restored insulin-stimulated glucose uptake in DM adipocytes, whereas DM ECM reduced insulin-stimulated glucose uptake in NDM adipocytes. Similarly, NDM ECM fully restored basal lipolysis and partially restored β-adrenergic–stimulated lipolysis in DM adipocytes, but, conversely, DM ECM had no effect on lipolysis in NDM adipocytes. Finally, no differences in basal glucose uptake or lipogenesis were observed with any combination of ECM adipocytes, suggesting that the ECM does not regulate these functions in this model system. Taken together, these results demonstrate that NDM ECM has the capacity to restore some, but not all, features of normal cellular metabolism in DM adipocytes, whereas DM ECM has the capacity to induce some but not all features of dysfunctional cellular metabolism in NDM adipocytes.
In our cohort, the subjects with DM were older and more of them were men than were the subjects without DM, requiring post hoc age and sex adjustment of data. Nonetheless, differences in metabolic outcomes between DM and NDM adipocytes in 2D and 3D-ECM culture remained significant after adjustment, except for insulin-stimulated glucose uptake in 3D-ECM culture. In contrast, the rescue effect of NDM ECM on lipolysis was strengthened by age and sex adjustment, primarily due to decreases in basal and β-adrenergic–stimulated lipolysis in DM/DM tissues. These observations suggest that age- and sex-related differences in ECM contribute to its effects on adipocyte metabolism independent of DM status. Subjects with DM had a higher prevalence of other metabolic diseases and medications, factors that may contribute to different patient-specific effects independent of DM. Subject heterogeneity is an inherent weakness of human studies, and larger studies are necessary to rigorously address these and other potential confounders. Limited access to lean tissues precluded inclusion of a lean control group, preventing study of obesity-specific ECM effects. Nonetheless, ECM effects differed based on the presence or absence of metabolic disease, supporting the clinical relevance of these data. The ECM model lacks multiple adipose tissue constituents but permits isolated study of specific effects of ECM on adipocytes. We focused study on VAT ECM and adipocytes because of the strong association of VAT with metabolic disease and limitations in the quantities of SAT available. Future research will incorporate other cell types (e.g., macrophages, leukocytes, endothelial cells) and adipose tissue depots into the ECM model, which provides a tractable system to study multiple aspects of adipose tissue biology.
The mechanisms underlying ECM-adipocyte interactions remain unknown. ECM may regulate preadipocyte differentiation with effects on ultimate adipocyte metabolic phenotype. Nonetheless, Oil Red-O staining and adipogenic gene expression were similar between DM and NDM adipocytes in standard 2D culture, and no difference in adipogenic gene expression was observed between groups in 3D-ECM culture. These data argue against a significant intrinsic difference in differentiation capacity between DM and NDM adipocytes or regulation of adipocyte differentiation by the ECM; further study of other aspects of differentiation is necessary to confirm these observations. ECM interactions with inflammatory cells may influence adipocytes; we have demonstrated differences in IL-4 expression, a profibrotic cytokine, between DM and NDM tissues that correlate with differences in ECM composition (4), suggesting a possible mechanism linking inflammation to fibrosis. Finally, DM-specific alterations in the ECM itself, such as increased advanced glycation end-product glycosylation, may regulate adipocyte metabolism. The ECM-adipocyte culture model provides a tool to study these and other putative mechanisms.
Conclusion
We demonstrate that human DM is associated with an adipocyte metabolic phenotype characterized by decreased glucose uptake and decreased lipolysis and that the ECM regulates adipocyte metabolism: NDM ECM partially rescues metabolic dysfunction in metabolically unhealthy adipocytes, whereas DM ECM impairs certain features of metabolic function in metabolically healthy adipocytes. These observations suggest the ECM as a target for research directed toward manipulating adipocyte metabolism.
Acknowledgments
The authors thank the University of Michigan Center for Statistical Consultation & Research for statistical consultation; Eric White, University of Michigan Department of Internal Medicine for advice and discussion; Colleen Buda, PA, Justin Fahey, PA, Danielle Guerin, NP, Kendra Rogers, PA, and Marilyn Woodruff, NP, for assistance with study coordination. Scanning electron microscopy was performed by University of Michigan Microscopy & Image Analysis Laboratory Biomedical Research Core Facility.
Acknowledgments
This work was supported by National Institutes of Health Grants R01DK097449 (to R.W.O.), R01DK090262 (to C.N.L.), and T32DK101357 (to L.A.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 2D
- 2-dimensional
- 3D
- 3-dimensional
- cpm
- counts per minute
- AD
- adipocytes
- DM
- diabetes mellitus
- DMEM
- Dulbecco’s modified Eagle medium
- ECM
- extracellular matrix
- FCS
- fetal calf serum
- HbA1c
- hemoglobin A1c
- NDM
- non–diabetes mellitus
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PMSF
- phenylmethanesulphonylfluoride
- SAT
- subcutaneous adipose tissue
- VAT
- visceral adipose tissue
References
- 1.Dankel SN, Svärd J, Matthä S, Claussnitzer M, Klöting N, Glunk V, Fandalyuk Z, Grytten E, Solsvik MH, Nielsen HJ, Busch C, Hauner H, Blüher M, Skurk T, Sagen JV, Mellgren G. COL6A3 expression in adipocytes associates with insulin resistance and depends on PPARγ and adipocyte size. Obesity (Silver Spring). 2014;22(8):1807–1813. [DOI] [PubMed] [Google Scholar]
- 2.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, Scherer PE, Seay SA, McCoin CS, Bonaldo P, Adams SH. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306(3):E233–E246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN, O’Rourke RW. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring). 2016;24(3):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299(6):E1016–E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga J, Ploug T, An Z, Scherer PE. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun. 2014;5:3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, Neuman A. Diagnosis and Management of diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164(8):542–552. [DOI] [PubMed] [Google Scholar]
- 8.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186(9):866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han TT, Toutounji S, Amsden BG, Flynn LE. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials. 2015;72:125–137. [DOI] [PubMed] [Google Scholar]
- 10.Porzionato A, Sfriso MM, Macchi V, Rambaldo A, Lago G, Lancerotto L, Vindigni V, De Caro R. Decellularized omentum as novel biologic scaffold for reconstructive surgery and regenerative medicine. Eur J Histochem. 2013;57(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akie TE, Cooper MP. Determination of fatty acid oxidation and lipogenesis in mouse primary hepatocytes. J Vis Exp. 2015;102:e52982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Diaz S, Johnson LA, DeKroon RM, Moreno-Navarrete JM, Alzate O, Fernandez-Real JM, Maeda N, Arbones-Mainar JM. Polymerase I and transcript release factor (PTRF) regulates adipocyte differentiation and determines adipose tissue expandability. FASEB J. 2014;28(8):3769–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MJ, Fried SK. Optimal protocol for the differentiation and metabolic analysis of human adipose stromal cells. Methods Enzymol. 2014;538:49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Rourke RW, Meyer KA, Gaston G, White AE, Lumeng CN, Marks DL. Hexosamine biosynthesis is a possible mechanism underlying hypoxia’s effects on lipid metabolism in human adipocytes. PLoS One. 2013;8(8):e71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, Kirkland JL. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55(9):2571–2578. [DOI] [PubMed] [Google Scholar]
- 16.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106(36):15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman MA, Peroni OD, Villoria J, Schön MR, Abumrad NA, Blüher M, Klein S, Kahn BB. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484(7394):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd PR, Kahn BB. Glucose transporters and insulin action: implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341(4):248–257. [DOI] [PubMed] [Google Scholar]
- 19.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, Fraterrigo G, Okunade AL, Patterson BW, Klein S. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125(2):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulain-Godefroy O, Lecoeur C, Pattou F, Frühbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R1–R7. [DOI] [PubMed] [Google Scholar]
- 21.Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52(5):882–890. [DOI] [PubMed] [Google Scholar]
- 22.Berndt J, Kovacs P, Ruschke K, Klöting N, Fasshauer M, Schön MR, Körner A, Stumvoll M, Blüher M. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50(7):1472–1480. [DOI] [PubMed] [Google Scholar]
- 23.Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab. 2014;25(5):255–262. [DOI] [PubMed] [Google Scholar]
- 24.Bougnères P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D. In vivo resistance of lipolysis to epinephrine: a new feature of childhood onset obesity. J Clin Invest. 1997;99(11):2568–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enoksson S, Talbot M, Rife F, Tamborlane WV, Sherwin RS, Caprio S. Impaired in vivo stimulation of lipolysis in adipose tissue by selective beta2-adrenergic agonist in obese adolescent girls. Diabetes. 2000;49(12):2149–2153. [DOI] [PubMed] [Google Scholar]
- 26.Acosta JR, Douagi I, Andersson DP, Bäckdahl J, Rydén M, Arner P, Laurencikiene J. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560–570. [DOI] [PubMed] [Google Scholar]
- 27.Andersson DP, Löfgren P, Thorell A, Arner P, Hoffstedt J. Visceral fat cell lipolysis and cardiovascular risk factors in obesity. Horm Metab Res. 2011;43(11):809–815. [DOI] [PubMed] [Google Scholar]
- 28.Berndt J, Kralisch S, Klöting N, Ruschke K, Kern M, Fasshauer M, Schön MR, Stumvoll M, Blüher M. Adipose triglyceride lipase gene expression in human visceral obesity. Exp Clin Endocrinol Diabetes. 2008;116(4):203–210. [DOI] [PubMed] [Google Scholar]
- 29.Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab. 2007;92(6):2292–2299. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin T, Craig C, Liu LF, Perelman D, Allister C, Spielman D, Cushman SW. Adipose cell size and regional fat deposition as predictors of metabolic response to overfeeding in insulin-resistant and insulin-sensitive humans. Diabetes. 2016;65(5):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest. 1994;93(6):2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, Samuel VT, Shulman GI, Wang Y, Kang C, Sul HS. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58(4):855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Kim KH, de Val S, Kang C, Sul HS. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15(2):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh W, Abu-Elheiga L, Kordari P, Gu Z, Shaikenov T, Chirala SS, Wakil SJ. Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci USA. 2005;102(5):1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clemente-Postigo M, Queipo-Ortuño MI, Fernandez-Garcia D, Gomez-Huelgas R, Tinahones FJ, Cardona F. Adipose tissue gene expression of factors related to lipid processing in obesity. PLoS One. 2011;6(9):e24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soronen J, Laurila PP, Naukkarinen J, Surakka I, Ripatti S, Jauhiainen M, Olkkonen VM, Yki-Järvinen H. Adipose tissue gene expression analysis reveals changes in inflammatory, mitochondrial respiratory and lipid metabolic pathways in obese insulin-resistant subjects. BMC Med Genomics. 2012;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Klöting N, Grégoire C, Lolmede K, Blüher M, Clément K. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99(8):E1466–E1470. [DOI] [PubMed] [Google Scholar]
- 38.Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60(5):1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]