Abstract
Context:
Lifestyle approaches for preventing gestational diabetes mellitus (GDM) have produced mixed results.
Objective:
The aim of the present study was to compare the effectiveness of 3 lifestyle interventions [healthy eating (HE), physical activity (PA), and both HE and PA (HE+PA)] with usual care (UC) in reducing GDM risk.
Design:
The present study was a multicenter randomized controlled trial conducted from 2012 to 2014 [the DALI (vitamin D and lifestyle intervention for GDM prevention) lifestyle study].
Setting:
The study occurred at antenatal clinics across 11 centers in 9 European countries.
Patients:
Consecutive pregnant women at <20 weeks of gestation with a body mass index (BMI) of ≥29 kg/m2 and without GDM using the International Association of Diabetes and Pregnancy Study Group criteria (n = 436). For the intervention, women were randomized, stratified by site, to UC, HE, PA, or HE+PA. The women received 5 face-to-face and ≤4 telephone coaching sessions using the principles of motivational interviewing. A gestational weight gain (GWG) <5 kg was targeted. The coaches received standardized training and an intervention toolkit tailored to their culture and language.
Main Outcome Measures:
The endpoints were the GWG at 35 to 37 weeks and the fasting glucose and insulin sensitivity [homeostasis model assessment insulin resistance (HOMA-IR)] at 24 to 28 weeks.
Results:
We randomized 108 women to HE+PA, 113 to HE, 110 to PA, and 105 to UC. In the HE+PA group, but not HE or PA alone, women achieved substantially less GWG than did the controls (UC) by 35 to 37 weeks (−2.02; 95% confidence interval, −3.58 to −0.46 kg). Despite this reduction, no improvements were seen in fasting or postload glucose levels, insulin concentrations, or HOMA-IR. The birthweights and large and small for gestational age rates were similar.
Conclusions:
The combined HE+PA intervention was able to limit GWG but did not reduce fasting glycemia. Thus, lifestyle changes alone are unlikely to prevent GDM among women with a BMI of ≥29 kg/m2.
We studied pregnant women in a large European multicenter randomized controlled trial of physical activity and/or healthy eating and found no effect on gestational diabetes mellitus risk, despite the significant gestational weight gain limitation.
Gestational diabetes mellitus (GDM), a high prepregnancy body mass index (BMI), and gestational weight gain (GWG) have been independently associated with an increased risk of adverse perinatal outcomes, including macrosomia, operative delivery, and shoulder dystocia (1). In GDM, such complications have a continuous relationship with maternal glucose concentrations during the oral glucose tolerance test (OGTT) (2). With the increasing prevalence of obesity in pregnancy and GDM (3), it has become increasingly important to develop evidence-based clinical interventions that prevent the development of GDM and minimize excess GWG.
The development of type 2 diabetes through intensive lifestyle interventions can be reduced by 58% within 4 years in nonpregnant women who have previously had GDM (4). However, whether GDM can be prevented through antenatal lifestyle interventions, even with the limitation of excess GWG, has been disputed (5). Randomized controlled trials (RCTs) have provided variable evidence that lifestyle interventions “work” (6), likely because of different intervention protocols and study populations. Furthermore, no studies have yet assessed, within the same population and with the same protocol, which intervention strategy is superior for the prevention of GDM.
The “vitamin D and lifestyle intervention for GDM prevention” (DALI) (7, 8) has harmonized some of these sources of variation. It was a European multicenter RCT testing different approaches for the reduction in GDM risk. The study was unique with 2 arms: (1) the DALI lifestyle study, which compared healthy eating (HE), physical activity (PA), and combined HE and PA (HE+PA) interventions with a control group; and (2) the DALI vitamin D study comparing vitamin D supplementation with and without an HE+PA intervention. In the present report, we report on the DALI lifestyle study, which tested which of the lifestyle interventions was most efficacious in reducing GWG and fasting glucose and improving insulin sensitivity measured using the homeostasis model assessment-insulin resistance (HOMA-IR) (9).
Methods
Design and participants
The DALI lifestyle study was a multicenter RCT with a factorial study design conducted in 9 European countries [United Kingdom, Ireland, Netherlands, Austria, Poland, Italy (Padua and Pisa), Spain, Denmark (Odense and Copenhagen), and Belgium] in 2012 to 2015 (7, 8). The trial registration number was ISRCTN70595832. The local ethics committees approved the study. Pregnant women with a prepregnancy BMI of ≥29 kg/m2, ≤19 ± 6 days of gestation, a singleton pregnancy, and aged ≥18 years were invited to participate. The BMI cutoff was based on data from across the DALI sites to allow recruitment of sufficient women into the trial from countries with lesser rates of maternal obesity (10). The exclusions included a diagnosis of GDM using the OGTT using the International Association of Diabetes and Pregnancy Study Group (IADPSG)/World Health Organization (WHO) 2013 criteria (fasting venous plasma glucose ≥5.1 mmol/L and/or 1-hour glucose of ≥10 mmol/L and/or 2-hour glucose of ≥8.5 mmol/L) (11, 12), preexisting diabetes, chronic medical conditions (local investigator defined; e.g., valvular heart disease) or a psychiatric disorder, inability to walk ≥100 m safely, requirement for a complex diet, and/or not fluent in the major language of the country of recruitment or unable to have a conversation with the lifestyle coach in another language for which the intervention materials were available.
Procedures
The main trial was performed after a pilot study (8), a protocol review, and a standardization startup site visit and included staff standardization workshops every 6 months. The review resulted in no major protocol changes, other than separating the lifestyle and vitamin D limbs, because of the low randomization rates (owing to a high prevalence of GDM at baseline and lower than projected recruitment rates).
After the women provided written informed consent, they underwent assessments, including a 75-g OGTT (samples taken at 0, 60, 120 minutes), questionnaire, and weight before 20 weeks of gestation (baseline), at 24 to 28 weeks (visit 2), at 35 to 37 weeks (visit 3), and 48 hours after birth, as previously described (7, 8).
Local laboratories rapidly provided the OGTT results to assess study eligibility and to support referral for clinical care, if needed. The blood samples were handled in a standardized manner and stored at −20°C or −80°C until further analysis in the central trial laboratory in Graz, Austria. If GDM developed after baseline, women were managed according to local practice. A diagrammatic overview of the assessments and intervention is shown in Supplemental Figure 1 (4.5MB, tif) .
Randomization and blinding
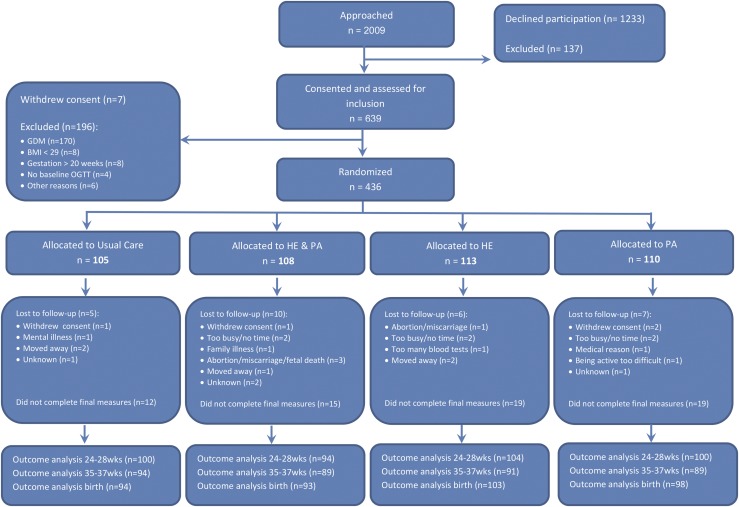
Randomization to either HE+PA, HE, PA, or UC (Fig. 1) was performed using a computerized electronic random number generator, prestratified for site. The trial coordinator (D.S.) prepared and distributed sealed opaque envelopes, containing group allocations to each site. The allocation outcome was communicated to the participants by the coach. The staff involved with measurements, but not the participants, were kept unaware of the intervention. Statistical analyses were performed blinded for allocation.
Figure 1.
CONSORT diagram of recruitment, randomization, and withdrawal of the DALI lifestyle trial.
Lifestyle interventions
After randomization, women were assigned a lifestyle coach, and the individual sessions were scheduled for each participant (7, 8). The coaching involved discussion of 7 HE and/or 5 PA “messages” according to previous work (13) (Supplemental Table 1 (14.1KB, docx) ). The HE intervention promoted a food-based, lower simple and complex carbohydrate, lower fat, higher fiber, higher protein diet, including a focus on portion size and, therefore, a more limited intake of total calories. The PA intervention promoted both aerobic and resistance physical activity. All interventions recommended a limitation in GWG to 5 kg. The messages were supported by a “toolkit” for each participant, including the participant handbook, educational materials [e.g., adapted FITT model (frequency, intensity, time, type)] based on the American College of Obstetricians and Gynecologists guidelines (14), pedometers (Digiwalker SW-200; Yamax, Tokyo, Japan), and flexible elastic Dyna-Bands (Thera-Band, Akron, OH). The message delivery was built on the principles of patient empowerment and cognitive behavioral techniques inspired by motivational interviewing (15). The number of contacts and time taken were expected to be the same, independent of the allocated intervention and included 5 face-to-face sessions of approximately 30 to 45 minutes duration, and ≤4 telephone calls of ≤20 minutes or contacts using electronic mail. Face-to-face sessions occurred largely in the hospital or midwife practice, depending on the local arrangements. At least 4 face-to-face coaching sessions were expected to occur before the second measurement session (24 to 28 weeks), and the intervention was completed by 35 weeks of gestation.
Standardization of the lifestyle intervention was achieved through a coach training program, which concluded with an observed session with an actor, provision of a desk-file including all materials and methods, and the use of a personal digital assistant (PDA; HTC HD7 Windows Phone 7; Microsoft Corp., Redmond, WA), with made to order software to provide a framework for the session. A “paper” PDA, including all fields, was available if any problems occurred with the PDA. The standardization sessions continued approximately every 6 months through the trial.
A key component was for women to strive to achieve a maximum GWG of 5 kg, as the lower limit of the recommended weight gain by the Institute of Medicine for those with a BMI of ≥30 kg/m2 (16), and the observation of better outcomes among Danish obese women with this degree of GWG (17). The same weight target was used for the women with a BMI of 29.0 to 29.9 kg/m2 to avoid complicating the intervention protocol. If the GWG was already beyond this before the start of, or during, the intervention, the advice was to maintain this weight throughout the remaining pregnancy. The coaches had scales available to assist women with their weight management, if weight scales were not available in the home.
Assessments
Information on demographics, prepregnancy weight, maternal/paternal smoking, alcohol consumption, medical and obstetric history, and medication use was gathered by questionnaire. Women underwent 3 fasting assessments and undertook a standardized, sitting, 75-g OGTT, with blood samples taken at 0, 60, and 120 minutes after glucose ingestion. The women completed the questionnaire and anthropometric measurements between the blood tests. Local laboratories were used to rapidly obtain the results to the OGTT to assess eligibility for the study and to support referral for clinical care, if needed. The blood samples were centrifuged and separate serum and plasma aliquots (1000 µL or 250 µL) placed in microrack tubes and stored at −20°C or −80°C until further analysis in the central trial laboratory in Graz, Austria, certified according to the International Organization for Standardization 9001 standards.
Glucose was measured using the hexokinase method (DiaSys Diagnostic Systems, Holzheim, Germany) with a lower limit of sensitivity of 0.1 mmol/L. The central values are used for trial reporting.
Insulin was quantified by a sandwich-immunoassay (ADVIA Centaur; Siemens Health Care Diagnostics Inc., Vienna, Austria) with an analytical sensitivity of 0.5 mU/L, intra-assay coefficient of variation of 3.3% to 4.6%, and interassay coefficient of variation of 2.6% to 5.9%. All assays were performed following the instructions of the manufacturer. The HOMA-IR was calculated as follows: (glucose × insulin)/22.5 (9).
Height was measured at baseline using a stadiometer (SECA Leicester Height Measure 206; SECA, Birmingham, UK), and the average value of 2 measurements was used. The women were weighed on calibrated electronic scales (SECA Measure 888; 877) wearing no shoes and light clothes to the nearest 0.1 kg. The average value of 2 measurements was used. Weight gain was defined as the change in objectively measured weight, and the weight was calculated for 3 periods: baseline to 24 to 28 weeks, baseline to 35 to 37 weeks, and from 24 to 28 to 35 to 37 weeks.
Data from the medical records were obtained regarding comorbidities, obstetric and perinatal outcomes, and birth weight.
Effectiveness of lifestyle interventions
The effectiveness of the PA intervention was assessed using the Pregnancy Physical Activity Questionnaire (18) at the 3 time points. The original Pregnancy Physical Activity Questionnaire consisted of 32 activities, including household/caregiving, occupational, sports/exercise, and inactivity measured during the current trimester. Open-ended questions allowed the respondent to add activities not already listed. In the DALI study, 2 questions for cycling to work [4.0 metabolic equivalent of task (MET)] and cycling for fun or exercise (8.0 MET) were added owing to the frequent performance of these activities in some European countries. Participants were asked to select the category that best approximated the amount of time spent in 1 of these activities. The duration of time spent in each activity was multiplied by its intensity such that an average weekly energy expenditure (MET hours per week) was calculated for each activity. For the open-ended reported activities, a compendium of physical activities was used to obtain MET values for intensity (19). Activities were categorized by intensity (sedentary, light, moderate, vigorous), type and total activity (sum of all activity with an intensity >1.5 MET). Self-reported moderate and vigorous activity were summed and presented as MET hours per week of moderate-to-vigorous PA (MVPA).
Nutrition was assessed using a made to order short food frequency questionnaire covering key foods linked to the intervention messages and based on previous work (20). The number of portions per week for each key food component was calculated as the product of the frequency consumed per week and the number of portions each episode.
Outcomes
The GWG at 35 to 37 weeks and fasting glucose and HOMA-IR at 24 to 28 weeks were primary outcomes. GWG was defined as the change in objectively measured weight from baseline, because some women were unable to recall their prepregnancy weight accurately. The HOMA-IR was then calculated (9).
The secondary outcomes included physical activity, nutrition, glucose concentrations 1 and 2 hours after glucose ingestion, fasting insulin concentrations, insulin levels at 1 and 2 hours after glucose ingestion, GDM, birth weight, gestational age, and small-for-gestational age (SGA) or large-for-gestational age (LGA) infants.
The data from the medical records included comorbidity, obstetric, birthweight and perinatal outcomes, and resource data.
Statistical analysis
The sample size calculations have been previously reported (7) but were repeated based on the mean ± standard deviation found in the pilot study (8). Based on the pilot study data, the numbers needed in each intervention arm were 41 women to detect a GWG difference of 4 kg (mean, 10 ± 5.8 kg); 19 women to measure a fasting glucose difference of 0.3 mmol/L (mean, 4.8 ± 0.4 mmol/L), and 220 women to find a difference of 0.44 for the HOMA-IR (mean, 3.0 ± 1.8).
The insulin level and HOMA-IR were log transformed because of skewness. For the 35- to 37-week assessment, fasting glucose and HOMA-IR were carried forward from 24 to 28 weeks if GDM was diagnosed, and women were excluded from the GWG analyses, because the treatments (e.g., diet and/or insulin treatment) could influence weight gain. For sensitivity analyses of the intervention effects, missing data were multiply imputed, stratified by treatment group (21). Using predictive mean matching, 20 complete data sets were created (i.e., loss of efficiency <0.05) (21). The data sets were analyzed separately, and the pooled estimates were calculated using Rubin’s rules (22).
Data were analyzed according to intention to treat and according to an a priori statistical analysis plan. Differences between subjects withdrawing from the study and those who stayed in the study were assessed using the t test (normally distributed continuous variables), Mann-Whitney U test (skewed continuous variables), or χ2 test (categorical variables). To assess differences in outcomes at 24 to 28 and 35 to 37 weeks of gestation between intervention groups, multilevel analyses were performed, using 2 levels (site and individual). The analyses with glucose and insulin as the outcomes were adjusted for baseline values. Models with weight gain variables were adjusted for maternal BMI at baseline. The birth weight was adjusted for gestational age at birth. Two-sided P < .05 was taken as statistically significant. Comparisons between the intervention groups demonstrated interactions regarding GWG; hence, the factorial analysis (7) was not reported.
Analyses were performed in SPSS, version 22, except for multilevel analyses, which were performed using MLwiN, version 2.22.
Results
Figure 1 shows the Consolidated Standards of Reporting Trials diagram. The numbers randomized and the baseline characteristics in each group were comparable (Table 1). The gestational age on entry ranged from 8 to 19 ± 6 weeks. Women who withdrew before the final OGTT (n = 73; 17%) had higher rates of chronic hypertension and previous GDM (Supplemental Table 2 (16.4KB, docx) ).
Table 1.
Baseline Characteristics of All Women Included per Intervention Group
| Variable | UC (n = 105) | HE+PA (n = 108) | HE (n = 113) | PA (n =110) | Total (N = 436) |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 31.8 ± 5.6 | 31.9 ± 5.3 | 32.5 ± 5.5 | 31.7 ± 5.1 | 32.0 ± 5.4 |
| Multiparous, n (%) | 50 (48) | 56 (52) | 64 (57) | 51 (46) | 221 (51) |
| European descent, n (%) | 94 (90) | 95 (88) | 95 (84) | 94 (86) | 378 (87) |
| Lives with partner, n (%) | 100 (95) | 99 (92) | 108 (96) | 103 (94) | 410 (94) |
| Higher education, n (%) | 55 (52) | 59 (55) | 65 (58) | 60 (55) | 239 (55) |
| Maternal smoking, n (%) | 18 (17) | 11 (10) | 20 (18) | 18 (17) | 67 (15) |
| Paternal smoking, n (%) | 33 (32) | 29 (27) | 42 (38) | 39 (36) | 143 (33) |
| History of GDM, n (%) | 3 (5) | 4 (6) | 6 (7) | 4 (6) | 17 (6) |
| First degree FH DM, n (%) | 28 (27) | 18 (17) | 28 (25) | 27 (25) | 101 (23) |
| Chronic hypertension, n (%) | 9 (9) | 12 (11) | 17 (15) | 17 (16) | 55 (13) |
| Gestation on entry (wk), mean ± SD | 15.2 ± 2.4 | 15.2 ± 2.2 | 15.3 ± 2.5 | 15.5 ± 2.3 | 15.3 ± 2.3 |
| Prepregnancy weight (kg), mean ± SD | 92.0 ± 11.5 | 93.3 ± 13.7 | 92.5 ± 13.6 | 92.7 ± 13.4 | 92.6 ± 13.1 |
| Weight at entry (kg), mean ± SD | 94.2 ± 12.6 | 95.2 ± 13.8 | 94.9 ± 13.2 | 94.6 ± 12.8 | 94.7 ± 13.1 |
| Height (cm), mean ± SD | 165.9 ± 6.7 | 166.0 ± 6.6 | 165.2 ± 6.6 | 165.6 ± 7.2 | 165.7 ± 6.8 |
| BMI prepregnancy (kg/m2), mean ± SD | 33.4 ± 3.5 | 33.8 ± 3.9 | 33.9 ± 4.4 | 33.7 ± 4.0 | 33.7 ± 4.0 |
| BMI at entry (kg/m2), mean ± SD | 34.2 ± 3.9 | 34.5 ± 4.0 | 34.7 ± 4.2 | 34.4 ± 3.8 | 34.5 ± 4.0 |
| Fasting glucose (mmol/L), mean ± SD | 4.69 ± 0.35 | 4.62 ± 0.34 | 4.61 ± 0.38 | 4.55 ± 0.39 | 4.6 ± 0.4 |
| 1-h Glucose (mmol/L), mean ± SD | 7.0 ± 1.3 | 6.8 ± 1.2 | 6.7 ± 1.5 | 6.6 ± 1.4 | 6.8 ± 1.4 |
| 2-h Glucose (mmol/L), mean ± SD | 5.9 ± 1.1 | 5.9 ± 1.1 | 5.9 ± 1.1 | 5.7 ± 1.1 | 5.8 ± 1.1 |
| Fasting insulin (mU/L), median (rangea) | 12.9 (9.6–17.7) | 12.8 (10.3–17.2) | 13.0 (9.0–17.8) | 11.7 (10.1–15.3) | 12.7 (9.9–17.1) |
| 1-h Insulin (mU/L), median (rangea) | 89.9 (56.7–157.8) | 85.6 (60.6–158.8) | 87.3 (47.6–149.7) | 85.7 (53.1–143.7) | 87.8 (55.3–153.3) |
| 2-h Insulin (mU/L), median (rangea) | 59.4 (39.9–91.0) | 60.4 (46.8–100.0) | 53.5 (39.9–88.0) | 55.8 (40.0–77.8) | 57.0 (41.3–90.0) |
| HOMA-IR, median (rangea) | 2.8 (2.0–3.7) | 2.6 (2.1–3.5) | 2.7 (1.8–3.8) | 2.4 (2.0–3.1) | 2.6 (2.0–3.5) |
Abbreviations: FH DM, family history of diabetes; SD, standard deviation.
Range from 25th to 75th percentile.
The GWG, glucose, insulin, and HOMA-IR at 24 to 28 and 35 to 37 weeks of gestation are listed in Table 2. Compared with UC, the HE+PA intervention was associated with significantly lower GWG but similar fasting glucose and HOMA-IR at both 24 to 28 and 35 to 37 weeks (Table 3). Neither HE nor PA alone achieved substantial improvements in GWG, fasting glucose, or HOMA-IR compared with UC (Table 3). The fasting glucose was greater in the HE compared with US at both time points. The results of the sensitivity analysis with imputed data were fully in line with these findings (data not shown).
Table 2.
Primary and Secondary Outcomes in 4 Intervention Groups and Intervention Effects at 24 to 28 and 35 to 37 Weeks and at Birth
| Variable |
UC Group |
HE+PA Group |
HE Group |
PA Group |
||||
|---|---|---|---|---|---|---|---|---|
| n | Value | n | Value | n | Value | n | Value | |
| 24-28 wk | ||||||||
| Weight gain T1-T2 (kg), mean ± SDa | 100 | 4.3 ± 2.6 | 97 | 3.1 ± 2.4 | 102 | 3.5 ± 2.9 | 102 | 4.3 ± 3.0 |
| Fasting glucose (mmol/L), mean ± SD | 97 | 4.61 ± 0.41 | 89 | 4.63 ± 0.39 | 104 | 4.72 ± 0.45 | 98 | 4.58 ± 0.41 |
| HOMA-IR, median (rangeb) | 94 | 2.9 (2.2–4.2) | 87 | 2.9 (2.4–3.6) | 103 | 3.0 (2.2–4.1) | 97 | 2.9 (2.2–4.2) |
| Secondary variables | ||||||||
| 1-h Glucose (mmol/L), mean ± SD | 99 | 7.8 ± 1.5 | 91 | 7.9 ± 1.6 | 104 | 7.8 ± 1.8 | 96 | 7.6 ± 1.8 |
| 2-h Glucose (mmol/L), mean ± SD | 100 | 6.3 ± 1.2 | 90 | 6.3 ± 1.2 | 101 | 6.3 ± 1.2 | 95 | 6.2 ± 1.3 |
| Fasting insulin (mU/L), median (rangeb) | 95 | 14.1 (11.3–20.8) | 88 | 14.2 (11.6–17.6) | 103 | 14.6 (10.6–19.4) | 98 | 14.8 (11.5–20.1) |
| 1-h Insulin (mU/L), median (rangeb) | 97 | 128.8 (76.6–187.8) | 90 | 143.4 (76.7–182.2) | 102 | 132.1 (71.8–182.1) | 94 | 112.3 (67.2–186.8) |
| 2-h Insulin (mU/L), median (rangeb) | 96 | 78.5 (54.4–135.6) | 89 | 82.3 (52.2–131.0) | 101 | 69.0 (50.9–136.7) | 93 | 70.6 (49.3–121.1) |
| GDM, n (%) | 100 | 19/100 (19) | 92 | 18/92 (20) | 106 | 26/106 (25) | 99 | 21/99 (21) |
| 35-37 wk | ||||||||
| Weight gain T1-T3 (kg), mean ± SD | 79 | 8.8 ± 4.7 | 75 | 6.5 ± 3.8 | 74 | 8.0 ± 4.7 | 76 | 8.5 ± 5.0 |
| Fasting glucose (mmol/L), mean ± SDc | 94 | 4.60 ± 0.50 | 89 | 4.56 ± 0.51 | 91 | 4.73 ± 0.48 | 87 | 4.51 ± 0.41 |
| HOMA-IR, median (rangeb)c | 93 | 3.3 (2.2–5.4) | 85 | 3.1 (2.4–4.1) | 90 | 3.5 (2.7–4.6) | 87 | 3.3 (2.2–4.3) |
| Secondary variables | ||||||||
| Weight gain T2-T3 (kg), mean ± SD | 79 | 4.4 ± 2.8 | 75 | 3.4 ± 2.2 | 71 | 4.5 ± 2.7 | 76 | 4.2 ± 2.9 |
| Weight gain <5 kg, n (%) | 79 | 16/79 (20) | 75 | 27/75 (36) | 74 | 19/74 (26) | 76 | 18/76 (24) |
| Weight gain not exceeding IOM, n (%) | 79 | 19/79 (24) | 75 | 30/75 (40) | 74 | 21/74 (28) | 76 | 21/76 (28) |
| 1-h Glucose (mmol/L), mean ± SD | 90 | 8.2 ± 1.4 | 87 | 8.0 ± 1.3 | 88 | 8.2 ± 1.5 | 87 | 8.0 ± 1.5 |
| 2-h Glucose (mmol/L), mean ± SD | 90 | 6.5 ± 1.2 | 86 | 6.4 ± 1.1 | 89 | 6.9 ± 1.2 | 87 | 6.4 ± 1.1 |
| Fasting insulin (mU/L), median (rangeb)c | 93 | 16.5 (11.2–24.3) | 86 | 15.5 (12.4–20.6) | 90 | 16.8 (13.0–22.0) | 89 | 16.3 (12.1–22.2) |
| 1-h Insulin (mU/L), median (rangeb) | 90 | 187.8 (112.7–225.2) | 86 | 173.9 (117.8–217.6) | 88 | 181.5 (107.9–232.2) | 87 | 152.4 (95.5–211.2) |
| 2-h Insulin (mU/L), median (rangeb) | 89 | 118.7 (59.4–174.3) | 86 | 110.4 (68.5–176.1) | 89 | 134.5 (66.0–194.1) | 85 | 110.7 (61.6–164.9) |
| GDM, n (%) | 94 | 35/94 (37) | 84 | 27/84 (32) | 91 | 40/91 (44) | 89 | 30/89 (34) |
| Birth outcomes (secondary variables) | ||||||||
| Gestational age at birth (wk), mean ± SD | 93 | 39.8 ± 1.6 | 93 | 39.8 ± 1.4 | 103 | 39.4 ± 2.1 | 98 | 39.5 ± 1.6 |
| Birth weight (g), mean ± SD | 94 | 3571 ± 517 | 91 | 3472 ± 498 | 103 | 3448 ± 643 | 96 | 3456 ± 502 |
| SGA <10th percentile, n (%) | 90 | 5/90 (6) | 86 | 7/86 (8) | 101 | 10/101 (10) | 87 | 5/87 (6) |
| LGA >90th percentile, n (%) | 90 | 16/90 (18) | 86 | 8/86 (9) | 101 | 15/101 (15) | 88 | 12/88 (14) |
Abbreviations: IOM, Institute of Medicine recommended maximum gestational weight gain; SD, standard deviation; T1-T2, difference between 24 to 28 weeks and baseline; T1-T3, difference between 35 to 37 weeks and baseline; T2-T3, difference between 35 to 37 weeks and 24 to 28 weeks.
Adjusted for BMI at baseline and number of weeks between measurements.
Range from 25th to 75th percentile.
Value of 24 to 28 weeks was carried forward when, based on local oral glucose tolerance test results, women had developed GDM.
Table 3.
Adjusted Differences or Odds Ratio of Primary and Secondary Outcomes; Comparisons of Intervention Groups and Usual Care Group
| Variable | HE+PA vs UC | HE vs UC | PA vs UC |
|---|---|---|---|
| 24–28 wk | |||
| Weight gain T1-T2 (kg) | −1.19 (−1.90 to −0.49)a | −0.64 (-1.33 to 0.06) | 0.12 (−0.58 to 0.82) |
| Fasting glucose (mmol/L) | 0.03 (−0.07 to 0.12) | 0.15 (0.05 to 0.24)a | 0.03 (−0.07 to 0.12) |
| HOMA-IRb | 0.002 (−0.11 to 0.11) | 0.04 (−0.07 to 0.14) | 0.07 (−0.04 to 0.17) |
| Secondary variables | |||
| 1-h Glucose (mmol/L) | 0.24 (−0.17 to 0.65) | 0.08 (−0.33 to 0.48) | 0.08 (−0.33 to 0.48) |
| 2-h Glucose (mmol/L) | 0.06 (−0.26 to 0.37) | −0.01 (−0.32 to 0.30) | −0.03 (−0.33 to 0.28) |
| Fasting insulin (mU/L)b | 0.00 (−0.10 to 0.10) | 0.01 (−0.09 to 0.11) | 0.07 (−0.03 to 0.17) |
| 1-h Insulin (mU/L)b | 0.05 (−0.11 to 0.20) | 0.06 (−0.09 to 0.21) | −0.01 (−0.16 to 0.15) |
| 2-h Insulin (mU/L)b | −0.06 (−0.23 to 0.11) | 0.00 (−0.17 to 0.17) | 0.001 (−0.17 to 0.17) |
| GDM (%) | OR 1.10 (0.48 to 2.49) | OR 1.48 (0.69 to 3.15) | OR 1.21 (0.55 to 2.67) |
| 35–37 wk | |||
| Weight gain T1-T3 (kg)c,d | −2.02 (−3.58 to −0.46)a | −0.28 (−1.67 to 1.12) | 0.01 (−1.38 to 1.39) |
| Fasting glucose (mmol/L)e | −0.03 (−0.15 to 0.09) | 0.16 (0.03 to 0.28)a | −0.03 (−0.16 to 0.09) |
| HOMA-IRb,c | −0.03 (−0.17 to 0.12) | 0.11 (−0.03 to 0.25) | 0.06 (−0.08 to 0.20) |
| Secondary variables | |||
| Weight gain T2-T3 (kg)c,d | −0.81 (−1.76 to −0.14)a | 0.27 (−0.56 to 1.10) | −0.11 (−0.92 to 0.70) |
| Weight gain <5 kg (%)c,d | OR 2.26 (1.02 to 4.98)a | OR 1.14 (0.50 to 2.64) | OR 1.10 (0.47 to 2.54) |
| Weight gain not exceeding IOM (%)c,d | OR 2.13 (1.05 to 4.33)a | OR 1.10 (0.52 to 2.32) | OR 1.13 (0.54 to 2.37) |
| 1-h Glucose (mmol/L)e | −0.04 (−0.46 to 0.38) | 0.10 (−0.33 to 0.53) | 0.16 (−0.26 to 0.58) |
| 2-h Glucose (mmol/L)e | −0.17 (−0.51 to 0.17) | 0.21 (−0.14 to 0.55) | 0.02 (−0.32 to 0.36) |
| Fasting insulin, mU/L)a,e | −0.003 (−0.13 to 0.13) | 0.07 (−0.06 to 0.20) | 0.08 (−0.05 to 0.21) |
| 1-h Insulin (mU/L)a,e | −0.01 (−0.18 to 0.16) | 0.03 (−0.14 to 0.20) | −0.07 (−0.24 to 0.10) |
| 2-h Insulin (mU/L)a,e | −0.06 (−0.25 to 0.13) | 0.12 (−0.07 to 0.31) | −0.01 (−0.20 to 0.18) |
| GDM (%) | OR 0.80 (0.43 to 1.49) | OR 1.33 (0.73 to 2.40) | OR 0.86 (0.47 to 1.58) |
| Birth outcomes (secondary variables) | |||
| Gestational age at birth (wk) | 0.02 (−0.62 to 0.66) | −0.66 (−1.27 to −0.04)a | −0.36 (−0.99 to 0.27) |
| Birth weight (g)f | −105 (-243 to 32) | −65 (−199 to 69) | −65 (−202 to 72) |
| SGA <10th percentile (%) | 1.51 (0.46 to 4.94) | 1.87 (0.61 to 5.69) | 1.04 (0.29 to 3.71) |
| LGA >90th percentile (%) | 0.47 (0.19 to 1.18) | 0.81 (0.37 to 1.76) | 0.73 (0.32 to 1.66) |
Data presented as adjusted differences or ORs (95% confidence intervals). All regression analyses were adjusted for the baseline values of the outcome variable, except for GDM.
Abbreviations: IOM, Institute of Medicine recommended maximum gestational weight gain; OR, odds ratio; SD, standard deviation; T1-T2, difference between 24 to 28 weeks and baseline; T1-T3, difference between 35 to 37 weeks and baseline; T2-T3, difference between 35 to 37 weeks and 24 to 28 weeks.
Statistically significant difference (P < 0.05).
Variable was log transformed for the regression analyses; adjusted differences between UC and intervention group should be interpreted as the percentage of difference in the outcome variable.
Adjusted for BMI at baseline and number of weeks between measurements.
Excluding women who developed GDM at 24 to 28 weeks according to local oral glucose tolerance test (n = 60).
Value of 24 to 28 weeks was carried forward when, according to local oral glucose tolerance test results, women had developed GDM.
Adjusted for gestational age.
The HE and HE+PA interventions were associated with substantial improvements in healthy eating, and the PA intervention was associated with significantly greater MVPA at 24 to 28 weeks (Table 4). Sedentary behavior was lower with the HE+PA intervention at 24 to 28 and 35 to 37 weeks and with HE at 24 to 28 weeks.
Table 4.
Physical Activity and Healthy Eating in 4 Intervention Groups at 24 to 28 and 35 to 37 Weeks and Adjusted Differences Compared With Usual Care
| Variable | UC | HE+PA | HE | PA |
Adjusted Differences OR
(95% CI) |
||
|---|---|---|---|---|---|---|---|
| HE+PA vs UC | HE vs UC | PA vs UC | |||||
| 24–28 wk | |||||||
| Total PA (MET h/wk), median (range)a,b | 130 (100 to 189) | 133 (98 to 202) | 141 (103 to 198) | 165 (117 to 199) | 0.00 (−0.12 to 0.12) | 0.02 (−0.10 to 0.14) | 0.08 (−0.04 to 0.20) |
| MVPA (MET h/wk), median (range)a,b | 30 (15 to 63) | 44 (21 to 77) | 36 (17 to 80) | 51 (27 to 89) | 0.24 (−0.01 to 0.50) | 0.09 (0.16 to 0.33) | 0.36 (0.13 to 0.60)c |
| Sedentary time (MET h/wk), mean ± SD | 13 ± 10 | 11 ± 9 | 12 ± 8 | 12 ± 7 | −3.0 (−4.47 to −0.45)c | −2.18 (−4.14 to −0.21)c | −1.96 (−3.93 to 0.02) |
| Sugared drinks (n), mean ± SD | 5.3 ± 5.2 | 5.2 ± 7.7 | 3.2 ± 4.5 | 6.9 ± 7.3 | −0.9 (−2.7 to 0.9) | −2.0 (−3.8 to −0.2)c | 0.6 (−1.2 to 2.4) |
| Vegetables, mean ± SD | 12.0 ± 9.4 | 13.8 ± 8.1 | 15.5 ± 10.1 | 13.2 ± 9.1 | 2.6 (0.1 to 5.0)c | 4.5 (2.0 to 7.0)c | 0.2 (−2.2 to 2.7) |
| Fiber, mean ± SD | 34.1 ± 17.9 | 35.1 ± 17.6 | 35.6 ± 17.0 | 33.9 ± 18.0 | −0.0 (−4.9 to 4.8) | 2.8 (−2.1 to 7.7) | −1.6 (−6.4 to 3.1) |
| Portion size, mean ± SD | 16.4 ± 12.4 | 15.7 ± 12.3 | 13.4 ± 9.9 | 19.1 ± 12.2 | −4.0 (−7.4 to −0.6)c | −5.3 (−8.6 to −1.9)c | −0.9 (−4.3 to 2.4) |
| Protein, mean ± SD | 8.7 ± 6.2 | 9.8 ± 6.5 | 9.6 ± 5.9 | 9.1 ± 5.2 | 1.4 (−0.2 to 3.1) | 1.0 (−0.7 to 2.7) | 0.2 (−1.4 to 1.8) |
| Fat, mean ± SD | 6.1 ± 5.8 | 5.6 ± 4.8 | 5.0 ± 6.4 | 6.5 ± 5.8 | −1.1 (−2.9 to 0.6) | −1.7 (−3.5 to 0.02) | −0.2 (−1.9 to 1.6) |
| Carbohydrates, mean ± SD | 36.6 ± 18.8 | 33.7 ± 16.3 | 31.6 ± 15.0 | 36.3 ± 19.0 | −4.8 (−9.0 to 0.5) | -4.8 (−9.6 to −0.1)c | −2.8 (−7.5 to 1.9) |
| 35–37 wk | |||||||
| Total PA (MET h/wk), median (range)a | 105 (76 to 141) | 111 (88 to 163) | 116(93 to 169) | 119 (86 to 180) | 0.09 (−0.06 to 0.23) | 0.10 (−0.04 to 0.24) | 0.09 (−0.06 to 0.23) |
| MVPA, (MET h/wk), median (range)a | 22 (11 to 42) | 29 (8 to 55) | 21 (10 to 59) | 35 (16 to 60) | 0.16 (−0.14 to 0.47) | 0.11 (−0.20 to 0.41) | 0.31 (−0.002 to 0.61) |
| Sedentary time (MET h/wk), mean ± SD | 14 ± 10 | 11 ± 8 | 13 ± 10 | 14 ± 8 | −2.98 (−5.29 to −0.67)c | −0.76 (−3.05 to 1.54) | −0.50 (−2.79 to 1.80) |
| Sugared drinks (portions/wk), mean ± SD | 5.0 ± 5.7 | 3.6 ± 4.8 | 3.3 ± 5.3 | 6.1 ± 6.8 | −2.0 (−3.7 to −0.4)c | −1.6 (−3.2 to 0.1) | 0.4 (−1.3 to 2.1) |
| Vegetables (portions/wk), mean ± SD | 13.1 ± 9.6 | 12.3 ± 8.0 | 15.6 ± 11.2 | 12.8 ± 11.1 | 0.3 (−2.4 to 3.0) | 3.5 (0.8 to 6.3)c | −1.1 (−3.7 to 1.6) |
| Fiber (portions/wk), mean ± SD | 35.0 ± 17.9 | 31.8 ± 17.5 | 33.3 ± 18.0 | 31.6 ± 19.3 | −4.0 (−9.2 to 1.2) | 0.0 (−5.2 to 5.3) | −4.2 (−9.3 to 1.0) |
| Portion size, mean ± SD | 17.3 ± 11.4 | 15.9 ± 10.1 | 13.9 ± 10.8 | 19.5 ± 13.6 | −2.9 (−6.6 to 0.8) | −4.5 (−8.2 to −0.9)c | 0.2 (−3.4 to 3.8) |
| Protein (portions/wk), mean ± SD | 8.8 ± 7.1 | 8.7 ± 5.7 | 9.8 ± 8.6 | 8.8 ± 4.3 | 0.2 (−1.7 to 2.1) | 0.8 (−1.1 to 2.7) | −0.4 (−2.3 to 1.5) |
| Fat (portions/wk), mean ± SD | 6.6 ± 8.5 | 5.7 ± 5.7 | 4.7 ± 4.1 | 6.4 ± 4.3 | −1.6 (−3.4 to 0.2) | −2.5 (−4.3 to (−0.7)c | −0.8 (−2.6 to 0.9) |
| Carbohydrates (portions/wk), mean ± SD | 35.6 ± 18.3 | 31.9 ± 18.7 | 28.2 ± 14.2 | 35.0 ± 18.5 | −5.2 (−10.2 to −0.3)c | −7.0 (−11.9 to −2.0)c | −3.0 (−7.9 to 1.8) |
All regression analyses assessing differences between intervention and control group were adjusted for baseline values of the outcome variable.
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
Range from 25th to 75th percentile.
Variable was log transformed for the regression analyses; adjusted differences between control and intervention group should be interpreted as the percentage of difference in the outcome variable.
Statistically significant (P < 0.05).
No statistically significant differences among the groups in the development of GDM, SGA, or LGA infants, other than a small, but significantly, lower gestational age at birth in the HE group compared with UC (Tables 2 and 3).
Discussion
The DALI lifestyle trial was a pan-European study designed to find the most effective lifestyle intervention for preventing GDM in preparation for a larger RCT. The primary outcomes chosen to define “effective” were GWG, fasting glucose, and HOMA-IR. The effects of advice on healthy eating, physical activity, and the combination of the 2 on these 3 outcomes were evaluated, allowing the separation of the effects of the 2 intervention modalities. Women with glucose levels greater than the IADPSG/WHO criteria at baseline were excluded, making it a true trial for the prevention of incident GDM. The outcomes were evaluated at 2 time points in pregnancy: 24 to 28 weeks, the usual gestation at which testing for GDM occurs, and at 35 to 37 weeks, the latest gestation we believed an OGTT could be reliably undertaken to assess the sustainability of the intervention effect. Being across multiple countries, with recruitment largely through nonspecialized antenatal clinics, DALI is representative of the wider pregnant, obese, European population, without evidence of elevated glucose concentrations at 9 to 20 weeks of gestation. The intensive interventions, based on motivational interviewing, resulted in substantial lifestyle improvements at both time periods of their assessment. However, neither PA nor HE alone had any substantial beneficial effect on GWG or metabolic outcomes (HE was associated with an increased fasting glucose in the main trial, the converse to that found in our pilot study). An improvement in both lifestyle interventions, such as in the combined intervention group, resulted in substantial GWG limitation but still with no effect on fasting glucose or insulin resistance. Despite the important changes in GWG, the postload glucose and insulin concentrations remained unchanged, and this occurred with and without the PA intervention.
Of the large number of early lifestyle RCTs, only 2 (1 HE and 1 HE+PA intervention) showed a substantial (60% to 77%) reduction in the proportion of women developing GDM (23, 24). Both RCTs were also associated with substantial GWG reductions (3.4 kg in obese participants and 6.8 kg in nonobese participants) that were greater than the average GWG reduction of 1.4 kg achieved in lifestyle RCTs (25). The recent RADIEL study (gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish gestational diabetes prevention study) showed a 39% adjusted reduction in GDM, although with a modest reduction in GWG of 0.5 kg (26). A finding potentially explained by the inclusion of about 30% women with previous GDM. However, the 2 largest recent RCTs of HE+PA interventions were negative; the UPBEAT (UK pregnancies better eating and activity trial) (27) and LIMIT (limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: a randomised trial) (28) studies showed a reduction in GWG of only 0.55 kg and 0.05 kg, respectively, which did not result in a reduction in GDM at 24 to 28 weeks. Furthermore, no previous trial, with the exception of the RADIEL study (26), excluded women with “abnormal” glucose tolerance at baseline and, therefore, were not genuine GDM prevention trials.
Although not 1 of our primary outcomes, the birthweight was not significantly lower with the interventions nor were their reductions in LGA or increases in SGA infants. A post hoc analysis did show reductions in infants with a birthweight ≥4.5 kg in the LIMIT study (28); however, the DALI lifestyle study, along with other studies (25), did not find a similar effect on LGA rates. We were concerned about the SGA rates with our limitation in GWG; however, again, no statistically significant difference was found.
Previously, doubts existed regarding whether the negative findings in previous studies resulted from interventions that had not achieved a sufficient GWG difference (5), either because of the nature of the intervention involved or because of controls gaining less weight than expected after successful adoption of their own lifestyle changes (6). However, the relatively high GWG reduction, such as in the DALI lifestyle study, did not affect the metabolic parameters. Future studies might need even more intensive interventions, with greater support for women, leading to greater lifestyle changes. Perhaps even more imaginative tools, such as tele-health approaches, might be included (5). It is important to note that the need for intensive support during lifestyle interventions was clearly shown in the Diabetes Prevention Program (4). However, for the practical purposes of knowledge transfer, the DALI study has demonstrated that a substantial reduction in GWG through lifestyle changes alone does not affect glycemia in obese mothers. Although further escalation of the rigor of lifestyle intervention might start to affect HOMA, the cost/benefit value should be assessed to determine whether the effect on GDM is limited. Also, the future health trajectory of both mother and child will require analysis.
A further question is whether lifestyle changes starting from, or before, conception could be more effective than those beginning at the start of the second trimester. However, testing such an intervention before or in early pregnancy could prove challenging. The former could be investigated among women with previous GDM who are planning further pregnancies (although lifestyle interventions should be in place in any case). Women in early pregnancy are often not scheduled for antenatal care, and multiple practical reasons exist for why beginning a trial so early in pregnancy could be problematic.
The present study had several strengths, including its European multicenter randomized controlled trial design, its robust approach to intervention fidelity, and inclusion of a health economic analysis. No increase in SGA rate was seen, consistent with the findings among obese women with a GWG of 0 to 5 kg in Denmark (17). A concern exists that inadequate weight gain could lead to the fetal “thrifty phenotype” predisposing to long-term metabolic risk (29, 30).
Several limitations are noteworthy. The DALI lifestyle study was funded as an exploratory trial and was not large enough to evaluate differences in, for example, GDM. We sought to overcome this using a 2×2 factorial design, assuming we would be able to compare 2 groups at a time, providing more power. However, given the greater interaction between the interventions than anticipated, we did not combine the intervention groups. The initial power calculations were based on both between-group and between-factor analyses; thus, this decision remained within the original study design. From the pilot data, the power for HOMA was too low in the present study. However, given the small differences among the intervention groups, these would not have been considered clinically relevant, even when statistically significant. We have not adjusted the significance level for multiple comparisons, because the comparisons followed our a priori statistical analysis plan, and the differences in GWG are in line with (or better than) than those from other RCTs, which speaks against the role of chance. Some withdrawals and missing data occurred; however, the missing data did not influence the results of the effects analysis much, because when using imputed data, the GWG difference was −2.3 kg (95% confidence interval, −3.7 to −0.9 kg). Fasting glucose was inexplicably, although marginally, increased in the HE group. Others have suggested that diets higher in complex carbohydrates and low in fat might improve insulin sensitivity (31), and the DALI HE recommendation included lowering both carbohydrate and fat intake. Further work is required to understand a possible metabolic basis for these findings. However, because the pilot study found a marginally lower fasting glucose with HE, we suggest that both likely resulted from chance.
Excluding women with glucose levels greater than the IADPSG/WHO 2013 criteria at baseline was both a strength and a weakness. The conversion rates were still very high (19% by 24 to 28 weeks and 37% by 35 to 37 weeks), even after excluding approximately 20% of women with “hyperglycemia” in early pregnancy. Because the criteria for GDM diagnosis early in pregnancy might need to be higher (32), it could be argued that some women who would have benefited from the DALI intervention were excluded. However, it has made the DALI study 1 of the few true trials for the prevention of incident GDM, rather than intervening among a group in which a proportion at least had pre-existing GDM. GDM is clearly a heterogeneous condition with, for example, a substantial proportion of women with “early GDM,” who are more insulin resistant (33), and different anthropometry and glucose tolerance test profiles between women with GDM from different ethnic groups (34). Whether the effect of lifestyle approaches would be more effective in subgroups (as perhaps occurred in the RADIEL study with its greater proportion of women with previous GDM), warrants further study.
We conclude that, although the DALI HE+PA intervention is an effective intervention compared with UC for pregnant women with a BMI of ≥29 kg/m2 for limiting GWG, it is unlikely to prevent GDM. Further studies of lifestyle interventions should probably concentrate on the first trimester, the prepregnancy period, and special subgroups.
Acknowledgments
We thank the participants, coaches, research midwives/nurses, and health professionals collaborating in the recruitment.
Acknowledgments
The project described received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 242187. In the Netherlands, additional funding was provided by the Netherlands Organisation for Health Research and Development (ZonMw) (grant 200310013). In Poland, additional funding was obtained from Polish Ministry of Science (grants 2203/7, PR/2011/2). In Denmark, additional funding was provided by Odense University Free Research Fund. In the United Kingdom, the DALI team acknowledge the support received from the NIHR Clinical Research Network: Eastern, especially the local diabetes clinical and research teams based in Cambridge. In Spain, additional funding was provided by CAIBER 1527-B-226. The funders had no role in any aspect of the study beyond funding.
Acknowledgments
Author contributions: All authors contributed to the conception and/or design of the trial, read and corrected draft versions of the report, and approved the final report. D.S. wrote the first draft of the paper, M.N.M.v.P. undertook the statistical analyses, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. D.S. was guarantor.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- DALI
- vitamin D and lifestyle intervention for GDM prevention
- GDM
- gestational diabetes mellitus
- GWG
- gestational weight gain
- HE
- healthy eating
- HE+PA
- healthy eating plus physical activity
- HOMA-IR
- homeostasis model assessment-insulin resistance
- IADPSG
- International Association of Diabetes and Pregnancy Study Group
- LGA
- large for gestational age
- MET
- metabolic equivalent of task
- MVPA
- moderate-to-vigorous physical activity
- OGTT
- oral glucose tolerance test
- PA
- physical activity
- PDA
- personal digital assistant
- RCT
- randomized controlled trial
- SGA
- small for gestational age
- UC
- usual care
- WHO
- World Health Organization
References
- 1.Simmons D. Diabetes and obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):25–36. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 3.Simmons D. Epidemiology of diabetes in pregnancy. In: McCance D, Maresh M, eds. Practical Management of Diabetes in Pregnancy. London, United Kingdom: Blackwell Publishing; 2010;1–16. [Google Scholar]
- 4.Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons D, van Poppel MN; DALI Consortium . UPBEAT, RADIEL, and DALI: what’s the difference? Lancet Diabetes Endocrinol. 2015;3(10):761. [DOI] [PubMed] [Google Scholar]
- 6.Simmons D. Prevention of gestational diabetes mellitus: where are we now? Diabetes Obes Metab. 2015;17(9):824–834. [DOI] [PubMed] [Google Scholar]
- 7.Jelsma JG, van Poppel MN, Galjaard S, Desoye G, Corcoy R, Devlieger R, van Assche A, Timmerman D, Jans G, Harreiter J, Kautzky-Willer A, Damm P, Mathiesen ER, Jensen DM, Andersen L, Dunne F, Lapolla A, Di Cianni G, Bertolotto A, Wender-Oegowska E, Zawiejska A, Blumska K, Hill D, Rebollo P, Snoek FJ, Simmons D. DALI: vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial—study protocol. BMC Pregnancy Childbirth. 2013;13:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons D, Jelsma JG, Galjaard S, Devlieger R, van Assche A, Jans G, Corcoy R, Adelantado JM, Dunne F, Desoye G, Harreiter J, Kautzky-Willer A, Damm P, Mathiesen ER, Jensen DM, Andersen LL, Lapolla A, Dalfra M, Bertolotto A, Wender-Ozegowska E, Zawiejska A, Hill D, Rebollo P, Snoek FJ, van Poppel MN. Results from a European multicenter randomized trial of physical activity and/or healthy eating to reduce the risk of gestational diabetes mellitus: the DALI lifestyle pilot. Diabetes Care. 2015;38(9):1650–1656. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 10.Vellinga A, Zawiejska A, Harreiter J, Buckley B, Di Cianni G, Lapolla A, Corcoy R, Simmons D, Adelantado JM, Damm P, Desoye G, Devlieger R, Hill D, Kautzky-Willer A, Klemetti M, Mathiesen E, Rebollo P, Snoek F, Tikkanen M, Timmerman D, van Assche A, van Poppel M, Wender-Oegowska E, Dunne F. Associations of body mass index (maternal BMI) and gestational diabetes mellitus with neonatal and maternal pregnancy outcomes in a multicentre European database (diabetes and pregnancy vitamin D and lifestyle intervention for gestational diabetes mellitus prevention). ISRN Obes. 2012;2012:424010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO/NMH/MND/13.2. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 13.Simmons D, Rush E, Crook N; Te Wai o Rona: Diabetes Prevention Strategy Team . Development and piloting of a community health worker-based intervention for the prevention of diabetes among New Zealand Maori in Te Wai o Rona: diabetes prevention strategy. Public Health Nutr. 2008;11(12):1318–1325. [DOI] [PubMed] [Google Scholar]
- 14.Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller WR, Rollnick S Motivational Interviewing, Preparing People to Change Addictive Behavior. New York, NY: The Guildford Press; 1991. [Google Scholar]
- 16.Rasmussen KM, Yaktine AL Weight Gain During Pregnancy. Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 17.Jensen DM, Ovesen P, Beck-Nielsen H, Mølsted-Pedersen L, Sørensen B, Vinter C, Damm P. Gestational weight gain and pregnancy outcomes in 481 obese glucose-tolerant women. Diabetes Care. 2005;28(9):2118–2122. [DOI] [PubMed] [Google Scholar]
- 18.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–1760. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 20.Simmons D, Mandell C, Fleming C, Gatland B, Leakehe L. Evaluation of a diabetes knowledge and behaviour (DKB) questionnaire. Asia Pac J Clin Nutr. 1994;3(4):193–200. [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004. Available at: https://books.google.com/books?hl=en&lr=&id=bQBtw6rx_mUC&pgis=1. Accessed 30 Nov 2015.
- 23.Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol. 2011;51(2):141–146. [DOI] [PubMed] [Google Scholar]
- 24.Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, Facchinetti F. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med. 2014;27(13):1348–1352. [DOI] [PubMed] [Google Scholar]
- 25.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, Kunz R, Mol BW, Coomarasamy A, Khan KS. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, Kaaja RJ, Pöyhönen-Alho M, Tiitinen A, Huvinen E, Andersson S, Laivuori H, Valkama A, Meinilä J, Kautiainen H, Eriksson JG, Stach-Lempinen B. Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care. 2016;39(1):24–30. [DOI] [PubMed] [Google Scholar]
- 27.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E, Pasupathy D, Patel N, Robson SC, Sandall J, Sanders TA, Sattar N, Seed PT, Wardle J, Whitworth MK, Briley AL; UPBEAT Trial Consortium . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–777. [DOI] [PubMed] [Google Scholar]
- 28.Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, Crowther CA, Wittert G, Owens JA, Robinson JS; LIMIT Randomised Trial Group . Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ. 2014;348:g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM Jr, Saade G, Sorokin Y, Peaceman AM, Tolosa JE; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014;211(2):137.e1–137.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernandez TL, Van Pelt RE, Anderson MA, Daniels LJ, West NA, Donahoo WT, Friedman JE, Barbour LA. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care. 2014;37(5):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntyre HD, Sacks DA, Barbour LA, Feig DS, Catalano PM, Damm P, McElduff A. Issues with the diagnosis and classification of hyperglycaemia in early pregnancy. Diabetes Care. 2016;39(1):53–54. [DOI] [PubMed] [Google Scholar]
- 33.Harreiter J, Simmons D, Desoye G, Corcoy R, Adelantado JM, Devlieger R, van Assche A, Galjaard S, Damm P, Mathiesen ER, Jensen DM, Andersen LLT, Dunne F, Lapolla A, Dalfra MG, Bertolotto A, Mantaj U, Wender-Ozegowska E, Zawiejska A, Hill D, Jelsma JGM, Snoek FJ, Worda C, Bancher-Todesca D, van Poppel MNM, Kautzky-Willer A; DALI Core Investigator Group . IADPSG and WHO 2013 gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy. Diabetes Care. 2016;39(7):e90–e92. [DOI] [PubMed] [Google Scholar]
- 34.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ, Persson B, Trimble ER; HAPO Study Cooperative Research Group . Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care. 2012;35(3):526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]