Abstract
Background
Atherosclerosis occurs as a result of low-density lipoprotein (LDL) deposits oxidation. Endothelial dysfunction is an early process of atherosclerosis. Restoring endothelial lining back to normal by endothelial progenitor cells (EPCs) is critical for slowing or reversing vascular disease progression. Oxidative stress from hydrogen peroxide (H2O2) is increased in dyslipidemia so that antioxidant agent is required to prevent destruction of blood vessels.
Objectives
This study aims to report Ganoderma lucidum polysaccharide peptide (PsP) effects in atherogenic process by measuring H2O2 level, IL-10 level, and EPC number in blood serum, and also intima-media thickness of aorta in dyslipidemia Wistar rat model by giving them a hypercholesterol diet (HCD).
Materials and methods
The study was an experimental in vivo post-test with control group design. Thirty-five Wistar rats (Rattus norwegicus) were divided into five groups (normal diet group, HCD group, and hypercholesterol groups that received 50 mg/kg, 150 mg/kg, and 300 mg/kg bodyweight PsP).
Results
Each treatment group showed significant results for the administration of PsP using the one-way analysis of variance test (p<0.050) for the reduction of H2O2 (p = 0.003), levels of IL-10 (p = 0.027), number of EPC in the blood serum (p = 0.011), and the intima-media thickness of the aorta (p = 0.000). PsP from G. lucidum is a potent antioxidant and may prevent atherogenesis process in patients with dyslipidemia.
Conclusions
The optimum doses of PsP in this study is 300 mg/kg bodyweight. Further studies are required to determine the antioxidant effects of PsP G. lucidum and its benefits in the management of dyslipidemia.
Keywords: Atherogenesis, Dyslipidemia, EPC, PsP
Introduction
Mortality from coronary heart disease (CHD) caused by atherosclerosis increases sharply in both industrial and developing countries (1). Prevalence of atherosclerotic CHD has been increasing yearly and has been declared by the World Health Organization (WHO) as a global threat (2). It is estimated that 1.9 billion people, or one-third of the world’s population, are affected by this disease. In Indonesia, morbidity and mortality from CHD are likely to increase and the mortality rate has reached 50% of population (2).
Risk factors for atherosclerosis include dyslipidemia, free radicals, endothelial dysfunction, and inflammation (3). Signs of inflammation occurred from the beginning until the development of atherosclerosis. Inflammation showed an important role in the development of atherosclerotic lesions affecting the coronary arteries (4). Increased levels of cholesterol in blood, especially low-density lipoprotein (LDL) cholesterol, is harmful because of the peroxidation process (auto-oxidation) of lipids which are exposed to oxygen. An excessive intake of calories can lead to an increase in the activity of the citric acid cycle, which produces reactive oxygen species (ROS) (5). Macrophages activated by the presence of ROS produce various pro-inflammatory cytokines, such as TNFα, IL-1 and IL-6, as well as anti-inflammatory cytokines such as IL-10. IL-10 is an inhibitor of macrophages and dendritic cells, and is mainly produced by Th2 cells and activated macrophages (6). IL-10 attains significant role in controlling non-specific immune response and cellular immunity (7).
Lipid peroxidation is a chain reaction that continues to produce ROS, such as OH-, RO, and hydrogen peroxide (H2O2) (5). H2O2 activates nuclear factor kappa beta (NF-кB). NF-кB is a transcription factor that plays an important role in controlling various biological effects such as inflammation, immune system, cell proliferation, cell differentiation, tumorigenesis, and cell apoptosis (8). Activation of NF-кB is a process when translocation of NF-кB subunit from the cytoplasm into the nucleus happens and stimulates the expression of pro-inflammatory genes such as cytokines (TNF-α and interleukins), adhesion molecules (ICAM-1 and VCAM-1) and chemokines. NF-κB activation products initiate the process of atherosclerosis and endothelial dysfunction characterized by the increase of inducible nitric oxide synthase (iNOS), platelet adhesion, migration and proliferation of smooth muscle cells (9).
Vascular endothelial damage (endothelial dysfunction) is the initial process of atherosclerosis formation. Underlying mechanisms of endothelial dysfunction associated with production and bioavailability of nitric oxide (NO), oxidative stress, adhesion molecules and production of chemokines such as macrophage chemoattractant protein-1 (MCP-1) and plasminogen activating factor inhibitor (PAI-1), all of which contribute to enhance the inflammatory response involved in the development of thrombus either directly or indirectly (10). It further increases the exposure of adhesion molecules on endothelial cells and decreases the ability of the endothelium to release NO and other substances that help to prevent attachment of macromolecules, platelets, and monocytes to the endothelium (11). The loss of NO can cause smooth muscle proliferation in the intima-media of a blood vessel. These conditions can be triggered by hypercholesterolemia that leads to intima-media thickening (12). Nowadays, the thickness of the intima-media of a blood vessel, is used as an early screening of atherosclerosis (13).
Endothelial dysfunction is a hallmark of cardiovascular and cerebrovascular disease. Restoring the endothelial lining to normal is critical for slowing or reversing the progression of vascular disease (14-15-16). Recent studies suggest that a damaged endothelial lining can be restored by endothelial progenitor cells (EPCs). Circulating EPCs derived from bone marrow contribute to the repopulation by seeding the intimal lining with new cells. These circulating EPCs home in on injured areas and accelerate the regeneration of new endothelium (17-18-19). EPCs also play a role in neovascularization and determine the prognosis of cardiovascular disease. The number of functional EPCs is a reflection of the body’s ability to repair blood vessel’s inner lining after injury due to exposure of the risk factors of cardiovascular disease (20).
A source of antioxidants such as polysaccharide peptides (PsPs), is required to compensate the increased oxidative stress. PsPs are protein-bound polysaccharide extracts that may be obtained from the extraction of plants and fungi, one of which is Ganoderma lucidum (21). There are several bioactive substances that can be identified and isolated from G. lucidum, such as triterpenoids, polysaccharide, nucleosides, sterols, and alkaloids, which show its function and role as an anti-inflammatory, antioxidant, anti-tumor, immunomodulatory, and radioprotector in previous studies (22). PsP is also reported for its scavenger activity against free radicals, although its effect is still 100 times less potent than ascorbic acid (Vitamin C) (23). This study aims to report the effects of G. lucidum PsP in atherogenic process by measuring levels of H2O2, levels of IL-10 and numbers of EPCs in the blood serum, and also the intima-media thickness of the aorta, in Wistar rats model of dyslipidemia, by giving them HCD.
Materials and methods
Ganoderma lucidum polysaccharide peptide
PsP was supplied by Sahabat Lingkungan Hidup Partner Labs, Surabaya, Indonesia, in the form of capsules containing 250 mg of an extract of G. lucidum. Each gram of PsP contained 200 mg of β-D-glucan. PsP was administered to the animal models daily via oral gavage in the last 4 weeks of the study. The HCD was obtained from the Laboratory of Pharmacology, Medical Faculty, Brawijaya University and was given to the rats for 12 weeks.
Animals
We used Guidelines on the Care and Use of Animals for Scientific Purposes National Advisory Committee for Laboratory Animal Research for care of the animals in this study. Thirty-five Wistar rats (Rattus norwegicus), 8 weeks of age and weighing 150-200 g, were obtained from CV Gamma Scientific Biolab (Malang, Indonesia). These rats were divided into five groups: negative control group (a normal diet group); positive control group (a group fed with HCD); and three groups fed an HCD and administered with three different doses of G. lucidum PsP (50 mg/kg, 150 mg/kg, and 300 mg/kg bodyweight). Those three groups were named HCD + PsP 50 mg, HCD + PsP 150 mg and HCD + PsP 300 mg. Each treatment group consisted of seven rats.
Hypercholesterol diet (HCD)
HCD was given throughout this study (12 weeks). HCD consisted of: high amounts of cholesterol, colic acid and lard to increase LDL cholesterol levels in rat’s blood (24). Lard contain high cholesterol levels compared to the other animal oil. Colic acid was given because without this the administration of high-fat diet will not increase cholesterol levels and atherosclerotic plaque development in 12 weeks. HCD was administered to create a dyslipidemic condition, which can lead to atherosclerosis.
Blood sampling
Blood samples were obtained via cardiac puncture. The blood samples were preserved in the Laboratory of Physiology, Brawijaya University, Malang, Indonesia.
H2O2 measurement
Levels of H2O2 in rat plasma were measured using a colorimetric hydrogen peroxide kit (Assay Design, Abcam, Cambridge, MA, USA) and observed at 570 nm using an enzyme-linked immunosorbent assay reader (ELISA Kit, Boehringer-Mannheim GMBH, Mannheim, Germany).
EPC measurement
Numbers of EPCs in the blood circulation were measure with Flowcytometry. EPCs were analyzed by CD34 per CP Santa Cruz SC-19621 and CD 133 FITC (fluorescein isothiocyanate) Bioss bs-0395R-FITCmarker.
Interleukin (IL)-10 measurement
The levels of IL-10 in rats’ blood serum were measured by ELISA test. Three different ELISA kits (BT Lab, Gwang-dong, South Korea) were used for measuring each parameter.
Measurement of tunica intima-media thickness
Aortic intima-media thickness measurements in this study, used Dot Slide Microscope. The image results obtained in this study were read by OlyVIA software with 400× magnification. Wall thickness can be measured by drawing a perpendicular line from the line of the inner intima to the outer media.
Ethics
We obtained ethical approval for the animal treatment and experimental processes in this study from the Ethics Committee for Health Research Number 462/EC/KEPK/08/2013. Euthanasia was administered to the rats using ether, then the rats were dissected and aortas were taken. The aorta was placed in 10% formalin for preservation before the making of the slide.
Statistical analysis
The one-way analysis of variance (ANOVA) test was used to determine the effects of PsP on the levels of H2O2 and the levels of IL-10 in Wistar rats with dyslipidemia. This analysis was continued by the post hoc test using the Duncan method to detect differences in parameters in each treatment group. SPSS software version 20 (IBM Corporation, New York, NY, USA) was used for data analysis.
Results
The number of circulating EPCs, measured through CD 34 (+) marker in all groups in this study ranged from 0 to 96 cells. HCD + PsP 300 mg group had the highest average number of EPCs (25.80 cells), compared to the other groups. The positive control group had the lowest average number of EPCs (0.75 cells). PsP administration significantly increased the average number of circulating EPCs in the blood circulation. It was proven from the average number of EPCs in the groups administered with PsP, which have higher number of EPCs, compared to positive and negative control groups. Therefore, the increased dose of PsP is in synergy with the increase of average number of circulating EPCs in the blood circulation.
Based on one-way ANOVA test in the 95% confidence interval, it was concluded that the PsP administration significantly affects the increased of EPC numbers (p = 0.011). The results of post hoc analysis showed that the number of EPCs in the negative control group and HCD + PsP in various doses have different average numbers of EPCs. This suggests that the administration of PsP at doses of 50, 150 and 300 mg may increase the number of EPCs significantly, in the group of rats with dyslipidemia.
H2O2 levels in experimental groups ranged from 9.25-145.50 nmol. The highest average levels of H2O2 were in the positive control group, while the lowest average levels of H2O2 were in the negative control group. Rats in the treatment groups which were fed with HCD and 50 mg/kg, 150 mg/kg and 300 mg/kg dose of PsP had H2O2 levels around 10.38-119 nmol. The increased dose of PsP tends to decrease the average levels of H2O2. Based on one-way ANOVA test in the 95% confidence interval, it was concluded that there are significant differences in the levels of H2O2 (p = 0.003). The results of post hoc analysis showed that the levels of H2O2 in the positive controls and HCD + PsP 50 mg groups are significantly different with the negative control and the HCD + PsP 300 mg group. Based on these results, the administration of PsP at a dose of 300 mg in rats with dyslipidemia, significantly affect the decreased levels of H2O2 to its normal level.
The levels of IL-10 in the experimental group ranged from 68.66 to 311.94 pg/mL. The highest average level of IL-10 was in the positive control group and the lowest average levels of IL-10 were in the HCD + PsP 300 mg group. In the treatment groups that were fed with HCD and 50 mg/kg, 150 mg/kg and 300 mg/kg dose of PsP had IL-10 levels about 68.66-206.54 pg/mL. The increased doses of PsP tend to be followed by the decrease of IL-10 average levels. The results of one-way ANOVA test and post hoc analysis in the 95% confidence interval, showed that the administration of 50, 150 and 300 mg doses of PsP, can reduce the levels of IL-10 significantly in the rats with dyslipidemia (p = 0.027).
Aortic intima-media thickness in the experimental group ranged between 68.53 µm to 104.25 μm. The lowest average value of the aortic intima-media thickness obtained in the HCD + PsP 300 mg group (70.66 µm) and highest was in the positive control group (90.80 µm). Statistical analysis showed that there were significant differences (p<0.05) between the negative control group and the positive control group. It suggests that there was no atherogenesis process in rats; thus, aortic intima-media thickness is much smaller than the positive control group. Meanwhile, when the aortic intima-media thickness of HCD + PsP 300 mg group (70.66 ± 0.53 µm) was compared to the positive control group (90.80 ± 8:28 µm), aortic intima-media thickness in this group was much smaller, as well as when compared with the other treatment groups. This suggests that higher dose of PsP can reduce intima-media thickness more significantly (p<0.05). Based on one-way ANOVA test in the 95% confidence interval, it was concluded that there were significant differences of intima-media thickness at least in two treatment groups (p = 0.000). The results of post hoc analysis showed that administration of PsP 300 mg in rats with dyslipidemia, significantly affects the decline of intima-media thickness to its normal thickness. The hematoxylin and eosin (HE) staining results of the intima-media thickness in each treatment group can be seen in Figure 1.
Fig. 1 -.
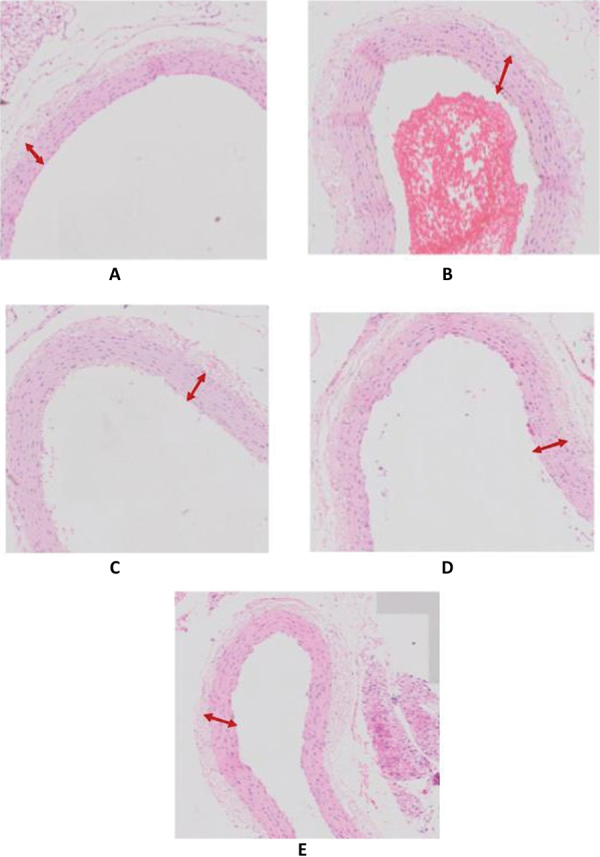
Intima-media thickness in each treatment group: (A) rat aorta in negative control group; (B) rat aorta in positive control group; (C) rat aorta in HCD + PsP 50 mg/kg bodyweight (BW) group; (D) rat aorta in HCD + PsP 150 mg/kg BW group; (E) rat aorta in HCD + PsP 300 mg/kg BW group. Hematoxylin and eosin staining. 400× magnification.
The results of HE staining were observed using dot scan OlyVIA with 40 times magnification, and it shows rat aortic intima-media thickness, as seen in Figure 1.
Discussion
Although endothelial dysfunction is associated with the development of atherosclerosis, the function of EPCs as prognostic markers has only recently been demonstrated (25). In a study of 44 patients with coronary artery disease (CAD) and 33 patients with acute coronary syndrome followed up for a median of 10 months’ duration, a reduced number of EPCs was associated with a significantly higher incidence of cardiovascular events (25). In another larger study with 519 patients with stable CAD, increased levels of EPCs were related to reduced risk of death from cardiovascular causes, a first major cardiovascular event, revascularization and hospitalization (26).
Currently, EPCs are defined as cells positive for both hematopoietic stem-cell markers such as CD34 and an endothelial marker protein such as VEGFR2. CD34 is not exclusively expressed on hematopoietic stem cells, but also on mature endothelial cells. Other studies have used the more immature hematopoietic stem-cell marker CD133 and demonstrated that purified CD133-positive cells can differentiate endothelial cells in vitro (27, 28). Thus, CD133 + VEGFR2 + cells are more likely to reflect immature progenitor cells, whereas CD34 + VEGFR2 + may represent shed cells of the vessel walls (28). Fadini et al first demonstrated that the number of EPCs marked by CD34+/CD133+/KDR+ is significantly decreased in diabetic patients with peripheral artery disease (PAD) compared to those who suffered diabetes alone (29,30). This finding was further supported by another paper from Fadini where they reported significantly lower CD34+/KDR + EPCs in PAD patients compared to healthy controls (30). Research conducted by Fadini et al, supports the results of this study, which showed the average number of EPCs in the positive control group receiving HCD is lower than the negative control group and the treatment groups with 50, 150 and 300 mg/kg doses of PsP.
Some studies showed conflicting results and this may be due to the varying definition of EPCs using different methods of identification, different timing of blood sampling, different severity of native disease and concomitant medication and comorbidities that may affect EPC numbers and functions. EPCs have also been shown to be useful prognostic markers in predicting events in patients with CAD (31). The administration 50, 150 and 300 mg/kg of PsP can increase the average number of circulating EPCs in the blood vessels, even until it exceeds the average number of EPCs in the negative control group. It proves that the administration of antioxidants contained in PsP is able to significantly increase the number of EPCs in rats (p = 0.011), although these groups also received HCD. This study also reveals the antioxidant properties of high doses of PsP (300 mg/kg bodyweight) to EPC compared to other treatment groups. This result is supported by one previous study of anti-oxidative and anti-atherogenic effects of high dosages of PsP against all parameters of atherogenic processes; which are foam cells, intimal-medial thickness, and insulin resistance, and thus, confirms the absolute antioxidant benefits of PsP (32).
H2O2, a particular variant of reactive oxygen species (ROS), plays a role in the activation of inflammatory genes, including NF-кB translocation to the nucleus (32). The oxidant status also shows an enhancement of GSH and SOD as antioxidant parameters and MDA reduction as oxidative marker (33). In the early stages of atherosclerosis, endothelial cells contribute to the formation of ROS. ROS formation from smooth muscle cells and fibroblasts occurs after endothelial cell dysfunction. Table I shows that the levels of H2O2 in the various treatment groups ranged from 9.25 to 145.50 nmol. Negative control group, had lower levels of H2O2 ranged from 9.25 to 18.00 nmol, whereas the positive control group, which was fed with HCD, had H2O2 levels ranged from 62.13 to 145.50 nmol. The results of this study showed an increase in H2O2 levels, especially in the group which was fed with HCD. The increased levels of H2O2 in this study were due to HCD that increased LDL cholesterol levels in rats. Hypercholesterolemic conditions over a long period will lead to the increased production of ROS.
TABLE I -.
Measurement results of parameters
| Parameter | Treatment groups | P (one-way ANOVA p<0.050) | ||||
|---|---|---|---|---|---|---|
| Positive control Mean ± SD (min-max) | Negative control Mean ± SD (min-max) | HCD + PsP 50 mg/kg BW Mean ± SD (min-max) | HCD + PsP 150 mg/kg BW Mean ± SD (min-max) | HCD + PsP 300 mg/kg BW Mean ± SD (min-max) | ||
| Data are presented as mean ± standard deviation (range) values. All the values of the parameters have been corrected into International Standard of Mathematics (decimals). | ||||||
| p<0.05 indicates the significant difference. | ||||||
| ANOVA = analysis of variance; PsP = polysaccharide peptide. | ||||||
| H202 (nmol) | 84.2780 ± 35.20506 (62.13-145.50) | 14.25 ± 3.30246 (9.25-18.00) | 64.6760 ± 40.61848 (10.38-119) | 40.9260 ± 23.05566 (26.50-80.13) | 28.47 ± 8.00618 (21.75-41.88) | 0.003 |
| IL-10 (pg/mL) | 202.518 ± 73.29135 (134.86-311.94) | 127.362 ± 17.05956 (107.33-150.38) | 131.722 ± 45.24811 (68.66-187.08) | 120.086 ± 51.50449 (68.66-206.54) | 103.616 ± 13.63951 (80.83-116.78) | 0.027 |
| Tunica intima-media thickness (µm) | 90.80 ± 8.28 (77.84-115.53) | 72.31 ± 1.18 (71.09-74.06) | 89.34 ± 6.95 (73.52-99.46) | 80.62 ± 3.35 (69.36-92.88) | 70.66 ± 0.53 (70.02-71.50) | 0.000 |
| EPC (cells) CD 34 (+) | 0.75 ± 0.5 (0.00-1.00) | 2.60 ± 4.722287581 (0.00-11.00) | 4.20 ± 1.483239697 (2.00-6.00) | 4.33 ± 2.516611478 (2.00-7.00) | 25.80 ± 41.39082024 (0.00-96.00) | 0.011 |
PsP from G. lucidum is a source of antioxidants to reduce the level of oxidative stress. PsP is able to act as a scavenger against free radicals. In addition, PsP also has been reported to have anti-inflammatory activity (33). G. lucidum PsP prevents endothelial damage through its activity as an antioxidant that inhibits oxidative stress, especially from H2O2 (34). The treatment group fed with HCD and PsP had H2O2 levels which are lower than H2O2 levels in the positive control group. The average levels of H2O2 tend to decline to normal levels with the administration of higher doses of PsP. In addition, the results of one-way ANOVA test showed that PsP administration significantly reduced H2O2 levels (pV = 0.003) to their normal level. Based on these results, the administration of PsP 300 mg in rats with HCD significantly affects the decreased levels of H2O2 to their normal level.
IL-10 is an anti-inflammatory cytokine that may play a protective role in atherosclerosis (35). The results of this study are consistent with the theory that the increased levels of IL-10 play an important role in anti-inflammatory mechanism. The results of this study showed an increase in IL-10 levels, especially in the group fed with HCD. The increased levels of IL-10 are influenced by HCD, which can increase the production of ROS, which can then activate macrophages to release anti-inflammatory cytokines such as IL-10 as a defense mechanism of the body to control the excessive inflammation process (36-37-38). The one-way ANOVA test and post hoc analysis results also showed that the administration of PsP in rats fed with HCD significantly affect the decreased level of IL-10 as an anti-inflammatory cytokine (pV = 0.027). PsP as an antioxidant can reduce IL-10 production because it prevents harmful and excessive inflammatory processes induced by HCD administration (39, 40).
According to Arhana (2000), endothelial cell damage causes vascular smooth muscle cell hyperplasia, because of the inability of the vascular endothelial cells to synthesize NO (41). This reaction occurs in the formation of Ox-LDL, which activates the transcription factor (NF-κβ), which can induce immune system’s proteins and intermediate molecules to cause atherosclerosis progression (42, 43). The aortic intima-media thickness in the experimental group in this study ranged between 68.53 μm to 104.25 μm. The highest average of aortic intima-media thickness was in the positive control group, while the lowest is in HCD + PsP 300 mg group. Administration of PsP significantly reduced the aortic intima-media thickness (p = 0.000). Significant differences of intima media thickness were found if the HCD + PsP 300 mg group compared with the positive control, HCD + PsP 50 mg/kg, and HCD + PsP 150 mg/kg groups. It shows that high doses of PsP given to rats were able to lower the aortic intima-media thickness.
Previous studies have reported the benefits of high concentrations of β-glucan as antiseptic, antioxidant, anti-aging, immune-system activator, protection against radiation, anti-inflammatory, anti-cholesterol, and antidiabetic (44). According to Jia (45), and You (46) in previous studies, antioxidants contained in the G. lucidum polysaccharide can prevent the decrease in tissue antioxidant enzymes and can provide cellular protection against ROS through the destruction of macrophages in animal models with oxidative stress. Antioxidants contained in PsP will react with free radicals to form stable and harmful molecules and prevent the formation of activated macrophages; that there are no substance and chemoattractant cytokines released to increase the intima media thickness (24, 47).
This study proves that the PsP can reduce rat aortic intima-media thickness in the atherogenesis process. As an antioxidant, PsP can inhibit the formation of ROS in the LDL oxidation stage and reduce the number of foam cells created by high stress oxidative condition. Further research should be done to find out every substance contained in PsP and toxicity tests to determine the safety of PsP in higher doses. β-Glucan has been proven scientifically as a biological defense modifier (BDM) and included in the category of “Generally Recognized As Safe” (GRAS) by the FDA, and has no toxic or side effects (48-49-50).
Conclusion
PsP of G. lucidum is able to increase the EPC quantity by inhibiting atherogenic process in aortic tissues of rats with dyslipidemia. PsP prevents endothelial damage through its activity as an antioxidant that inhibits oxidative stress, especially from H2O2, increases anti-inflammatory cytokines such as IL-10, increases circulating EPC numbers and decreases intima-media thickness of aorta. The most effective doses of PsP in inhibiting atherogenesis process in this study was 300 mg/kg bodyweight. Future research on PsP in G. lucidum is required to further explore the advantages of PsP in the treatment of dyslipidemia.
Disclosures
Financial support: No grants or funding have been received for this study.
Conflict of interest: None of the authors has financial interest related to this study to disclose.
References
- 1.Reynolds LA, Tansey EM. 27th ed. London:: Wellcome Trust Centre for the History of Medicine; Cholesterol, atherosclerosis and coronary disease in the UK, 1950-2000. In: Wellcome witnesses to twentieth century medicine. 2006; 15-25. Available from http://www.histmodbiomed.org/sites/default/files/44850.pdf. Accessed Feb 22, 2017. [Google Scholar]
- 2.World Health Organization. 2014. New initiative launched to tackle cardiovascular disease, the world’s number one killer. Available from http://www.who.int/cardiovascular_diseases/en/. Accessed Feb 22, 2017. [Google Scholar]
- 3.Wihastuti TA, Sargowo D, Tjokroprawiro A, Permatasari N, Widodo MA, Soeharto S. Vasa vasorum anti-angiogenesis through H₂O₂, HIF-1α, NF-κB, and iNOS inhibition by mangosteen pericarp ethanolic extract (Garcinia mangostana Linn) in hypercholesterol-diet-given Rattus norvegicus Wistar strain. Vasc Health Risk Manag. 2014;10:523–531. doi: 10.2147/VHRM.S61736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cybulsky MI, Iiyama K, Li H et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray RK, Granner DK, Mayes PA, Rodwell VW., eds . 25th ed. Stamford:: Appleton & Lange; Harper’s illustrated biochemistry. 2000:221-227. [Google Scholar]
- 6.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KW, de Waal Malefyt R, Coffman RL, OGarra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19(1):683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 8.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Andreakos E, Smith C, Kiriakidis S et al. Heterogeneous requirement of IkappaB kinase 2 for inflammatory cytokine and matrix metalloproteinase production in rheumatoid arthritis: implications for therapy. Arthritis Rheum. 2003;48(7):1901–1912. doi: 10.1002/art.11044. [DOI] [PubMed] [Google Scholar]
- 10.Lesnik P, Chapman MJ. A new dimension in the vasculoprotective function of HDL: progenitor-mediated endothelium repair. Arterioscler Thromb Vasc Biol. 2006;26(5):965–967. doi: 10.1161/01.ATV.0000219613.90372.c1. [DOI] [PubMed] [Google Scholar]
- 11.Guyton AC, Hall JE. 11th Ed. Jakarta:: EGC; Textbook of medical physiology. 2007; 125-138. Available from http://vet.uokufa.edu.iq/staff/falah/Textbook of Medical Physiology.pdf. Accessed Feb 22, 2017. [Google Scholar]
- 12.Ou J, Wang J, Xu H et al. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ Res. 2005;97(11):1190–1197. doi: 10.1161/01.RES.0000190634.60042.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo KS, Chook P, Yu CW et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28(7):852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 14.Shimokawa H, Aarhus LL, Vanhoutte PM. Porcine coronary arteries with regenerated endothelium have a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin. Circ Res. 1987;61(2):256–270. doi: 10.1161/01.res.61.2.256. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa H, Flavahan NA, Vanhoutte PM. Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries. Possible dysfunction of a pertussis toxin-sensitive G protein. Circ Res. 1989;65(3):740–753. doi: 10.1161/01.res.65.3.740. [DOI] [PubMed] [Google Scholar]
- 16.Borg-Capra C, Fournet-Bourguignon MP, Janiak P et al. Morphological heterogeneity with normal expression but altered function of G proteins in porcine cultured regenerated coronary endothelial cells. Br J Pharmacol. 1997;122(6):999–1008. doi: 10.1038/sj.bjp.0701475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med (Berl) 2004;82(10):671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 18.Walter DH, Rittig K, Bahlmann FH et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 19.Griese DP, Ehsan A, Melo LG et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108(21):2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch EZ, Chisolm GM, III, White HM. Reendothelialization and maintenance of endothelial integrity in longitudinal denuded tracks in the thoracic aorta of rats. Atherosclerosis. 1983;46(3):287–307. doi: 10.1016/0021-9150(83)90179-x. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Zhang Y, Zhong Z. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int J Mol Sci. 2011;12(9):6135–6145. doi: 10.3390/ijms12096135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z, Chen X, Zhong Z, Chen L, Wang Y. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39(1):15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- 23.Seto SW, Lam TY, Tam HL et al. Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine. 2009;16(5):426–436. doi: 10.1016/j.phymed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Baraas F. Jakarta:: Kardia Iqratama; Molecular cardiology. 2006. [In Indonesian]. [Google Scholar]
- 25.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 26.Werner N, Kosiol S, Schiegl T et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 27.Gehling UM, Ergün S, Schumacher U et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–3112. [PubMed] [Google Scholar]
- 28.Handgretinger R, Gordon PR, Leimig T et al. Biology and plasticity of CD133+ hematopoietic stem cells. Ann N Y Acad Sci. 2003;996(1):141–151. doi: 10.1111/j.1749-6632.2003.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 29.Fadini GP, Sartore S, Albiero M et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 30.Fadini GP, Sartore S, Baesso I et al. Endothelial progenitor cells and the diabetic paradox. Diabetes Care. 2006;29(3):714–716. doi: 10.2337/diacare.29.03.06.dc05-1834. [DOI] [PubMed] [Google Scholar]
- 31.Losordo DW, Kibbe MR, Mendelsohn F et al; Autologous CD34+ Cell Therapy for Critical Limb Ischemia Investigators. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circ Cardiovasc Interv. 2012;5(6):821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyle CH, Martinez LJ, Coleman MC, Spitz DR, Weintraub NL, Kader KN. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic Biol Med. 2006;40(12):2206–2213. doi: 10.1016/j.freeradbiomed.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Chen X, Zhong Z, Chen L, Wang Y. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39(1):15–27. doi: 10.1142/S0192415X11008610. [DOI] [PubMed] [Google Scholar]
- 34.Mallat Z, Besnard S, Duriez M et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85(8):e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 35.Mallat Z, Heymes C, Ohan J, Faggin E, Lesèche G, Tedgui A. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 1999;19(3):611–616. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- 36.Uyemura K, Demer LL, Castle SC et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97(9):2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101(8):1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19(3):734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 40.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109(6):745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arhana GET. The role of nitrogen oxide and infection. Sari Pediatri. 2000;2(2):113–119. [In Indonesian]. [Google Scholar]
- 42.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest. 2001;107(3):255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mello JM, Orsi AM, Padovani CR et al. Structure of the aortic wall in the guinea pig and rat. Braz J Morphol Sci. 2004;21(1):35–38. Available from http://jms.org.br/PDF/v21n1a06.pdf. Accessed Feb 22, 2017. [Google Scholar]
- 44.Kusmiati Tamat SR, Isnaini N. Jakarta:: Jurnal Ilmu Kefarmasian Indonesia.; Production and determination of β-glucan levels from three of Saccharomyces cerevisiae strains in medium containing molasses. 2007;5(1):7-16. [In Indonesian]. [Google Scholar]
- 45.Jia J. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009;155(1):32–36. [Google Scholar]
- 46.You Y-H. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by ROS. Acta Pharmacol Sin. 2002;23(09):787–791. [PubMed] [Google Scholar]
- 47.Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 2009;84(10):917–938. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thontowi A, Nuswantara S. Glucan production of Saccharomyces cerevisiae in medium with different nitrogen sources in air-lift fermentor. Biodiversitas. 2007;8(4):253–256. [In Indonesian]. [Google Scholar]
- 49.Wihastuti TA, Heriansyah T, Merika S et al. Inhibition of oxidative stress in hypercolesterolemic rats by soy milk. J Cardiovasc Disease Res. 2016;7(2):74–82. [Google Scholar]
- 50.Heriansyah T, Wihastuti T, Sargowo D et al. Reduction of histopathological images through a decrease in H2O2 levels in diabetic rats with pollysaccharide peptides. Biomarkers and Genomic Medicine. 2015;7(1):31–37. [Google Scholar]


