Abstract
Background and objectives
Kidney length is often measured during routine abdominal ultrasonography and may be of use to identify patients at high vascular and renal risk. We aimed to explore patient characteristics related to kidney length, from which reference values were derived, and evaluate the relationship between kidney length and the risk of cardiovascular events and ESRD in high-risk patients.
Design, setting, participants, & measurements
The study population consisted of 10,251 patients with clinical manifest arterial disease or vascular risk factors included in the Second Manifestations of ARTerial disease (SMART) Study cohort between 1996 and 2014. Linear regression was used to explore patient characteristics of kidney length. The relationship between kidney length and cardiovascular events (myocardial infarction, stroke, and cardiovascular mortality), all-cause mortality, and ESRD was analyzed using Cox regression. Kidney length was analyzed in tertiles, using the second tertile as the reference category.
Results
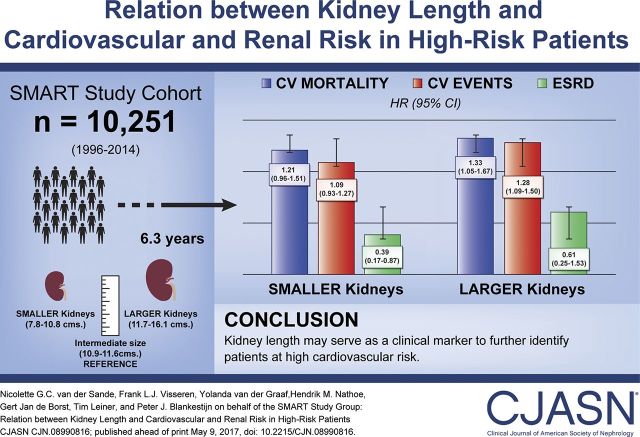
Kidney length was strongly correlated with body surface area (2.04 mm; 95% confidence interval [95% CI], 1.95 to 2.13 per 0.1 m2 increase) and eGFR (1.62 mm; 95% CI, 1.52 to 1.73 per 10 ml/min per 1.73 m2 increase). During the median follow-up of 6.3 years, 1317 patients experienced a cardiovascular event, including 711 myocardial infarctions, 369 strokes, and 735 vascular cause deaths. A total of 1462 patients died of any cause and 52 patients developed ESRD. Irrespective of eGFR, patients in the third tertile of kidney length (11.7–16.1 cm) were at higher risk of cardiovascular mortality (hazard ratio, 1.33; 95% CI, 1.05 to 1.67) and cardiovascular events (hazard ratio, 1.28; 95% CI, 1.09 to 1.50). Patients in the first tertile of kidney length (7.8–10.8 cm) were not at higher risk of cardiovascular adverse events.
Conclusions
Large kidney length is related to higher risk of cardiovascular events and mortality in high-risk patients, irrespective of eGFR. Kidney length may serve as a clinical marker to further identify patients at high cardiovascular risk.
Keywords: cardiovascular disease; end-stage renal disease; kidney; Biomarkers; Body Surface Area; Cardiovascular Diseases; Cause of Death; Follow-Up Studies; Humans; Kidney Failure, Chronic; Linear Models; Myocardial Infarction; Reference Values; Renal Insufficiency, Chronic; risk factors; Stroke
Introduction
CKD has been identified as an independent cardiovascular risk factor (1,2). This can, in part, be explained by risk factors related to both development of cardiovascular disease and CKD (e.g., hypertension and diabetes). Conversely, atherosclerosis leads to CKD, as is seen in patients with renal artery stenosis. Atherosclerosis also effects kidney size because it is related to an accelerated decrease in kidney length with age (3). This could be the direct effect of atherosclerosis on the kidney by inducing microvascular and glomerular injury (4). Kidney length is measured for diagnostic purposes to discriminate between acute or CKD, or is provided as additional information during radiologic imaging for other diagnostic purposes. Besides having a direct diagnostic value, kidney length may identify patients at high risk for cardiovascular disease. A small kidney length may be a marker of subclinical atherosclerotic disease. In addition, a large kidney length may also be related to higher cardiovascular risk. Large kidney length, presumably because of glomerular hyperfiltration, is related to progression of CKD in patients with diabetic nephropathy (5). Therefore, it could be relevant to know which factors influence kidney length in a high cardiovascular risk population.
For proper interpretation of kidney length, reference values are needed. Previous studies in healthy populations or populations with low prevalence of cardiovascular disease reported references values of kidney length for men and women separately, without taking body height into account, and these studies had small sample sizes (6–8).
Therefore, the aims of this study were (1) to explore patient characteristics of kidney length in patients at high cardiovascular risk, from which reference values for kidney length can be derived; and (2) to determine the relationship between kidney length and risk of cardiovascular events and ESRD.
Materials and Methods
Study Participants
The study population was obtained from the Second Manifestations of ARTerial disease (SMART) Study, an ongoing prospective single-center cohort study at the University Medical Center Utrecht that started in September of 1996. Patients with clinical manifest arterial disease (cerebrovascular disease, coronary heart disease, peripheral arterial disease, or abdominal aortic aneurysm) or with higher risk for atherosclerotic vascular disease (hypertension, diabetes, or hyperlipidemia) were included. Patients were approached either via the outpatient clinic or during the weeks after hospital discharge. All patients were clinically stable during recruitment and had no clinical signs of GN, kidney cancer, or AKI. All study patients gave their written informed consent. The SMART Study was approved by the Ethics Committee at the University Medical Center Utrecht. Detailed information on the rationale and design has been described elsewhere (9).
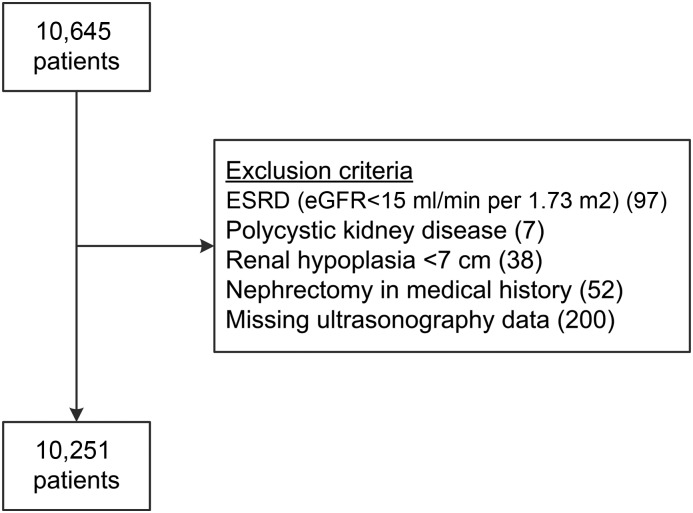
A total of 10,645 patients were enrolled between 1996 and 2014. Patients with abnormal kidney size because of underlying kidney disease were excluded, including ESRD (eGFR<15 ml/min per 1.73 m2), polycystic kidney disease, hypoplasia of the kidneys (<7 cm), and nephrectomy (Figure 1). Another 200 patients were excluded because of missing data on ultrasonography of the kidneys.
Figure 1.
In total, 394 patients with abnormal kidney size because of underlying kidney disease, or missing data on ultrasonography were excluded.
Registration of coincidental findings on ultrasonography in the SMART Study started in 2004. A total of 6654 patients were included between 2004 and 2014. We found renal masses (eight malignancies) in 30 patients and hydronephrosis in six patients.
Measurements
All patients filled in a questionnaire regarding medical history and underwent a physical examination. Fasting urine and blood samples were obtained. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 creatinine equation (10). Albuminuria was defined by an albumin-to-creatinine ratio >30 mg/g in a morning urine sample. Diabetes was defined as fasting serum glucose ≥126 mg/dl, self-reported diabetes, and/or use of insulin or oral hypoglycemic drugs. Body surface area was estimated using the Du Bois formula (11).
Ultrasonography was performed at study enrollment, according to protocol to register kidney size, using an ATL HDI 3000 (Advanced Technology Laboratories, Bothell, WA) equipped with 4–2 MHz curved array transducer. These measurements are routinely performed by well trained and experienced technicians at the diagnostic vascular laboratory of the Radiology Department. Kidney length was measured as the maximum pole-to-pole length in the oblique sagittal plane. Carotid intima media thickness was measured in the anterolateral, posterolateral, and mediolateral directions with an ATL Ultramark 9 (Advanced Technology Laboratories) equipped with a 10-MHz linear array transducer. Carotid intima media thickness was defined as the mean of the left and right common carotid artery measurements.
Follow-Up and Outcomes
Patients were biannually asked to complete a standardized questionnaire regarding newly diagnosed diseases and hospital admissions. Complete information of each reported event was collected and independently reviewed by three members of the SMART end point committee. The outcomes of interest included the composite of major cardiovascular events (including myocardial infarction, stroke, and cardiovascular mortality), all-cause mortality, and ESRD (need for long-term dialysis or RRT) (Supplemental Table 1). In total, 633 patients (6.2%) were lost to follow-up.
Data Analyses
Mean kidney length was the average of left and right kidney length. Baseline characteristics are presented as tertiles of mean kidney length. Differences across tertiles of kidney length were assessed using one-way ANOVA for continuous variables and chi-squared test for categorical variables. Linear regression analyses were used to determine the relationship between each patient characteristic and mean kidney length separately. Potential patient characteristics included risk factors for cardiovascular disease and anthropomorphic measurements. Results were adjusted for confounders, including age and sex. In a second exploratory model, eGFR was added to evaluate which patient characteristics were related to mean kidney length independent of eGFR. To directly compare the influence of continuous patient characteristics on kidney length, the standardized coefficient of each continuous patient characteristic was calculated. We evaluated model assumptions, including homogeneity of residuals and multicollinearity. No violation was observed. Reference values were on the basis of kidney length measurements in the study population. Reference values for mean kidney length were presented as the fifth, 50th, and 95th percentiles according to body height and age, using separate values for men and women.
The relationship between mean kidney length and major cardiovascular events (composite and individual components), all-cause mortality, and ESRD was evaluated by Cox proportional hazard models. Potential confounders were chosen on the basis of literature, including age, sex, smoking, body height, waist circumference, fasting glucose, systolic BP, and albuminuria (3,12–14). First, kidney length was modeled with a restricted cubic spline to determine if there was a nonlinear relationship between kidney length and cardiovascular events and mortality. There appeared to be a U-shaped relationship between kidney length and adverse outcomes, indicating that small and large kidney length were related to a higher risk of adverse outcomes (Supplemental Figure 1). Kidney length was divided into tertiles to ensure a sufficient number of events in each tertile for the outcome of ESRD. The second tertile of kidney length was chosen as the reference category. Two models were made, and the first model included all potential confounders. The second model included all potential confounders and eGFR because the relationship between kidney length and adverse outcomes may simply reflect the relationship between eGFR and adverse outcomes. Results are presented as hazard ratios (HRs) with 95% confidence intervals (95% CIs).
Because the relationship between kidney length and adverse outcomes might be different in patients with clinically manifest vascular disease compared with patients with cardiovascular risk factors, the modifying effect of clinically manifest vascular disease was examined on a multiplicative scale by incorporating interaction terms in the second model. The modifying effect of eGFR on the relationship between kidney length and adverse outcomes was assessed similarly.
Sensitivity analyses were performed to determine whether the relationship between mean kidney length and adverse outcomes was influenced by the proportion of patients with large differences in left and right kidney length. Kidney length differences may arise from underlying kidney disease, such as unilateral atrophy because of severe renal artery stenosis (15). A decrease in kidney length of −1.9 cm (range, −1.2 to −3.4) has been reported in patients with severe renal artery stenosis (16). Therefore, we excluded patients with a kidney length difference of >2 cm. Lastly, patients with diabetes were excluded to assess the robustness of our results.
Proportional hazard assumptions were evaluated using Schoenfeld residuals, and no violation was observed. Missing data for covariates were imputed using bootstrapping and predictive mean matching (17). Imputed variables included smoking (0.8%), systolic BP (0.2%), and albuminuria (4.5%). Probability values <0.05 were considered significant. Analyses were performed with R statistical software, version 3.0.3 (http://R-project.org).
Results
Baseline Characteristics
Information on 10,251 patients and data on 20,502 kidneys were used for analyses. Mean kidney length ranged from 7.8 to 16.1 cm (Table 1). Patients in the third tertile of kidney length were more likely to be men, on average younger, more likely to have diabetes, had larger body height, higher BMI, and higher eGFR compared with patients in the first tertile of kidney length.
Table 1.
Baseline characteristics of all participants by tertiles of mean kidney length
| Characteristics | Mean Kidney Length | P Value | ||
|---|---|---|---|---|
| First Tertile (n=3567) | Second Tertile (n=3562) | Third Tertile (n=3122) | ||
| Range of kidney length, cm | 7.8–10.8 | 10.9–11.6 | 11.7–16.1 | |
| Men | 1773 (50%) | 2438 (68%) | 2604 (83%) | <0.001 |
| Age, yr | 59 (13) | 56 (12) | 54 (11) | <0.001 |
| History of vascular disease | ||||
| Cerebrovascular disease | 813 (23%) | 699 (20%) | 537 (17%) | <0.001 |
| Coronary heart disease | 1415 (40%) | 1474 (41%) | 1317 (42%) | 0.10 |
| Peripheral arterial disease | 483 (14%) | 433 (12%) | 397 (13%) | 0.21 |
| Abdominal aortic aneurysm | 203 (6%) | 193 (5%) | 190 (6%) | 0.50 |
| Diabetes mellitus | 531 (15%) | 635 (18%) | 808 (26%) | <0.001 |
| Smoking, current or past | 2455 (69%) | 2597 (74%) | 2413 (78%) | <0.001 |
| Body height, cm | 170 (9) | 175 (8) | 179 (8) | <0.001 |
| Waist circumference, cm | 89 (12) | 94 (12) | 101 (13) | <0.001 |
| Body mass index, kg/m2 | 26 (4) | 27 (4) | 28 (5) | <0.001 |
| Body surface area, m2 | 1.8 (0.2) | 2.0 (0.2) | 2.1 (0.2) | <0.001 |
| Systolic BP, mmHg | 142 (23) | 141 (21) | 142 (20) | <0.01 |
| Diastolic BP, mmHg | 82 (13) | 83 (12) | 84 (12) | <0.001 |
| Intima media thickness, mm | 0.85 [0.70–1.00] | 0.83 [0.70–0.98] | 0.83 [0.70–0.98] | 0.14 |
| Cholesterol, mg/dl | 200 (52) | 198 (53) | 197 (56) | 0.07 |
| Triglycerides, mg/dl | 23 (17–34) | 25 (18–36) | 28 (19–42) | <0.001 |
| LDL cholesterol, mg/dl | 120 (46) | 119 (46) | 117 (45) | 0.02 |
| HDL cholesterol, mg/dl | 52 (16) | 49 (15) | 45 (14) | <0.001 |
| Creatinine, mg/dl | 1.1 (0.3) | 1.0 (0.2) | 1.0 (0.2) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 72 (18) | 80 (17) | 86 (17) | <0.001 |
| Albuminuria | 520 (15%) | 496 (15%) | 506 (17%) | 0.02 |
| HbA1c, % | 5.9 (1.0) | 6.0 (1.0) | 6.3 (1.3) | <0.001 |
| Glucose-lowering drugs | 316 (9%) | 373 (10%) | 483 (15%) | <0.001 |
| BP-lowering drugs | 2412 (68%) | 2309 (65%) | 2118 (68%) | 0.01 |
| ACE inhibitors | 969 (27%) | 982 (28%) | 914 (29%) | 0.13 |
| Angiotensin II receptor blockers | 405 (11%) | 384 (11%) | 368 (12%) | 0.43 |
| Lipid-lowering drugs | 1933 (54%) | 1981 (56%) | 1759 (56%) | 0.19 |
Values are presented as number (percentage), mean (SD), or median [interquartile range]. HbA1c, hemoglobin A1c; ACE, angiotensin-converting enzyme.
Patient Characteristics Correlated with Kidney Length
The relationship between each patient characteristic and kidney length was analyzed separately. Sex correlated with a larger kidney length for men compared with women (6.50 mm; 95% CI, 6.15 to 6.85) (Table 2). A 10-year age increase correlated with a smaller kidney length (−1.59 mm; 95% CI, −1.73 to −1.46). Presence of diabetes correlated with a larger kidney length (3.00 mm; 95% CI, 2.59 to 3.42). A higher body height (3.36 mm; 95% CI, 3.12 to 3.59 per 10 cm) and body surface area (2.04 mm; 95% CI, 1.95 to 2.13 per 0.1 m2) correlated with a larger kidney length. Furthermore, albuminuria (0.83 mm; 95% CI, 0.38 to 1.29) and eGFR (1.62 mm; 95% CI, 1.52 to 1.73 per 10 ml/min per 1.73 m2) correlated with a larger kidney length. Additional adjustment of eGFR yielded similar results.
Table 2.
Patient characteristics correlated with mean kidney length in patients with cardiovascular disease and risk factors (n=10,251)
| Characteristics | Model 1 | Model 2 |
|---|---|---|
| Mean Kidney Length in mm (95% CI) | Mean Kidney Length in mm (95% CI) | |
| Men | 6.50 (6.15 to 6.85)a | 5.92 (5.59 to 6.26)b |
| Age, per 10 yr | −1.59 (−1.73 to −1.46)c | −0.18 (−0.34 to −0.02)d |
| Cerebrovascular disease | −0.70 (−1.11 to −0.29) | −0.59 (−0.99 to −0.19) |
| Coronary heart disease | −0.01 (−0.36 to 0.35) | 0.05 (−0.29 to 0.39) |
| Abdominal aortic aneurysm | −0.06 (−0.55 to 0.43) | −0.09 (−0.57 to 0.38) |
| Peripheral arterial disease | 0.38 (−0.34 to 1.10) | 0.90 (0.21 to 1.59) |
| Diabetes mellitus | 3.00 (2.59 to 3.42) | 2.75 (2.36 to 3.15) |
| Smoking current or past | 1.06 (0.68 to 1.43) | 0.85 (0.49 to 1.21) |
| Body height, per 10 cm | 3.36 (3.12 to 3.59) | 3.41 (3.19 to 3.63) |
| Waist circumference, cm | 0.22 (0.21 to 0.24) | 0.22 (0.21 to 0.24) |
| Body mass index, kg/m2 | 0.56 (0.52 to 0.59) | 0.56 (0.53 to 0.60) |
| Body surface area, per 0.1 m2 | 2.04 (1.95 to 2.13) | 2.06 (1.98 to 2.15) |
| Systolic BP, per 10 mmHg | 0.23 (0.16 to 0.31) | 0.27 (0.20 to 0.35) |
| Diastolic BP, per 10 mmHg | 0.46 (0.32 to 0.59) | 0.53 (0.41 to 0.66) |
| Intima media thickness, per 0.1 mm | 0.13 (0.06 to 0.20) | 0.15 (0.08 to 0.22) |
| Total cholesterol, per 10 mg/dl | 0.001 (−0.03 to 0.03) | 0.01 (−0.02 to 0.04) |
| Log(triglycerides), mg/dl | 1.34 (1.06 to 1.62) | 1.65 (1.38 to 1.92) |
| LDL cholesterol, per 10 mg/dl | −0.03 (−0.07 to 0.01) | −0.02 (−0.06 to 0.01) |
| HDL cholesterol, mg/dl | −0.06 (−0.07 to −0.05) | −0.07 (−0.08 to −0.06) |
| eGFR, per 10 ml/min per 1.73 m2 | 1.62 (1.52 to 1.73) | — |
| Albuminuria | 0.83 (0.38 to 1.29) | 1.53 (1.10 to 1.97) |
| HbA1c, % | 1.28 (1.09 to 1.46) | 1.16 (0.98 to 1.33) |
Each patient characteristic was analyzed separately. The coefficients indicate the difference in mean kidney length (millimeter). For the categorical variables, this difference is correlated with the presence or absence of the characteristic. For the continuous variables, this difference is correlated with a one unit increase of the variable. Model 1: each patient characteristic was adjusted for age and sex. Model 2: each patient characteristic was adjusted for age, sex, and eGFR. 95% CI, 95% confidence interval; —, no change in coefficients because the model already included eGFR; HbA1c, hemoglobin A1c.
Adjusted for age.
Adjusted for age and eGFR.
Adjusted for sex.
Adjusted for sex and eGFR.
In a direct comparison of continuous variables on kidney length, a 1 SD higher body surface area (4.54 mm; 95% CI, 4.36 to 4.72 per 1 SD) and eGFR (2.97 mm; 95% CI, 2.77 to 3.16 per 1 SD) strongly correlated with kidney length (Supplemental Table 2).
Reference Values of Kidney Length according to Body Height and Age
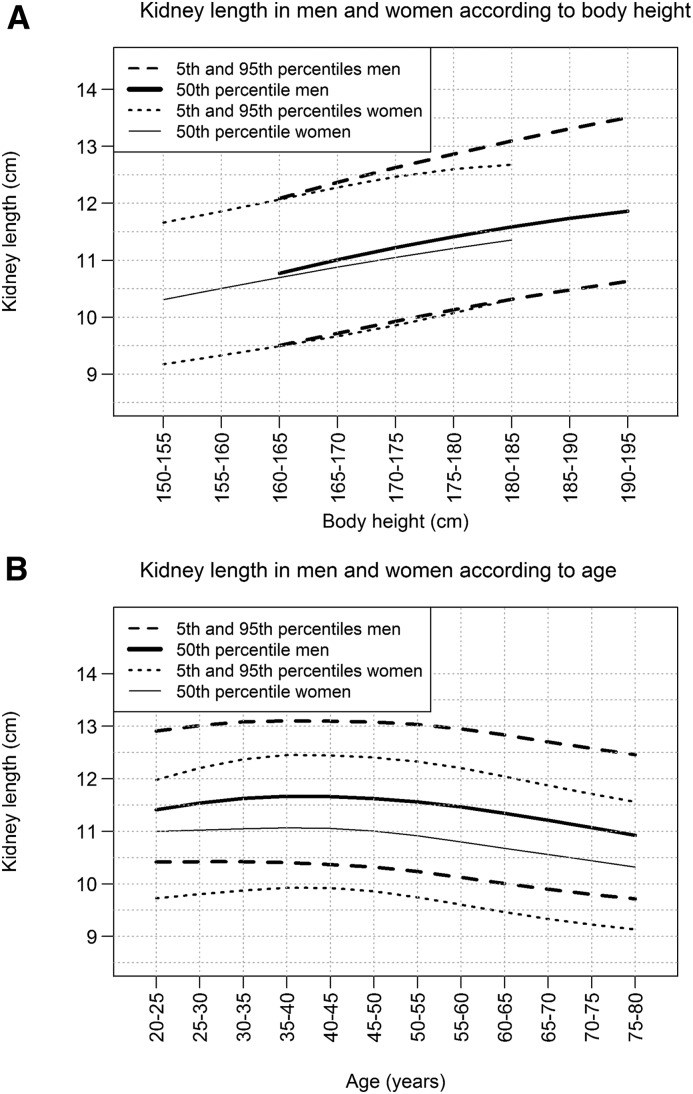
There was a linear relationship between kidney length in percentiles and body height (Figure 2). Kidney length differences between men and women seemed to be on the basis of variation in body height. There was a positive relationship between kidney length and age in patients <50 years, and a negative relationship in patients >50 years.
Figure 2.
There is a positive linear relationship between percentiles of kidney length and body height, whereas kidney length declines in patients >50 years.
Relationship between Kidney Length and Cardiovascular Events, Mortality, and ESRD
During the median follow-up of 6.3 years (interquartile range, 3.4–9.6 years), 1317 patients experienced a major cardiovascular event, including 711 myocardial infarctions, 369 strokes, and 735 vascular cause deaths. A total of 1462 patients died of any cause and 52 patients developed ESRD. The highest crude event rates were observed for all-cause mortality, which was 23.1 for patients in the first tertile, 17.6 for patients in the second tertile, and 17.2 for patients in the third tertile of kidney length (Table 3).
Table 3.
Crude event rates for cardiovascular disease, mortality, and ESRD according to tertiles of kidney length
| Outcomes | First Tertile (n=3567) | Second Tertile (n=3562) | Third Tertile (n=3122) |
|---|---|---|---|
| Range of kidney length cm | 7.8–10.8 | 10.9–11.6 | 11.7–16.1 |
| Events per 1000 py for | |||
| Myocardial infarction | 9.6 | 8.6 | 10.0 |
| Stroke | 5.6 | 4.6 | 4.3 |
| Cardiovascular mortality | 11.9 | 8.3 | 8.9 |
| Cardiovascular events | 19.4 | 15.6 | 17.2 |
| All-cause mortality | 23.1 | 17.6 | 17.2 |
| ESRD | 0.7 | 0.8 | 0.5 |
Py, person years.
The data were then analyzed using two models to account for potential confounders, one without eGFR (model 1) and one including eGFR (model 2). Patients in the first tertile of kidney length (7.8–10.8 cm) were at higher risk of cardiovascular mortality (HR, 1.38; 95% CI, 1.11 to 1.71) and cardiovascular events (HR, 1.20; 95% CI, 1.02 to 1.40) compared with patients in the second tertile of kidney length (10.9–11.6 cm) (Table 4, model 1). Patients in the third tertile of kidney length (11.7–16.1 cm) were at higher risk of cardiovascular events (HR, 1.19; 95% CI, 1.02 to 1.40) and at lower risk of ESRD (HR, 0.34; 95% CI, 0.14 to 0.82) compared with patients in the second tertile of kidney length.
Table 4.
Relationship between tertiles of kidney length and cardiovascular events, mortality, and ESRD
| Outcomes | First Tertile (n=3567) | Second Tertile (n=3562) | Third Tertile (n=3122) |
|---|---|---|---|
| Range, cm | 7.8–10.8 | 10.9–11.6 | 11.7–16.1 |
| Myocardial infarction | |||
| No. of events | 241 | 225 | 245 |
| Model 1, HR (95% CI) | 1.15 (0.93 to 1.43) | Reference | 1.20 (0.97 to 1.48) |
| Model 2, HR (95% CI) | 1.07 (0.86 to 1.33) | Reference | 1.26 (1.02 to 1.56) |
| Stroke | |||
| No. of events | 142 | 121 | 106 |
| Model 1, HR (95% CI) | 1.12 (0.84 to 1.50) | Reference | 1.06 (0.78 to 1.43) |
| Model 2, HR (95% CI) | 1.04 (0.77 to 1.39) | Reference | 1.12 (0.82 to 1.52) |
| Cardiovascular mortality | |||
| No. of events | 300 | 217 | 218 |
| Model 1, HR (95% CI) | 1.38 (1.11 to 1.71) | Reference | 1.22 (0.97 to 1.54) |
| Model 2, HR (95% CI) | 1.21 (0.96 to 1.51) | Reference | 1.33 (1.05 to 1.67) |
| Cardiovascular events | |||
| No. of events | 487 | 409 | 421 |
| Model 1, HR (95% CI) | 1.20 (1.02 to 1.40) | Reference | 1.19 (1.02 to 1.40) |
| Model 2, HR (95% CI) | 1.09 (0.93 to 1.27) | Reference | 1.28 (1.09 to 1.50) |
| All-cause mortality | |||
| No. of events | 582 | 460 | 420 |
| Model 1, HR (95% CI) | 1.13 (0.97 to 1.32) | Reference | 1.07 (0.92 to 1.26) |
| Model 2, HR (95% CI) | 1.06 (0.91 to 1.23) | Reference | 1.12 (0.96 to 1.32) |
| ESRD | |||
| No. of events | 18 | 22 | 12 |
| Model 1, HR (95% CI) | 1.21 (0.58 to 2.54) | Reference | 0.34 (0.14 to 0.82) |
| Model 2, HR (95% CI) | 0.39 (0.17 to 0.87) | Reference | 0.61 (0.25 to 1.53) |
Model 1 adjusted for age, sex, smoking, body height, waist circumference, fasting glucose, systolic BP, and albuminuria. Model 2 adjusted for age, sex, smoking, body height, waist circumference, fasting glucose, systolic BP, albuminuria, and eGFR. HR, hazard ratio; 95% CI, 95% confidence interval.
In exploratory analyses, patients in the first tertile of kidney length were at lower risk of ESRD (HR, 0.39; 95% CI, 0.17 to 0.87) compared with patients in the second tertile of kidney length, after additional adjustment for eGFR (model 2). Compared with patients in the second tertile of kidney length, there was a higher risk of myocardial infarction (HR, 1.26; 95% CI, 1.02 to 1.56), cardiovascular mortality (HR, 1.33; 95% CI, 1.05 to 1.67), and cardiovascular events (HR, 1.28; 95% CI, 1.09 to 1.50) for patients in the third tertile of kidney length after additional adjustment for eGFR.
No significant effect modification was observed for patients with clinically manifest vascular disease compared with patients without vascular disease (P value for interaction >0.05). Also, there was no effect modification by eGFR on the relationship between kidney length and adverse outcomes (P values for interaction >0.05). This means that the relationship between kidney length and adverse outcomes was not different for patients with low or high eGFR.
Sensitivity analyses were performed by excluding 412 patients with kidney length differences of >2 cm. Excluding these patients did not change the results (Supplemental Table 3). Excluding 1974 patients with diabetes did not substantially change the direction or the magnitude of the effects estimates, except that patients in the first tertile of kidney length were no longer at lower risk of ESRD (Supplemental Table 4).
Discussion
In this prospective cohort study of patients at high cardiovascular risk, kidney length is strongly correlated with body surface area and eGFR. Patients with large kidney length were at higher risk for cardiovascular events and mortality compared with patients with average kidney length. Patients with small kidney length were not at higher risk for adverse cardiovascular events.
For proper evaluation, kidney length needs to be interpreted taking body height of the patient into account because individual variation in kidney length is mainly correlated with anthropomorphic characteristics (18–21). The relationship between anthropometric characteristics, such as body height and body surface area, and kidney length was confirmed in this study. The differences in kidney length between sex seemed to be on the basis of variation in body height. However, a previous study on renal parenchymal volume demonstrated this was smaller in women, irrespective of body surface area (22). This observation suggests that differences in kidney size are not merely explained by differences in body size.
Kidney length could also potentially be used as a marker to identify patients who confer a higher risk of cardiovascular events and mortality. We demonstrated that large kidney length is related to relevant cardiovascular and mortality outcomes. Patients with small kidney length were no longer at higher risk of cardiovascular adverse outcomes after additional adjustment for eGFR. This might be because of the relationship between eGFR and kidney length. The relationship between kidney size and kidney function is in line with other studies that reported that eGFR is related to various measurements of kidney size (19,21,22). This finding suggests that the higher risk of cardiovascular events and mortality for patients with small kidney length is probably driven by low eGFR rather than small kidney length, which is an independent risk factor for cardiovascular disease (1). Furthermore, we found that there was no effect modification by eGFR, indicating that the relationship between kidney length and adverse events was not different for patients with low or high eGFR. Therefore, in patients who are considered to have a normal kidney function, large kidney length could be a marker of higher cardiovascular and renal risk.
The mechanism of how large kidneys are related to higher risk of vascular and renal adverse events remains unclear. Enlargement of the kidneys was first discovered in 1973 in diabetic patients and has been related to glomerular hyperfiltration (23,24). The presence of several pathophysiologic mechanisms are thought to play a role in the development of glomerular hyperfiltration, including hemodynamic changes such as increased glomerular capillary flow and glomerular capillary pressure (25). Glomerular hyperfiltration is considered to be an early manifestation of nephropathy and is associated with vascular risk factors, such as diabetes, hypertension, and smoking (26,27). In more advanced stages of diabetic nephropathy, large kidneys have been related to higher risk of dialysis, suggesting that patients with large kidneys are at risk of progressive kidney function loss (5). However, presence of diabetes cannot explain the observed higher cardiovascular risk in patients with large kidney length because the results were adjusted for fasting glucose, and in a sensitivity analysis, exclusion of diabetic patients yielded similar results. The relationship between large kidney length and cardiovascular events in the analyses without adjustment of eGFR could potentially be diluted by patients with normal to high eGFR because a relationship with myocardial infarction and cardiovascular mortality is observed after adjustment for eGFR. This suggests that the observed higher cardiovascular risk might be because of a discrepancy between kidney function and kidney size. It could therefore be speculated that large kidney length without corresponding normal to high eGFR may identify patients who are progressively declining in kidney function.
The strengths of this study include the design of a single-center prospective cohort study with substantial follow-up duration and a large number of clinical relevant outcomes. Kidney measurements were performed according to a protocol by well trained technicians.
Some limitations need to be considered. Computed tomography is a more precise method for measuring kidney length compared with ultrasonography (28). A previous study reported that the intra- and interobserver mean difference of the kidney length measurements were −0.01 cm (95% CI, −1.36 to 1.33) and −0.07 cm (95% CI, −1.27 to 1.13), respectively (29). This indicates that there might be a slight difference between repeated measurements. In clinical practice, ultrasonography is most often used in the initial evaluation of patients suspected of kidney disease and therefore enhances the applicability of our results. Furthermore, kidney function was estimated using the CKD-EPI equation, which can underestimate at high values (8). Because only 16 patients had an eGFR≥130 ml/min per 1.73 m2, and they were equally distributed across tertiles of kidney length, it is unlikely that this influenced the results. Lastly, the study population included patients with clinically manifest vascular disease and risk factors for vascular disease, which might decrease generalizability to the general population. It is not known whether the tertiles of kidney length, as observed in this study, fall within the distribution of kidney length in the general population. A study conducted within a general population cohort is needed to establish if these results are applicable to a low-risk population.
In conclusion, in patients at high cardiovascular risk, large kidney length is related to higher risk of cardiovascular events and mortality, irrespective of eGFR. In patients with normal kidney function, measurement of kidney length in clinical practice may serve as a marker to further identify patients at high cardiovascular risk.
Disclosures
None.
Supplementary Material
Acknowledgments
We gratefully acknowledge the contribution of the Second Manifestations of ARTerial disease (SMART) research nurses, R van Petersen (data manager), B.G.F. van Dinther (vascular manager), and the members of the SMART study group: A. Algra, D.E. Grobbee, G.E.H.M. Rutten, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands; and L.J. Kappelle, Department of Neurology, University Medical Center Utrecht, Utrecht, The Netherlands.
This work was supported by grant 1302-059 from Fonds NutsOhra, The Netherlands to P.B.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08990816/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Bax L, van der Graaf Y, Rabelink AJ, Algra A, Beutler JJ, Mali WP; SMART Study Group : Influence of atherosclerosis on age-related changes in renal size and function. Eur J Clin Invest 33: 34–40, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Chade AR, Lerman A, Lerman LO: Kidney in early atherosclerosis. Hypertension 45: 1042–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Rigalleau V, Garcia M, Lasseur C, Laurent F, Montaudon M, Raffaitin C, Barthe N, Beauvieux MC, Vendrely B, Chauveau P, Combe C, Gin H: Large kidneys predict poor renal outcome in subjects with diabetes and chronic kidney disease. BMC Nephrol 11: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emamian SA, Nielsen MB, Pedersen JF, Ytte L: Kidney dimensions at sonography: Correlation with age, sex, and habitus in 665 adult volunteers. AJR Am J Roentgenol 160: 83–86, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Cheong B, Muthupillai R, Rubin MF, Flamm SD: Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol 2: 38–45, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Miletić D, Fuckar Z, Sustić A, Mozetic V, Stimac D, Zauhar G: Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound 26: 185–189, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y: Second manifestations of ARTerial disease (SMART) study: Rationale and design. Eur J Epidemiol 15: 773–781, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–311, discussion 312–313, 1989 [PubMed] [Google Scholar]
- 12.Singh GR, Hoy WE: Kidney volume, blood pressure, and albuminuria: Findings in an Australian aboriginal community. Am J Kidney Dis 43: 254–259, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Päivänsalo MJ, Merikanto J, Savolainen MJ, Lilja M, Rantala AO, Kauma H, Reunanen A, Kesäniemi YA, Suramo I: Effect of hypertension, diabetes and other cardiovascular risk factors on kidney size in middle-aged adults. Clin Nephrol 50: 161–168, 1998 [PubMed] [Google Scholar]
- 14.Paajanen TA, Oksala NK, Kuukasjärvi P, Karhunen PJ: Short stature is associated with coronary heart disease: A systematic review of the literature and a meta-analysis. Eur Heart J 31: 1802–1809, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Caps MT, Zierler RE, Polissar NL, Bergelin RO, Beach KW, Cantwell-Gab K, Casadei A, Davidson RC, Strandness DE Jr: Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int 53: 735–742, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Guzman RP, Zierler RE, Isaacson JA, Bergelin RO, Strandness DE Jr: Renal atrophy and arterial stenosis. A prospective study with duplex ultrasound. Hypertension 23: 346–350, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Donders AR, van der Heijden GJ, Stijnen T, Moons KG: Review: A gentle introduction to imputation of missing values. J Clin Epidemiol 59: 1087–1091, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Glodny B, Unterholzner V, Taferner B, Hofmann KJ, Rehder P, Strasak A, Petersen J: Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol 9: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariyanna SS, Light RP, Agarwal R: A longitudinal study of kidney structure and function in adults. Nephrol Dial Transplant 25: 1120–1126, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Jovanović D, Gasic B, Pavlovic S, Naumovic R: Correlation of kidney size with kidney function and anthropometric parameters in healthy subjects and patients with chronic kidney diseases. Ren Fail 35: 896–900, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Ozbek O, Solak Y, Guler I, Ozbiner H, Ozbek S, Turkmen K, Nayman A, Biyik Z, Samur C, Turk S: Predictors of kidney dimensions measured by multi-detector computed tomography (MDCT) in 930 middle-aged and elderly patients. Ren Fail 34: 53–59, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Johnson S, Rishi R, Andone A, Khawandi W, Al-Said J, Gletsu-Miller N, Lin E, Baumgarten DA, O’Neill WC: Determinants and functional significance of renal parenchymal volume in adults. Clin J Am Soc Nephrol 6: 70–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogensen CE, Andersen MJ: Increased kidney size and glomerular filtration rate in early juvenile diabetes. Diabetes 22: 706–712, 1973 [DOI] [PubMed] [Google Scholar]
- 24.Christiansen JS, Gammelgaard J, Frandsen M, Parving HH: Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia 20: 451–456, 1981 [DOI] [PubMed] [Google Scholar]
- 25.Premaratne E, Verma S, Ekinci EI, Theverkalam G, Jerums G, MacIsaac RJ: The impact of hyperfiltration on the diabetic kidney. Diabetes Metab 41: 5–17, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S: Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 27: 1821–1825, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Maeda I, Hayashi T, Sato KK, Koh H, Harita N, Nakamura Y, Endo G, Kambe H, Fukuda K: Cigarette smoking and the association with glomerular hyperfiltration and proteinuria in healthy middle-aged men. Clin J Am Soc Nephrol 6: 2462–2469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang KY, Lee YJ, Park SC, Yang CW, Kim YS, Moon IS, Koh YB, Bang BK, Choi BS: A comparative study of methods of estimating kidney length in kidney transplantation donors. Nephrol Dial Transplant 22: 2322–2327, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, Beek FJ: Renal volume measurements: Accuracy and repeatability of US compared with that of MR imaging. Radiology 211: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.