Abstract
Background and objectives
To assess the association between self-reported sleep duration and quality and odds of having CKD in Chinese adults on the basis of a community study.
Design, setting, participants, & measurements
In this cross-sectional study, we included 11,040 Chinese adults who participated in an ongoing prospective study, the Kailuan cohort. Survey questionnaire items addressed insomnia, daytime sleepiness, snoring, and sleep duration during their 2012 interview. Overall sleep quality was evaluated by summarizing these four sleep parameters. Fasting blood samples and single random midstream morning urine samples were collected in 2012 and analyzed for serum creatinine and proteinuria. CKD was defined by eGFR<60 ml/min per 1.73 m2 or proteinuria >300 mg/dl. We also examined those at high or very high risk of having CKD, on the basis of the Kidney Disease Improving Global Outcomes recommendations. The association between sleep quality and CKD was assessed using logistic regression model.
Results
Worse overall sleep quality was associated with higher likelihood of being high or very high risk for CKD (multiadjusted odds ratio, 2.69; 95% confidence interval, 1.30 to 5.59 comparing two extreme categories; P trend <0.01), but not overall CKD (multiadjusted odds ratio, 1.58; 95% confidence interval, 0.89 to 2.80 comparing two extreme categories; P trend =0.46), after adjusting for potential confounders. Specifically, individuals with worse sleep quality were more likely to have proteinuria (multiadjusted odds ratio, 1.95; 95% confidence interval, 1.03 to 3.67 comparing two extreme categories; P trend =0.02), rather than lower eGFR level (multiadjusted mean eGFR levels were 96.4 and 93.6 ml/min per 1.73 m2 in the two extreme sleep categories, respectively; P trend =0.13). However, there was no statistically significant association between individual sleep parameters and CKD status.
Conclusions
Worse overall sleep quality was associated with higher odds of being high or very high risk for CKD and proteinuria in Chinese adults.
Keywords: Sleep; chronic kidney disease; cross-sectional study; Adult; creatinine; Cross-Sectional Studies; Fasting; glomerular filtration rate; Humans; Kidney Function Tests; Logistic Models; Prospective Studies; proteinuria; Renal Insufficiency, Chronic; Self Report; Sleep Initiation and Maintenance Disorders; Snoring; Surveys and Questionnaires
Introduction
The prevalence of CKD is estimated to be 8%–16% worldwide (1), and approximately 11% in China (2). Individuals with CKD generally have a higher risk of substantial complications, including progression to ESRD (3), cardiovascular disease (4), and mortality (5). In addition to genetic predisposition factors, risk factors for CKD include presence of certain conditions (e.g., obesity, diabetes, and hypertension) and unhealthy lifestyle factors (e.g., smoking and physical inactivity) (6).
Sleep disorders may be a potential risk factor for CKD because they directly result in chronobiologic alterations in the renin-angiotensin-aldosterone system and sympathetic nervous system activation (7). Furthermore, sleep disorders may indirectly contribute to risk of CKD through influences on the aforementioned diseases (8–10). The notion that sleep disorders may be a risk factor for CKD is supported by the observation that 50%–80% of patients undergoing conventional hemodialysis experience certain symptoms of sleep disorders, such as insomnia or excessive daytime sleepiness (11). However, only a few studies to date have examined the association between sleep and risk of CKD in the general adult population, and results are inconsistent. For example, in some retrospective studies, nonapnea sleep disorder or short sleep duration were associated with a higher risk of CKD or proteinuria (12,13). In contrast, some studies with modest sample sizes (n<500) reported that individuals with short sleep duration, poorer sleep quality, or obstructive sleep apnea (OSA) had a higher kidney filtration rate relative to others (14,15). Although these studies did not control for important confounders (e.g., socioeconomic status [12,13,15] and relevant biochemical markers [9,12,13]), were limited by small sample size (14,15), and had inconsistent results, there is suggestion of a potential link between sleep and kidney function.
We thus conducted a large-scale community-based study including >10,000 Chinese adults to comprehensively assess the association between sleep parameters (i.e., insomnia, daytime sleepiness, snoring, and sleep duration) and odds of having CKD, on the basis of the combined indices of eGFR and proteinuria (16). The overall sleep quality score represents a combination of different sleep parameters; thus, it may be a more powerful predictor of health outcomes than any single assessment alone. As a secondary outcome, we also examined four individual sleep parameters.
Materials and Methods
Participants
The current cross-sectional analysis was on the basis of a subset of the Kailuan cohort, a multicenter ongoing cohort including 101,510 Chinese adults (81,110 men and 20,400 women) aged 18–98 years, in 2006–2007, living in Tangshan City, China (17). In the Kailuan cohort, all participants underwent baseline questionnaire assessments of lifestyle and health status and clinical and laboratory examinations in 2006–2007; reassessments were conducted every 2 years. All assessments were conducted in 11 hospitals (referred to as centers) responsible for healthcare of the community. In 2012, information on sleep parameters (including insomnia, daytime sleepiness, snoring, and sleep duration) was collected among 12,990 participants (10,725 men and 2265 women) who underwent examination at one center (i.e., the Kailuan General Hospital). Participants with incomplete information on sleep parameters (n=265), serum creatinine or proteinuria (n=596), or covariates (n=989) were excluded, resulting in 11,040 participants (9197 men and 1843 women) in this analysis. Compared with participants included in the analysis, the participants who were not included in this analysis were older (58.3 versus 53.7 years; P<0.001), had lower education levels, higher prevalence of major chronic diseases (such as hypertension, diabetes, history of stroke, or cancer), and included a higher proportion of women (21.4% versus 16.7%; P<0.001). The study was jointly approved by the Ethics Committee of the Kailuan General Hospital and the Human Subjects Committee at Brigham and Women’s Hospital/Harvard Medical School.
Assessment of CKD
Twelve-hour fasting blood samples were collected before 9:00 a.m. at the face-to-face interview in 2012. Serum creatinine was assessed using the sarcosine oxidase assay method (creatinine kit; BioSino Bio-technology and Science Inc., Beijing, China), with a lower limit detection of 22 umol/L and an upper limit detection of 3000 umol/L (linear correlation coefficient ≥0.99). Within-laboratory intra- and interassay variable coefficients for serum creatinine were ≤5% and ≤6%, respectively. eGFR was computed using serum creatinine, sex, and age, according to the CKD Epidemiology Collaboration equation:
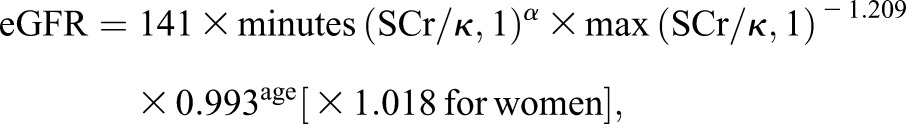 |
where SCr is serum creatinine (in milligram per deciliter), κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min is the minimum of SCr/κ or 1, and max is the maximum of SCr/κ or 1 (18).
A single random midstream morning urine sample was collected from each participant during their interview. Urine protein concentration was assessed using the dry-chemistry method and standard urinary sediment examination within 2 hours (H12-MA test strips; Changchun Dirui Medical Technology Co., Ltd., Changchun, China) with a lower limit detection of 15 mg/dl. All of the urine samples were measured using a urine analyzer (N-600; Changchun Dirui Medical Technology Co., Ltd.) at the central laboratory of the Kailuan General Hospital. The results of semiquantitative proteinuria were recorded as negative (<15 mg/dl), trace (15–29 mg/dl), 1+ (30–300 mg/dl), 2+ (300–1000 mg/dl), or 3+ (>1000 mg/dl).
CKD Outcome
In this study, CKD was defined and classified according to the status of eGFR and proteinuria. Participants with eGFR<60 ml/min per 1.73 m2 or proteinuria ≥2+ (>300 mg/dl) were considered as having CKD in this analysis. We also examined the association between sleep quality and high or very high risk of CKD, as defined by the Kidney Disease Improving Global Outcomes 2012 recommendations (16). Specifically, the eGFR categories were defined as eGFR≥90 ml/min per 1.73 m2 (G1), 60–89 ml/min per 1.73 m2 (G2), 45–59 ml/min per 1.73 m2 (G3a), 30–44 ml/min per 1.73 m2 (G3b), 15–29 ml/min per 1.73 m2 (G4), and <15 ml/min per 1.73 m2 (G5); proteinuria categories were defined as negative and trace (<30 mg/dl, A1), 1+ (30–300 mg/dl, A2), and ≥2+ (>300 mg/dl, A3). Participants with G1–2 and A3, or G3a and A2, or G3b and A1 were defined as being high risk for CKD. Participants with G4–5 and A1, or G3b–5 and A2, or G3a–5 and A3 were defined as being very high risk for CKD.
Assessment of Sleep Parameters (Exposure)
Sleep parameters in this study included insomnia, daytime sleepiness, sleep duration, and snoring. All information on sleep parameters was collected via questionnaires using verbal query by a trained interviewer at Kailuan General Hospital healthy examination center during their 2012 interview, as detailed below.
Insomnia
Insomnia status of the participants in the past month was assessed using the Chinese version of the Athens Insomnia Scale (AIS) (19). The AIS is a self-report questionnaire including eight items: the first five items estimate sleep procedure (sleep induction, night awakening, awakening in the early morning, total sleep duration, and total quality of sleep) and the last three items assess decreased sense of wellbeing, overall functioning, and daytime sleepiness. Each item was scored from 0 to 3 (0= no event, 1= mild, 2= moderate, and 3= severe). A total AIS score ≥6 was considered as insomnia (20). The AIS has been validated in China (20) and demonstrated good test-retest reliability (83%), sensitivity (96%), and specificity (76%) in Chinese adults, relative to the physician diagnosis on the basis of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (20).
Daytime Sleepiness
We used the Chinese version of Epworth Sleepiness Scale (ESS) to measure daytime sleepiness (21,22). The ESS includes eight questions, each scoring 0–3 with increasing scores signifying higher chance of falling asleep while engaged in specific situations of daily life. A total ESS score ≥10 was considered as excessive daytime sleepiness (21). In a validation study of the Chinese ESS, in which 31 Chinese bilingual adults completed both Chinese ESS and English ESS, the Chinese ESS demonstrated high internal consistency (Cronbach α =0.81), good test-retest reliability (rho =0.74), and significant correlation (Spearman rho =0.75) with English ESS in Chinese adults (22).
Sleep Duration and Snore Status
We collected information on sleep duration and snoring via questions. Participants were asked to report usual total hours of actual sleep during the night. In this analysis, we divided participants into four groups (<6, 6–7, 7–8, and ≥8 h/d) on the basis of their sleep duration; only approximately 1% of participants reported sleep duration of 9+ h/d.
Snoring was defined in this study as self-reported snoring plus self-reported breathing stops (i.e., apneas); breathing stops were explicitly defined as stops in breathing for >10 seconds before breathing resumed, occurring more than an estimated 50 times per night. The study definition of snoring was provided to participants and participants self-reported frequency of snoring as never/rare, occasional, or frequent.
Overall Sleep Quality Score
To comprehensively evaluate the association between sleep parameters and odds of having CKD, we further calculated an overall sleep quality score by combining all four sleep parameters, with a total range between 0 (best) and 8 (worst), as detailed in Supplemental Table 1. In this study, the overall sleep score was treated as a continuous variable and a categorical variable; a score of >5 was considered as worst sleep quality, 3–5 as poor sleep quality, and <3 as best sleep quality.
Assessment of Covariates
Information on covariates that was collected via questionnaires in 2012 included education level (primary, middle, or college), income level (<600, 600–1000, or >1000 RMB/mo), occupation (white collar, blue collar, or coalminer), physical activity (never, <4, or ≥4 times per week), smoking status (never, past smoker, or current smoker), alcohol consumption (never, past drinker, or current drinker), use of medications (e.g., sleep medications, hypoglycemic agents, and antihypertensives), and history of cancer, myocardial infarction (MI), and stroke.
Weight and height were measured by trained field workers during their 2012 interview; body mass index (BMI) was calculated as weight (kilogram)/height (square meters). Systolic and diastolic BPs were measured twice from the seated position using a mercury sphygmomanometer. The average of the two readings was used for the analyses. Hypertension was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg, or use of antihypertensive medications in last 2 weeks irrespective of BP status. Participants with systolic BP between 120 and 129 mmHg or diastolic BP of 80–89 mmHg were considered as having prehypertension.
The blood concentrations of glucose, triglyceride, LDL cholesterol, and HDL cholesterol were measured by an enzymatic method using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan General Hospital. Diabetes was defined as a fasting blood glucose concentration ≥7 mmol/L, or active treatment with insulin or any oral hypoglycemic agent. Impaired fasting glucose was defined as a fasting blood glucose concentration of 5.6–6.9 mmol/L.
Statistical Analyses
Statistical analyses were performed with SAS version 9.2 (SAS Institute, Inc., Cary, NC). Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) for the association between overall sleep quality score and individual sleep parameters and odds of having CKD or proteinuria. We adjusted for age, sex, education level, income level, occupation, physical activity, smoking, alcohol consumption, and BMI, diabetes, hypertension, MI, stroke, cancer, use of sleep medications, and plasma concentrations of triglyceride, LDL, and HDL. Because ORs tend to overestimate the effect size, we also calculated prevalence ratio (PR) using SAS procedure PROC GENMOD (23), adjusting for aforementioned covariates. The association between sleep quality and continuous eGFR was examined using SAS procedure PROC GLM, adjusting for potential confounders.
Because major chronic disease (e.g., diabetes, hypertension, MI, stroke, and cancer) and use of sleep medications might have effects on both sleep disorders and odds of having CKD, we conducted another separate sensitivity analysis by excluding participants with these diseases or conditions.
We also explored potential interactions between overall sleep quality score and age, sex, BMI, diabetes status, and between each sleep parameter, in relation to odds of having CKD or being high/very high risk for CKD, by including multiplicative terms in the multivariate logistic regression models.
Results
The characteristics of the cohort on the basis of CKD status in 2012 are shown in Table 1. Approximately 9% participants reported insomnia (AIS score ≥6), approximately 2.0% participants reported daytime sleepiness (ESS score ≥10), approximately 40% participants reported any snoring, and the mean sleep duration was 7 h/d (range, 4–14 h/d).
Table 1.
The basic characteristics in 2012 according to eGFR/proteinuria status
| Characteristics | eGFR<60 ml/min per 1.73 m2 or Proteinuria >300 mg/dl | P Value | ||
|---|---|---|---|---|
| No | Yes | |||
| N | 10,449 | 591 | ||
| Age, yr | 53.4±10.9 | 58.4±11.7 | <0.001 | |
| Overall sleep quality score | <3 | 7840 (75.0%) | 425 (71.9%) | 0.01 |
| 3–5 | 2479 (23.7%) | 151 (25.6%) | ||
| >5 | 130 (1.24%) | 15 (2.54%) | ||
| Athens Insomnia Scale score | <6 | 9372 (89.7%) | 514 (87.0%) | 0.04 |
| ≥6 | 1077 (10.3%) | 77 (13.0%) | ||
| The Epworth Sleepiness Scale score | <10 | 10257 (98.2%) | 567 (95.9%) | <0.001 |
| ≥10 | 192 (1.80%) | 24 (4.10%) | ||
| Sleep duration | <6 h/d | 1342 (12.8%) | 76 (12.9%) | 0.007 |
| 6–7 h/d | 2220 (21.3%) | 105 (17.8%) | ||
| 7–8 h/d | 1850 (17.7%) | 85 (14.4%) | ||
| ≥8 h/d | 5037 (48.2%) | 325 (54.9%) | ||
| Snoring | No | 6243 (59.8%) | 353 (59.7%) | 0.82 |
| Occasional | 2507 (23.9%) | 137 (23.2%) | ||
| Frequent | 1699 (16.3%) | 101 (17.1%) | ||
| Use of sleep medication | Yes | 1107 (10.6%) | 61 (10.3%) | 0.83 |
| Education level | Primary school | 560 (5.40%) | 51 (8.60%) | <0.001 |
| High school | 8909 (85.3%) | 511 (86.5%) | ||
| College | 980 (9.30%) | 29 (4.90%) | ||
| Income level, RMB/mo | <600 | 2352 (22.5%) | 146 (24.7%) | <0.001 |
| 600–1000 | 7422 (71.0%) | 348 (58.9%) | ||
| >1000 | 675 (6.5%) | 97 (16.4%) | ||
| Occupation | White collar | 697 (6.7%) | 41 (6.9%) | 0.25 |
| Blue collar | 5309 (50.8%) | 319 (54.0%) | ||
| Coalminer | 4443 (42.5%) | 231 (39.1%) | ||
| Physical activity, >20 min each time | Never | 5110 (48.9%) | 212 (35.9%) | <0.001 |
| <4 times/wk | 3766 (36.0%) | 280 (47.4%) | ||
| ≥4 times/wk | 1573 (15.1%) | 99 (16.8%) | ||
| Smoking status | Never | 5614 (53.7%) | 330 (55.8%) | 0.04 |
| Past smoker | 637 (6.1%) | 48 (8.20%) | ||
| Current smoker | 4198 (40.2%) | 213 (36.0%) | ||
| Alcohol consumption | Never | 6003 (57.5%) | 365 (61.8%) | 0.11 |
| Past drinker | 3216 (30.8%) | 167 (28.3%) | ||
| Current drinker | 1230 (11.8%) | 59 (9.9%) | ||
| Myocardial infarction history | Yes | 123 (1.18%) | 8 (1.35%) | 0.69 |
| Stroke history | Yes | 229 (2.19%) | 24 (4.06%) | 0.003 |
| Cancer | Yes | 21 (0.20%) | 1 (0.17%) | 0.87 |
| Hypertension | No | 1753 (16.8%) | 28 (4.74%) | <0.001 |
| Prehypertension | 3869 (37.0%) | 165 (27.9%) | ||
| Yes | 4827 (46.2%) | 398 (67.3%) | ||
| Diabetes | No | 6882 (65.9%) | 293 (49.6%) | 0.03 |
| Prediabetes | 2437 (23.3%) | 144 (23.4%) | ||
| Yes | 1130 (10.8%) | 154 (26.0%) | ||
| Body mass index, kg/m2 | 25.0±3.36 | 25.9±3.47 | <0.001 | |
| LDL cholesterol, mmol/L | 2.10±1.09 | 2.41±1.13 | <0.001 | |
| HDL cholesterol, mmol/L | 1.41±0.68 | 1.40±0.65 | 0.59 | |
| Triglyceride, mmol/L | 1.73±1.07 | 2.26±2.05 | <0.001 | |
Continuous variables were reported as means±SD. Categorical variables were reported as N (%).
We observed that worse overall sleep quality was associated with higher likelihood of being high or very high risk for CKD (multiadjusted OR, 2.69; 95% CI, 1.30 to 5.59 comparing two extreme categories; P trend <0.01), but not overall CKD (multiadjusted OR, 1.58; 95% CI, 0.89 to 2.80 comparing two extreme categories; P trend =0.46), after adjusting for potential confounders (Table 2). Participants with insomnia, daytime sleepiness, shorter/prolonged sleep duration, and frequent snoring also tended to have higher likelihood of being high or very high risk for CKD. However, the associations were not significant in the fully adjusted models (Table 2). We obtained similar results when PRs were used to estimate the association between sleep quality and CKD status. The multivariate-adjusted PR, comparing two extreme sleep quality categories, was 1.92 (95% CI, 1.08 to 3.42; P trend =0.03) for likelihood of being high or very high risk for CKD and 0.97 (95% CI, 0.66 for 1.43; P trend =0.88) for likelihood of CKD, after adjusting for potential confounders (Supplemental Table 2). Specifically, individuals with worse sleep quality were more likely to have proteinuria (multiadjusted OR, 1.95; 95% CI, 1.03 to 3.67 comparing two extreme sleep categories; P trend =0.02; Table 3), rather than lower eGFR level (adjusted mean eGFR levels were 96.4 and 93.6 ml/min per 1.73 m2 in two extreme sleep categories, respectively; P trend =0.13; Table 4).
Table 2.
The odds ratios (ORs) and 95% confidence intervals (95% CIs) of odds of having CKD, according to sleep status
| High or Very High Risk for CKD | eGFR<60 ml/min per 1.73 m2 or Proteinuria >300 mg/dl | ||||||
|---|---|---|---|---|---|---|---|
| Sleep parameters | Case/N | Age-Sex Adjusted | Fully Adjusteda | Case/N | Age-Sex Adjusted | Fully Adjusteda | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Overall sleep quality score | <3 | 146/8265 | Ref 1 | Ref 1 | 425/8265 | Ref 1 | Ref 1 |
| 3–5 | 69/2630 | 1.46 (1.09 to 1.95) | 1.35 (1.00 to 1.81) | 151/2630 | 1.07 (0.88 to 1.29) | 1.03 (0.82 to 1.22) | |
| >5 | 9/145 | 3.37 (1.68 to 6.78) | 2.69 (1.30 to 5.59) | 15/145 | 1.88 (1.09 to 3.25) | 1.58 (0.89 to 2.80) | |
| P trend | <0.001 | <0.01 | 0.12 | 0.46 | |||
| AIS score | <6 | 190/9886 | Ref 1 | Ref 1 | 514/9886 | Ref 1 | Ref 1 |
| ≥6 | 34/1154 | 1.47 (1.01 to 2.13) | 1.45 (0.96 to 2.06) | 77/1154 | 1.22 (0.95 to 1.56) | 1.18 (0.91 to 1.53) | |
| P difference | 0.04 | 0.08 | 0.12 | 0.22 | |||
| ESS score | <10 | 215/10,824 | Ref 1 | Ref 1 | 567/10,824 | Ref 1 | Ref 1 |
| ≥10 | 9/216 | 2.03 (1.03 to 4.02) | 1.62 (0.79 to 3.30) | 24/216 | 2.08 (1.34 to 3.22) | 1.48 (0.93 to 2.35) | |
| P difference | 0.04 | 0.19 | 0.001 | 0.10 | |||
| Sleep duration | <6 h/d | 39/1418 | 1.60 (0.99 to 2.59) | 1.62 (0.99 to 2.64) | 76/1418 | 1.09 (0.79 to 1.49) | 1.16 (0.84 to 1.61) |
| 6–7 h/d | 40/2325 | 1.05 (0.65 to 1.68) | 1.09 (0.67 to 1.75) | 105/2325 | 0.98 (0.73 to 1.32) | 1.06 (0.78 to 1.43) | |
| 7–8 h/d | 31/1935 | Ref 1 | Ref 1 | 85/1935 | Ref 1 | Ref 1 | |
| ≥8 h/d | 114/5362 | 1.22 (0.82 to 1.83) | 1.22 (0.80 to 1.84) | 325/5362 | 1.25 (0.97 to 1.59) | 1.19 (0.92 to 1.54) | |
| P trend | 0.78 | 0.85 | 0.03 | 0.21 | |||
| Snoring | No | 123/6596 | Ref 1 | Ref 1 | 353/6596 | Ref 1 | Ref 1 |
| Occasional | 52/2644 | 1.12 (0.81 to 1.56) | 1.01 (0.72 to 1.42) | 137/2644 | 1.36 (0.85 to 1.27) | 0.98 (0.79 to 1.22) | |
| Frequent | 49/1800 | 1.50 (1.07 to 2.11) | 1.26 (0.88 to 1.81) | 101/1800 | 1.05 (0.83 to 1.31) | 0.95 (0.75 to 1.22) | |
| P trend | 0.02 | 0.26 | 0.66 | 0.71 | |||
AIS, Athens Insomnia Scale; ESS, the Epworth Sleepiness Scale.
Adjusted for age, sex, education level (primary, high school, or college), income level (<600, 600–1000, or >1000 RMB/mo), occupation (white collar, blue collar, or coalminer), physical activity (never, <4, or ≥4 times/week), smoking status (never, past smoker, or current smoker), alcohol consumption (never, past drinker, or current drinker), myocardial infarction history (no, yes), stroke history (no, yes), cancer history (no, yes), hypertension (no, prehypertension, or hypertension), diabetes (no, prediabetes, or diabetes), use of sleep medication (no, yes), body mass index (<24, 24–28, or ≥28 kg/m2), triglyceride (<0.82, 0.82–1.22, 1.22–1.87, or ≥1.87 mmol/L), LDL (<1.76, 1.76–2.12, 2.12–2.65, or ≥2.65 mmol/L), and HDL (<1.31, 1.31–1.54, 1.54–1.79, or ≥1.79 mmol/L). Each set of sleep variables was modeled separately with adjustment for the covariates.
Table 3.
The odds ratios (ORs) and 95% confidence intervals (95% CIs) of odds of having proteinuria, according to sleep quality
| Overall Sleep Quality Score | Case/N | Multivariate Adjusted Model 1a | Multivariate Adjusted Model 2b |
|---|---|---|---|
| <3 | 240/8265 | Ref 1 | Ref 1 |
| 3–5 | 109/2630 | 1.21 (0.95 to 1.54) | 1.21 (0.95 to 1.54) |
| >5 | 12/145 | 1.95 (1.03 to 3.67) | 1.81 (0.95 to 3.44) |
| P trend | 0.02 | 0.03 |
Adjusted for age, sex, education level (primary, high school, or college), income level (<600, 600–1000, or >1000 RMB/mo), occupation (white collar, blue collar, or coalminer), physical activity (never, <4, or ≥4 times/week), smoking status (never, past smoker, or current smoker), alcohol consumption (never, past drinker, or current drinker), myocardial infarction history (no, yes), stroke history (no, yes), cancer history (no, yes), hypertension (no, prehypertension, or hypertension), diabetes (no, prediabetes, or diabetes), use of sleep medication (no, yes), body mass index (<24, 24–28, or ≥28 kg/m2), triglyceride (<0.82, 0.82–1.22, 1.22–1.87, or ≥1.87 mmol/L), LDL (<1.76, 1.76–2.12, 2.12–2.65, or ≥2.65 mmol/L), and HDL (<1.31, 1.31–1.54, 1.54–1.79, or ≥1.79 mmol/L).
Further adjusted for eGFR (ml/min per 1.73 m2).
Table 4.
Mean eGFR (ml/min per 1.73 m2) and 95% CIs, according to sleep quality
| Overall Sleep Quality Score | N | Multivariate Adjusted Model 1a | Multivariate Adjusted Model 2b |
|---|---|---|---|
| <3 | 8265 | 96.4 (92.3 to 100.6) | 89.4 (85.1 to 93.8) |
| 3–5 | 2630 | 96.5 (92.4 to 100.6) | 89.6 (85.2 to 93.9) |
| >5 | 145 | 93.6 (88.6 to 98.5)c | 87.3 (82.2 to 93.4) |
| P trend | 0.13 | 0.30 |
Adjusted for age, sex, education level (primary, high school, or college), income level (<600 600–1000, or >1000 RMB/mo), occupation (white collar, blue collar, or coalminer), physical activity (never, <4, or ≥4 times/week), smoking status (never, past smoker, or current smoker), alcohol consumption (never, past drinker, or current drinker), myocardial infarction history (no, yes), stroke history (no, yes), cancer history (no, yes), hypertension (no, prehypertension, or hypertension), diabetes (no, prediabetes, or diabetes), use of sleep medication (no, yes), body mass index (<24, 24–28, or ≥28 kg/m2), triglyceride(<0.82, 0.82–1.22, 1.22–1.87, or ≥1.87 mmol/L), LDL (<1.76, 1.76–2.12, 2.12–2.65, or ≥2.65 mmol/L), and HDL (<1.31, 1.31–1.54, 1.54–1.79, or ≥1.79 mmol/L).
Further adjusted for presence of proteinuria (yes/no).
P<0.05, relative to overall sleep quality score <3.
In the sensitivity analyses, exclusion of the participants with diabetes, hypertension, using sleep medications, MI, stroke, and cancer generated similar results (Supplemental Tables 3–5). We did not find significant interactions between age, sex, BMI, diabetes, and the overall sleep quality score, and between each sleep parameters, in relation to odds of having CKD or being high or very high risk for CKD (P interaction >0.05 for all).
Discussion
In this large-scale community-based study including >10,000 participants, we found that higher overall sleep quality score (i.e., poorer sleep quality) was associated with higher likelihood of being high or very high risk for CKD. The association appeared to be driven by proteinuria rather than lower eGFR. Likewise, participants with insomnia, daytime sleepiness, shorter/prolonged sleep duration, and frequent snoring also tended to have higher likelihood of CKD. However, the associations were not significant.
Our findings are consistent with previous studies that individuals with CKD had a higher prevalence of shorter and higher fragmented sleep and excessive daytime sleepiness, relative to those without CKD (24). In a recent meta-analysis including six observational studies, short sleepers tended to have higher risk of having CKD (pooled OR, 1.51; 95% CI, 0.99 to 2.55) (25). Excessive daytime sleepiness and short sleep duration were also found to be associated with greater 24-hour urinary albumin excretion (26). Similarly, a small sample study (n=91) linked snoring to proteinuria and high serum urea and creatinine concentration (27). High prevalence of OSA was found in nondialysis patients with CKD and the prevalence and severity of OSA increased with progression of CKD stage (28,29). It was also observed that severe OSA was also independently associated with higher serum cystatin C levels in patients without CKD; serum cystatin C is considered to be a biomarker reflecting clinically latent renal dysfunction (30).
Interestingly, several previous studies reported that objective shorter sleep duration or reported apnea-hypopnea index and desaturation index were associated with higher kidney filtration rates (14), or higher eGFR and urine albumin-to-creatinine ratio (15). Previous studies suggested that high levels of eGFR might be an early predictor of diabetes and hypertension (7,10), and were associated with higher incidence of coronary heart disease and mortality (31). In this context, sleep disorders might increase eGFR to abnormally high levels, and subsequently increase the risk for glomerular hypertension, renal injury, and the development of CKD (14). We thus performed sensitivity analyses by excluding participants with diabetes and hypertension, and observed similar relationships between overall sleep quality score and CKD. These findings, together with previous studies, suggest that sleep disorders could damage the renal function in a stepwise manner over a long-term period.
The exact underlying mechanism of association between sleep and CKD remains unknown. There were some lines of possible evidence in support of adverse effect of sleep on development of CKD. As described above, glomerular hyperfiltration, a mark of early kidney damage and risk factor for proteinuria, is observed in individuals with sleep disorders (e.g., OSA) (14,15). Sleep disorders abnormally activate the autonomic nervous system, a key regulator of kidney function, and then produce unfavorable effects on renal hemodynamics and BP (32). It has been observed that individuals with sleep disorders have excessively elevated daytime sympathetic nervous system activity (33) and fail to increase the cardiac vagal tone during the transition from wakefulness to nonrapid eye movement (34). Furthermore, snoring, a symptom of sleep-related breathing disorder, has a notable adverse effect on the regulation of renin-angiotensin-aldosterone system through pathways of hypoxemia, oxidative stress, and activated sympathetic activities, which result in endothelial dysfunction and lead to renal damage (10). Finally, short/prolonged sleep duration and sleep disorders could lead to systemic inflammation (35,36), which potentially results in glomerular endothelial dysfunction and subsequent renal dysfunction (37).
Our study had several limitations. First, this study was cross-sectional, which does not allow for establishing causality of the observed associations. The association between sleep and CKD might be bidirectional. Second, all sleep information was collected via self-report, and was subject to recall bias and misclassification, particularly for sleep duration and OSA (we used snoring as a surrogate). Third, the participants who were not included in this analysis were older and had high prevalence of major chronic diseases, which were associated with a higher risk of CKD. Therefore, we might underestimate the association between sleep and CKD. Fourth, the detection of proteinuria was on the basis of a semiquantitative method from single random urine samples rather than continuous quantitative method using 24-hour urine sample, which could lead to misclassification of CKD. Because of the high sensitivity (93.3%) and specificity (91.6%) of the semiquantitative method relative to the quantitative method (38), the approach used in this study remains a useful method for detecting proteinuria, especially in an epidemiologic study with large sample size.
In conclusion, in this large-scale community-based study we found that participants with lower overall sleep quality had a high likelihood of being high or very high risk for CKD. Future prospective studies are warranted to clarify the temporal relationship between objectively measured sleep disorders and CKD. If our findings are confirmed, sleep disorders as modifiable lifestyle factors may be a novel intervention target for preventing CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
Because G.C. was the Editor-in-Chief of the Clinical Journal of the American Society of Nephrology at the time of peer-review, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
This work is supported by the National Natural Science Foundation of China (grant nos. 81170244 and 81170090).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09270816/-/DCSupplemental.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H: Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 379: 815–822, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, James MT, Hemmelgarn BR; Alberta Kidney Disease Network : Risk of coronary events in people with chronic kidney disease compared with those with diabetes: A population-level cohort study. Lancet 380: 807–814, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallan S, de Mutsert R, Carlsen S, Dekker FW, Aasarød K, Holmen J: Obesity, smoking, and physical inactivity as risk factors for CKD: Are men more vulnerable? Am J Kidney Dis 47: 396–405, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Turek NF, Ricardo AC, Lash JP: Sleep disturbances as nontraditional risk factors for development and progression of CKD: Review of the evidence. Am J Kidney Dis 60: 823–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cepeda MS, Stang P, Blacketer C, Kent JM, Wittenberg GM: Clinical relevance of sleep duration: Results from a cross-sectional analysis using NHANES. J Clin Sleep Med 12: 813–819, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plantinga L, Lee K, Inker LA, Saran R, Yee J, Gillespie B, Rolka D, Saydah S, Powe NR; CDC CKD Surveillance Team : Association of sleep-related problems with CKD in the United States, 2005-2008. Am J Kidney Dis 58: 554–564, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Chaput JP, Després JP, Bouchard C, Astrup A, Tremblay A: Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: Analyses of the Quebec Family Study. Sleep Med 10: 919–924, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Chami H, Budhiraja R, Punjabi NM, Buysse D, Newman AB: Subjective and objective sleep quality in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the sleep heart health study. Am J Kidney Dis 52: 305–313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ST, Lin CL, Yu TM, Yang TC, Kao CH: Nonapnea sleep disorders and incident chronic kidney disease: A population-based retrospective cohort study. Medicine (Baltimore) 94: e429, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto R, Nagasawa Y, Iwatani H, Shinzawa M, Obi Y, Teranishi J, Ishigami T, Yamauchi-Takihara K, Nishida M, Rakugi H, Isaka Y, Moriyama T: Self-reported sleep duration and prediction of proteinuria: A retrospective cohort study. Am J Kidney Dis 59: 343–355, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Petrov ME, Kim Y, Lauderdale DS, Lewis CE, Reis JP, Carnethon MR, Knutson KL, Glasser SP: Objective sleep, a novel risk factor for alterations in kidney function: The CARDIA study. Sleep Med 15: 1140–1146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou YT, Lee PH, Yang CT, Lin CL, Veasey S, Chuang LP, Lin SW, Lin YS, Chen NH: Obstructive sleep apnea: A stand-alone risk factor for chronic kidney disease. Nephrol Dial Transplant 26: 2244–2250, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Wu Z, Jin C, Vaidya A, Jin W, Huang Z, Wu S, Gao X: Longitudinal patterns of blood pressure, incident cardiovascular events, and all-cause mortality in normotensive diabetic people. Hypertension 68: 71–77, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd , Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung KF, Kan KK, Yeung WF: Assessing insomnia in adolescents: Comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med 12: 463–470, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Sun JL, Chiou JF, Lin CC: Validation of the Taiwanese version of the Athens Insomnia Scale and assessment of insomnia in Taiwanese cancer patients. J Pain Symptom Manage 41: 904–914, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Johns MW: A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14: 540–545, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Chen NH, Johns MW, Li HY, Chu CC, Liang SC, Shu YH, Chuang ML, Wang PC: Validation of a Chinese version of the Epworth sleepiness scale. Qual Life Res 11: 817–821, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E: Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162: 199–200, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Light RP: Sleep and activity in chronic kidney disease: A longitudinal study. Clin J Am Soc Nephrol 6: 1258–1265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheungpasitporn W, Thongprayoon C, Gonzalez-Suarez ML, Srivali N, Ungprasert P, Kittanamongkolchai W, Caples SM, Erickson SB: The effects of short sleep duration on proteinuria and chronic kidney disease: A systematic review and meta-analysis [published online ahead of print April 21, 2016]. Nephrol Dial Transplant doi: 10.1093/ndt/gfw072 [DOI] [PubMed]
- 26.Afsar B: The relationship between self-reported nocturnal sleep duration, daytime sleepiness and 24-h urinary albumin and protein excretion in patients with newly diagnosed type 2 diabetes. Prim Care Diabetes 7: 39–44, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Agrawal V, Vanhecke TE, Rai B, Franklin BA, Sangal RB, McCullough PA: Albuminuria and renal function in obese adults evaluated for obstructive sleep apnea. Nephron Clin Pract 113: c140–c147, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Shanmugam GV, Abraham G, Mathew M, Ilangovan V, Mohapatra M, Singh T: Obstructive sleep apnea in non-dialysis chronic kidney disease patients. Ren Fail 37: 214–218, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Nigam G, Pathak C, Riaz M.. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath 20(3): 957–964, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Kato K, Takata Y, Usui Y, Shiina K, Asano K, Hashimura Y, Saruhara H, Nishihata Y, Tomiyama H, Yamashina A: Severe obstructive sleep apnea increases cystatin C in clinically latent renal dysfunction. Respir Med 105: 643–649, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J: Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617–2624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ: Sympathetic hyperactivity in chronic kidney disease: Pathogenesis, clinical relevance, and treatment. Kidney Int 65: 1568–1576, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Somers VK, Dyken ME, Clary MP, Abboud FM: Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roumelioti ME, Ranpuria R, Hall M, Hotchkiss JR, Chan CT, Unruh ML, Argyropoulos C: Abnormal nocturnal heart rate variability response among chronic kidney disease and dialysis patients during wakefulness and sleep. Nephrol Dial Transplant 25: 3733–3741, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM: Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R: Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J Clin Sleep Med 9(10): 1003–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Futrakul N, Sridama V, Futrakul P: Microalbuminuria--A biomarker of renal microvascular disease. Ren Fail 31: 140–143, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Lin CJ, Chen HH, Pan CF, Hsieh WS, Ho HT, Wu CJ: The characteristics of new semi-quantitative method for diagnosing proteinuria by using random urine samples. J Clin Lab Anal 25: 14–19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


