Abstract
Background and objectives
Use of diabetic donor kidneys has been a necessary response to the donor organ shortage. Recipients of diabetic donor kidneys have higher mortality risk compared with recipients of nondiabetic donor kidneys. However, the survival benefit of transplantation with diabetic donor kidneys over remaining on the waitlist has not been previously evaluated.
Design, setting, participants, & measurements
We performed an observational cohort study of 437,619 kidney transplant candidates from the Organ Procurement and Transplantation Network database, including 8101 recipients of diabetic donor kidneys and 126,560 recipients of nondiabetic donor kidneys. We used time-varying Cox proportional hazards modeling to assess the mortality risk of accepting a diabetic donor kidney compared with remaining on the waitlist or receiving a nondiabetic donor kidney.
Results
Among transplant recipients, median follow-up was 8.9 years and mortality rate was 35 deaths per 1000 person-years. Recipients of diabetic donor kidneys had 9% lower mortality compared with remaining on the waitlist or transplantation with a nondiabetic donor kidney (adjusted hazard ratio, 0.91; 95% confidence interval, 0.84 to 0.98). Although recipients of nondiabetic donor kidneys with a Kidney Donor Profile Index score >85% had lower mortality risk (adjusted hazard ratio, 0.86; 95% confidence interval, 0.81 to 0.91), recipients of diabetic donor kidneys with an index score >85% did not show any difference (adjusted hazard ratio, 1.09; 95% confidence interval, 0.97 to 1.22). Patients aged <40 years attained no survival benefit from transplantation with diabetic donor kidneys; diabetic patients at centers with long waitlist times attained the greatest survival benefit.
Conclusions
Diabetic donor kidneys appear associated with higher mortality risk compared with nondiabetic donor kidneys, but offer greater survival benefit compared with remaining on the waitlist for many candidates. Patients with high risk of mortality on the waitlist at centers with long wait times appear to benefit most from transplantation with diabetic donor kidneys.
Keywords: diabetes mellitus, transplant recipients, mortality, transplant donors, Confidence Intervals, Death, Follow-Up Studies, Humans, kidney transplantation, Tissue Donors, Tissue and Organ Procurement, Transplant Recipients, Waiting Lists
Introduction
In response to the growing demand for kidney transplantation, utilization of high risk or marginal donor kidneys is expanding in the United States (1). In particular, utilization of diabetic donor kidneys has more than doubled over the past decade (2). However, diabetic donor kidneys are associated with an increased risk of allograft failure and all-cause mortality compared with deceased nondiabetic donor kidneys. The risk associated with diabetic donor kidneys is particularly elevated for diabetic recipients (2).
In December of 2014, the new kidney allocation system was implemented using the Kidney Donor Profile Index (KDPI) and Estimated Post Transplant Survival (EPTS) score. KDPI assigns a weighted graft failure risk score to deceased donor kidneys on the basis of a broad range of donor characteristics (3,4). The EPTS score determines recipient mortality risk, taking into account recipient characteristics (3). Donor and recipient diabetes statuses are heavily weighted in the KDPI and EPTS scoring, respectively (5–7). With the new allocation process, diabetic recipients have limited access to low risk kidneys, increasing the likelihood that lower quality kidneys, including diabetic donor kidneys, will be transplanted into diabetic recipients.
The elevated risk associated with accepting marginal donor kidneys needs to be balanced with the risks of remaining on the waitlist. Previous studies demonstrated a survival benefit from transplantation with expanded criteria donor (ECD) kidneys [defined as kidneys from donors aged ≥60 years, or 50–59 years with at least two risk factors, including (1) cerebrovascular cause of death, (2) hypertension, or (3) creatinine>1.5 mg/dl] compared with remaining on the waitlist, particularly among diabetic recipients and in regions with longer waitlist times (8,9). Similarly, previous data demonstrated a survival benefit from transplantation with high-KDPI kidneys, particularly among patients at centers with longer median waitlist times (10). Nonetheless, no previous studies have evaluated the survival benefit of accepting a diabetic donor kidney specifically compared with remaining on the waitlist.
Accordingly, this study investigates the effect of donor and recipient diabetes status on mortality risk in eligible potential recipients on the transplant waiting list. Our goal was to identify which patients may benefit most from transplantation with diabetic donor kidneys as waiting times lengthen and demand for marginal donor kidneys increases, despite the previously demonstrated hazards associated with this type of organ offer.
Materials and Methods
Data Source
We used national registry data collected by the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The study was determined to meet institutional review board exemption authorized by Code of Federal Regulations §46.101, category 4 by the Institutional Review Board at the University of Pennsylvania (protocol no. 821021).
Cohort
The cohort included patients on the waitlist or who received a deceased donor kidney between January 1, 1994 (the year of the first diabetic donor kidney transplant) and March 31, 2015. Patient follow-up was through June 5, 2015, date of death, or date of deactivation from the waitlist, whichever was first. The study was restricted to incident waitlist candidates who were ≥18 years of age at the time of waitlisting, and to patients listed for their first kidney transplant (10). Patients who were never active on the waitlist during the study period and patients listed for multiorgan transplant were excluded (11).
Variables and Covariates
The primary outcome was all-cause mortality. Mortality data were provided as part of the OPTN dataset. All covariates for waitlisted patients (candidates) were ascertained from the forms submitted to the OPTN at the time of waitlisting. Candidate diabetes status was reported in the database as “DIAB.” The “DGN-TCR” variable in the database, which defines the diagnostic etiology of CKD, provided additional information regarding diabetes status in some candidates. Candidate sensitization was designated as panel reactive antibody >30%, on the basis of previous studies in this area (2,12). Dialysis vintage time was defined as duration of dialysis before waitlisting. Additional candidate-associated covariates used in the multivariable analyses included age, sex, race, year of waitlisting (in order to take into account era-effect), and ABO blood type (2,13–15). For categorical analyses of candidate age, locally weighted scatterplot smoothing demonstrated a cut-point for increased mortality at 40 years of age (16), which has also been utilized in existing literature (2,9). Donor diabetes status was reported as “DIABETES_DON.” Center median waitlist time tertiles were calculated by using the median waitlist times for all patients in the OPTN dataset at each transplant center (9,10).
Statistical Analyses
Descriptive Statistics.
Statistical analyses were performed using STATA version 13.0 (StataCorp., College Station, TX) with two-sided hypothesis testing and P<0.05 as the criteria for statistical significance. Descriptive statistics (mean, median, and proportion) were used to describe baseline clinical and demographic characteristics comparing recipients of diabetic donors, recipients of nondiabetic donors, and patients who were only ever on the waitlist. Continuous variables were compared using t test or rank-sum test for non-normally distributed variables. Categorical and binary variables were compared using chi-squared test.
Time-Varying Cox Models: Cumulative Mortality.
For the overall analysis, we compared the hazard of all-cause mortality of transplantation with a diabetic donor kidney compared with a composite reference group of transplantation with a nondiabetic donor kidney or remaining on the waitlist. Patients entered into the study at the time of waitlisting. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs). Time-varying exposure was incorporated at the time of transplant with either a diabetic or nondiabetic donor kidney (10,17,18).
Time-Varying Cox Models: Mortality Intervals Post-Transplant.
Multiple previous studies have demonstrated that transplantation with a deceased donor kidney is initially associated with an increased mortality hazard compared with remaining on the waitlist that declines over time (9,10,18). In order to address this, we performed analyses dividing the hazard of mortality into time intervals at 30 days post-transplant, 90 days post-transplant, 183 days (i.e., 6 months) post-transplant, 365 days (i.e., 1 year) post-transplant, 730 days (i.e., 2 years) post-transplant, and 1095 days (i.e., 3 years) post-transplant (10).
Model Parameters and Censoring.
Patients were censored at the time of removal from the waitlist or at the end of the follow-up period (June 5, 2015). For the multivariable regression, we selected recipient factors a priori that were known to be risk factors for mortality (2,13–15). On the basis of evidence from previous studies (2,9,10), we assessed for effect modification between donor and recipient diabetes status, donor diabetes status and median center waitlist time tertile, and recipient diabetes status and median center waitlist time tertile. The proportional hazards assumption was assessed via weighted versions of Kaplan–Meier curves using log-log plots as well as graphical displays on the basis of Schoenfeld and scaled Schoenfeld residuals (19).
Sensitivity Analyses
Sensitivity analyses were performed in which the donor kidney exposure groups were further divided into KDPI categories, using a KDPI cut-point of ≤85% versus >85% to distinguish the degree of quality of the diabetic donor kidneys (5). Sensitivity analyses were also performed limiting the cohort to patients who indicated that they were willing to accept an ECD donor kidney. Willingness to accept an ECD donor kidney was used as a surrogate marker for willingness to accept a high risk donor kidney, such as a diabetic donor kidney. The purpose of this sensitivity analysis was to exclude patients who would not have been offered a diabetic donor kidney. Additional sensitivity analyses were performed in which the cohort was limited to patients who were always active on the waitlist (11), limited to patients who were waitlisted on or after 2004 to assess for era effect (2), and stratified by candidate age <40 or ≥40 years.
Handling of Covariate Missingness
Waitlist candidates were excluded from the study if candidate or donor diabetes status was missing (n=30,596; 4.2% of patients assessed for eligibility in the study), as these were the primary exposure variables being evaluated in the cohort (2). Covariates included in the multivariate models were <5% incomplete. Given the large size of the cohort, missing data were addressed with a complete case analysis (20).
Results
Patient and Donor Characteristics
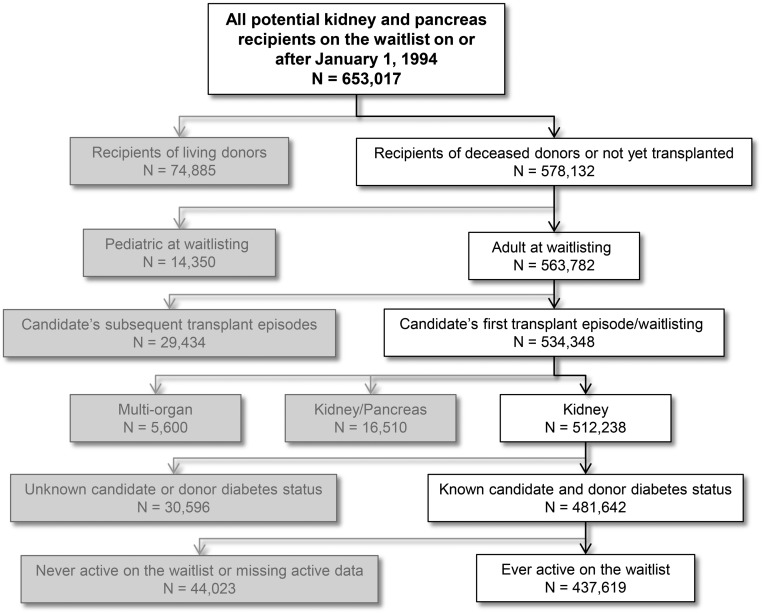
There were 437,619 patients eligible for inclusion in the study (see Figure 1). Among these patients, there were 8101 recipients of diabetic donor kidneys, 126,560 recipients of nondiabetic donor kidneys, and 302,958 waitlisted patients who never achieved transplantation. There was a significant difference between recipients of diabetic donor kidneys, recipients of nondiabetic donor kidneys, and patients who remained on the waitlist in all clinical and demographic characteristics evaluated (Table 1). Recipients of diabetic donor kidneys were older at the time of waitlisting (median age, 55 versus 51 years), had longer waitlist times (median, 675 versus 600 days), and were more likely to have delayed graft function (30% versus 24%) than recipients of nondiabetic donor kidneys. Diabetic donor kidneys had a higher KDPI (taking into account inclusion of diabetes in the calculation; median KDPI, 81% versus 44%) and came from donors with a higher body mass index (30 versus 25 kg/m2) compared with nondiabetic donor kidneys. Patients who remained on the waitlist were more likely to be at centers in the longest tertile of median center waitlist time (43% were at centers in the longest tertile compared with 29% of recipients of diabetic donor kidneys and 27% of recipients of nondiabetic donor kidneys), and had a higher rate of candidate diabetes (50% compared with 39% of recipients of diabetic donor kidneys and 34% of recipients of nondiabetic donor kidneys). During the follow-up period, 79% of transplant centers performed at least one transplant in which a candidate received a diabetic donor kidney.
Figure 1.
Cohort selection for evaluation of recipients of diabetic versus nondiabetic donor kidneys versus patients who were only on the waitlist.
Table 1.
Baseline characteristics of recipients of diabetic donor kidneys versus nondiabetic kidneys versus candidates who were only on the waitlist
| Baseline Characteristics | Diabetic Donor Recipients n=8101 | Nondiabetic Donor Recipients n=126,560 | Waitlist Only n=302,958 | P Value |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Median age, yr (IQR) | 55 (46–63) | 51 (40–59) | 51 (41–60) | <0.001 |
| Men, % | 62 | 61 | 59 | <0.001 |
| Ethnicity, % | <0.001 | |||
| White | 46 | 48 | 47 | |
| Black | 33 | 31 | 30 | |
| Hispanic | 13 | 13 | 15 | |
| Asian | 6 | 6 | 6 | |
| Other | 2 | 2 | 2 | |
| Cause of ESRD, % | <0.001 | |||
| Diabetes | 30 | 27 | 39 | |
| Hypertension | 30 | 27 | 19 | |
| Glomerular disease | 15 | 18 | 11 | |
| Cystic disease | 9 | 10 | 5 | |
| Other | 10 | 12 | 16 | |
| Missing | 6 | 6 | 10 | |
| Candidate diabetes, % | 39 | 34 | 50 | <0.001 |
| Maximum PRA ≥30%, % | 14 | 16 | 27 | <0.001 |
| Median dialysis vintage, d (IQR) | 327 (80–732) | 322 (91–730) | 271 (0–695) | <0.001 |
| Prewaitlist dialysis, % | 77 | 78 | 75 | <0.001 |
| Median waitlist time, d (IQR) | 675 (291–1184) | 600 (241–1121) | 758 (357–1364) | <0.001 |
| Patients listed at a center with the longest tertile of median wait time, % | 29 | 27 | 43 | <0.001 |
| Donor characteristics | ||||
| Expanded criteria donor, % | 39 | 16 | NA | <0.001 |
| Median KDPI percentile (IQR) | 81 (62–92) | 44 (21–67) | NA | <0.001 |
| Donor hepatitis C positive, % | 2 | 3 | NA | <0.001 |
| Median donor age, yr (IQR) | 51 (43–58) | 39 (23–51) | NA | <0.001 |
| Median donor body mass index, kg/m2 (IQR) | 30 (26–35) | 25 (22–29) | NA | <0.001 |
| Transplant characteristics | ||||
| Zero HLA mismatch, % | 6 | 10 | NA | <0.001 |
| Median cold ischemia time, h (IQR) | 18 (13–24) | 18 (12–24) | NA | <0.001 |
| Delayed graft function, % | 30 | 24 | NA | <0.001 |
IQR, Interquartile range; PRA, Panel reactive antibody; KDPI, Kidney Donor Profile Index.
Multivariable Regression Analyses for All-Cause Mortality
Median follow-up of transplant recipients in the primary analysis was 8.9 years. Compared with patients who either remained on the waitlist or received a nondiabetic donor kidney, patients who received a diabetic donor kidney had a significantly decreased cumulative hazard of all-cause mortality (Table 2; HR, 0.91; 95% CI, 0.84 to 0.98; see Supplemental Material, Supplemental Table 1). When taking into account KDPI compared with remaining on the waitlist or transplantation with a nondiabetic low-KDPI (KDPI≤85%) kidney, recipients of diabetic low-KDPI kidneys (Table 2; HR, 0.76; 95% CI, 0.68 to 0.85) and nondiabetic high-KDPI kidneys (HR, 0.86; 95% CI, 0.81 to 0.91) had a significantly lower hazard of mortality. However, recipients of diabetic high-KDPI kidneys had no reduction in mortality (Table 2; HR, 1.09; 95% CI, 0.97 to 1.22; see Supplemental Table 2). These findings persisted after restricting the cohort to patients willing to accept an ECD kidney, always active on the waitlist, waitlisted on or after 2004, and age 40 years or older at the time of waitlisting. Candidates aged <40 years at the time of waitlisting had no significant reduction in mortality from accepting a diabetic donor kidney, regardless of KDPI, nor from accepting a high-KDPI nondiabetic donor kidney.
Table 2.
Time-varying multivariable Cox proportional hazards for cumulative mortality in patients who received diabetic donor kidneys compared with the strategy of remaining on the waitlist or receiving a nondiabetic donor kidney (A) in the overall cohort, (B) divided by KDPI category, (C) when restricting to only patients willing to accept an ECD kidney, (D) when restricting to only patients who are always active on the waitlist, (E) when restricting to only patients who were waitlisted on or after 2004, (F) in candidates aged <40 years at the time of waitlisting, and (G) in candidates aged ≥40 years at the time of waitlisting
| Exposure Groups | HRa | 95% CI | P Value |
|---|---|---|---|
| (A) Overall cohort (n=437,619) | |||
| Waitlist or nondiabetic donor | REF | ||
| Diabetic donor | 0.91 | 0.84 to 0.98 | 0.02 |
| (B) Divided by KDPI risk category (n=437,619) | |||
| Waitlist or nondiabetic donor, KDPI≤85% | REF | ||
| Diabetic donor, KDPI≤85% | 0.76 | 0.68 to 0.85 | <0.001 |
| Diabetic donor, KDPI>85% | 1.09 | 0.97 to 1.22 | 0.15 |
| Nondiabetic donor, KDPI>85% | 0.86 | 0.81 to 0.91 | <0.001 |
| (C) Candidate willing to accept ECD kidney (n=304,701) | |||
| Waitlist or nondiabetic donor, KDPI≤85% | REF | ||
| Diabetic donor, KDPI≤85% | 0.75 | 0.66 to 0.84 | <0.001 |
| Diabetic donor, KDPI>85% | 1.10 | 0.98 to 1.24 | 0.10 |
| Nondiabetic donor, KDPI>85% | 0.86 | 0.81 to 0.92 | <0.001 |
| (D) Candidate always active on the waitlist (n=320,282) | |||
| Waitlist or nondiabetic donor, KDPI≤85% | REF | ||
| Diabetic donor, KDPI≤85% | 0.79 | 0.69 to 0.90 | <0.001 |
| Diabetic donor, KDPI>85% | 1.19 | 1.04 to 1.36 | 0.01 |
| Nondiabetic donor, KDPI>85% | 0.93 | 0.87 to 0.99 | 0.04 |
| (E) Candidate waitlisted in 2004 or later (n=271,403) | |||
| Waitlist or nondiabetic donor, KDPI≤85% | REF | ||
| Diabetic donor, KDPI≤85% | 0.68 | 0.56 to 0.83 | <0.001 |
| Diabetic donor, KDPI>85% | 1.17 | 0.98 to 1.40 | 0.09 |
| Nondiabetic donor, KDPI>85% | 0.77 | 0.69 to 0.86 | <0.001 |
| (F) Candidate age <40 yr (n=98,652) | |||
| Waitlist or nondiabetic donor, KDPI≤85% | REF | ||
| Diabetic donor, KDPI≤85% | 0.82 | 0.61 to 1.09 | 0.18 |
| Diabetic donor, KDPI>85% | 1.12 | 0.76 to 1.65 | 0.57 |
| Nondiabetic donor, KDPI>85% | 0.84 | 0.68 to 1.02 | 0.09 |
| (G) Candidate age ≥40 yr (n=338,287) | |||
| Waitlist or nondiabetic donor, KDPI≤85% | REF | ||
| Diabetic donor, KDPI≤85% | 0.74 | 0.66 to 0.84 | <0.001 |
| Diabetic donor, KDPI>85% | 1.04 | 0.92 to 1.17 | 0.51 |
| Nondiabetic donor, KDPI>85% | 0.83 | 0.78 to 0.88 | <0.001 |
HR, hazard ratio; 95% CI, 95% confidence interval; REF, Reference group; KDPI, Kidney Donor Profile Index; ECD, Expanded criteria donor.
All multivariable models were adjusted for candidate diabetes status, center median waitlist time tertile, three-way interaction between donor diabetes status, candidate diabetes status, and center median waitlist time tertile, in addition to candidate age, sex, waitlist year, race, ABO blood type, panel reactive antibody, and dialysis vintage.
Multivariable Regression Analyses for All-Cause Mortality: Stratified Analyses
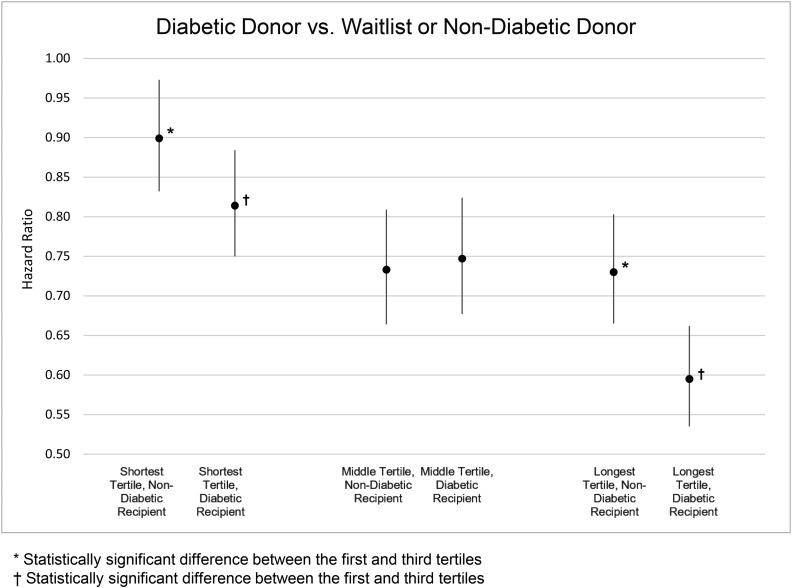
There was significant three-way interaction between donor diabetes status, recipient diabetes status, and center median waitlist time tertile. Analyses were stratified by donor diabetes status, recipient diabetes status, and center median waitlist time tertile. Compared with remaining on the waitlist or receiving a nondiabetic donor kidney, diabetic recipients of diabetic donor kidneys in the longest center median waitlist time tertile exhibited 40% lower mortality (Figure 2; HR, 0.60; 95% CI, 0.54 to 0.66 for diabetic recipients in the longest center median waitlist time tertile compared with HR, 0.81; 95% CI, 0.75 to 0.88 in the shortest tertile; see Supplemental Figure 1) and nondiabetic recipients exhibited 27% lower mortality (HR, 0.73; 95% CI, 0.67 to 0.80 for nondiabetic recipients in the longest tertile compared with HR, 0.90; 95% CI, 0.83 to 0.97 in the shortest tertile).
Figure 2.
Candidates at centers with the longest median waitlist times had lower cumulative hazard of mortality when they were transplanted with a diabetic donor kidney compared with remaining on the waitlist or receiving a nondiabetic donor kidney, particularly among diabetic candidates.
Multivariable Regression Analyses for All-Cause Mortality: Post-Transplant Time-Point Analyses
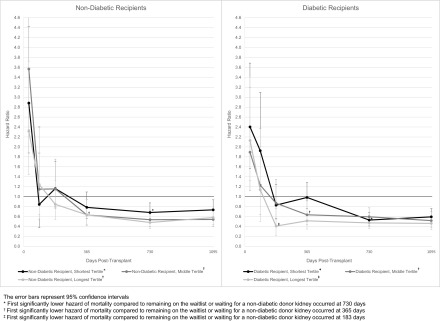
Compared with either remaining on the waitlist or receiving a nondiabetic donor kidney, diabetic recipients in the shortest waitlist tertile had a significantly higher mortality risk until 6 months post-transplant; all other recipients only had a significantly higher mortality risk until 30 days post-transplant (Figure 3, Supplemental Figure 2). Diabetic recipients of diabetic donor kidneys in the longest waitlist tertile had significantly lower all-cause mortality within 6 months post-transplant compared with either remaining on the waitlist or receiving a nondiabetic donor kidney, in contrast to a minimum of 1 year in all other exposure strata.
Figure 3.
Candidates at centers with the longest median waitlist times who received a diabetic donor kidney transplant had the earliest significantly lower post-transplant hazard of mortality compared with the strategy of remaining on the waitlist or receiving a nondiabetic donor kidney, particularly among diabetic candidates. The error bars represent 95% confidence intervals.
Discussion
Our study demonstrates a considerable survival benefit among patients who were transplanted with a diabetic donor kidney compared with patients who remained on the waitlist. Diabetic recipients at centers with the longest waitlist times achieve the earliest benefit from transplantation with diabetic donor kidneys compared with either remaining on the waitlist or receiving a nondiabetic donor kidney. Recipients of low-KDPI (KDPI≤85%) diabetic donor kidneys and high-KDPI (KDPI>85%) nondiabetic donor kidneys have lower mortality compared with remaining on the waitlist or transplantation with a low-KDPI nondiabetic donor kidney; however, recipients transplanted with a high-KDPI diabetic donor kidney have no reduction in mortality. Additionally, candidates aged <40 years at the time of waitlisting have no survival benefit from transplantation with a diabetic donor kidney, regardless of KDPI.
This is the first study to evaluate the risk of transplantation with a diabetic donor kidney in contrast to the risk of dying on the waitlist. Our previous work demonstrated a modest but significantly increased hazard of mortality and graft failure among recipients of diabetic donor kidneys compared with recipients of nondiabetic donor kidneys (2). However, the results of this study demonstrate a markedly greater survival benefit in patients who are transplanted with a diabetic donor kidney compared with remaining on the waitlist, particularly among patients at centers with the longest waitlist times. This study also demonstrates that high-KDPI diabetic donor kidneys contribute greater mortality risk compared with high-KDPI nondiabetic donor kidneys. Taken together, these data enhance the risk information provided by the KDPI calculation and can help to optimize physician and patient decision-making regarding organ offer acceptance.
These results compliment previous studies evaluating the risk of transplantation with deceased donor kidneys, and particularly marginal donor kidneys, compared with remaining on the waitlist (8,9,18). In a 2001 OPTN registry study, Ojo et al. (8) demonstrated that transplantation with a marginal donor kidney increased patients’ life expectancy by up to 10 years compared with patients who remained on the waitlist, depending on recipient risk factors. Using Scientific Registry of Transplant Recipients data in 2005, Merion et al. (9) demonstrated that longer organ procurement organization waitlist tertile was associated with significantly greater reduction in relative risk for mortality from transplantation with an ECD kidney compared with remaining on the waitlist (except in patients aged <40 years, similar to our findings with diabetic kidneys). In 2014, Massie et al. (10) found that transplantation with a high-KDPI kidney was associated with increased short-term but decreased long-term mortality risk compared with remaining on the waitlist or receiving a low-KDPI kidney; the lowest mortality risk was among patients at centers with the longest median waitlist times. Consistent with these previous studies, our analysis demonstrated that transplantation with a diabetic donor kidney was associated with an initial increase in perioperative mortality risk, but decrease in long-term mortality risk; the earliest survival benefit and greatest magnitude of cumulative survival benefit were among diabetic recipients at centers with the longest waitlist times.
Our study is strengthened by the large sample size, extensive generalizability, and long duration of follow-up. Given the comprehensiveness of the OPTN registry data, a broad range of recipient and donor characteristics were incorporated into the descriptive and multivariable analyses. Notably, the use of time-varying exposure to transplantation, taking into account donor diabetes status, permitted direct comparison of transplanted patients to those remaining on the waitlist, giving appropriate recognition to relative duration of time on the waitlist.
There were also limitations to this study. Although degree of diabetic injury in the donor kidney likely plays an important role in decisions about accepting these organs, OPTN data lack consistent, detailed proteinuria or procurement biopsy data (only 30% of donor kidneys had biopsies performed at the time of transplant, with no consistent reporting of typical findings appreciated in diabetic nephropathy, such as nodularity). Additionally, duration of donor diabetes is not reliably recorded. Furthermore, the considerably greater mortality risk among patients on the waitlist may have been reflective in part of greater burden of illness among patients who were not transplanted. To address this potential selection bias, we restricted the analyses to patients who were waitlisted for the first time and who were not listed for multiorgan transplant, in addition to adjusting for important risk factors for mortality. We also performed sensitivity analyses, restricting the analysis to only include patients who were always active on the waitlist.
Although diabetic donor kidneys contribute additional mortality risk compared with nondiabetic deceased donor kidneys, this study highlights the importance of continuing to use marginal donor kidneys in appropriate circumstances (21). Given that a substantial percentage of transplant centers still do not transplant diabetic donor kidneys into potential candidates, there evidently continues to be reticence to use diabetic kidneys, above and beyond the information provided by KDPI. Patients in areas with the longest waitlist times and with the highest risk of dying on the waitlist have the greatest potential benefit from receipt of diabetic donor kidneys. The increased use of marginal donor kidneys over the past several decades has been a necessary response to the increased demand for deceased donor kidneys (22). The new allocation system has generated heightened attention to the need to appropriately allocate high risk donor kidneys and optimize utilization of these organs (23). When accepted by appropriate candidates, diabetic donor kidneys have the potential to shorten waitlist times, and ideally reduce the risk of death on the waitlist. Future studies are needed to continue to improve our understanding of the interactions between specific recipient and donor risk factors, and to help guide decision-making in the allocation of high risk donor kidneys.
Disclosures
J.E.L. serves as a clinical consultant for Infusion Pharma and Sanofi.
Supplementary Material
Acknowledgments
We gratefully acknowledge input and advice from Justine Shultz, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
This research was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (grant no. K23-HL133843; Principal Investigator: J.B.C.) and National Institute of Diabetes and Digestive and Kidney Diseases (grant no. K23-DK103918; Principal Investigator: J.E.L., and grant no. K23-DK090209; Principal Investigator: K.A.F.).
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the National Institutes of Health.
This study was also supported in part by Health Resources and Service Administration.The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Opportunities to Increase Availability of Deceased Donor Kidneys,” on pages 871–873.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10280916/-/DCSupplemental.
References
- 1.Mohan S, Tanriover B, Ali N, Crew RJ, Dube GK, Radhakrishnan J, Hardy MA, Ratner LE, McClellan W, Cohen D: Availability, utilization and outcomes of deceased diabetic donor kidneys; analysis based on the UNOS registry. Am J Transplant 12: 2098–2105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JB, Bloom RD, Reese PP, Porrett PM, Forde KA, Sawinski DL: National outcomes of kidney transplantation from deceased diabetic donors. Kidney Int 89: 636–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedewald JJ, Samana CJ, Kasiske BL, Israni AK, Stewart D, Cherikh W, Formica RN: The kidney allocation system. Surg Clin North Am 93: 1395–1406, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, Wang X, Shteyn E, Cherikh W, Stewart D, Samana CJ, Chung A, Hart A, Kasiske BL: New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol 25: 1842–1848, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organ Procurement and Transplantation Network : Estimated Post Transplant Survival Calculator, Allocation Calculators, Richmond, VA, 2015 [Google Scholar]
- 7.Schold JD, Buccini LD, Reese PP, Poggio ED, Goldfarb DA: Effect of dialysis initiation for preemptively listed candidates in the revised kidney allocation policy. Am J Transplant 14: 2855–2860, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK: Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 12: 589–597, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL: Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 14: 2310–2316, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Grams ME, Massie AB, Schold JD, Chen BP, Segev DL: Trends in the inactive kidney transplant waitlist and implications for candidate survival. Am J Transplant 13: 1012–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Süsal C, Opelz G: Kidney graft failure and presensitization against HLA class I and class II antigens. Transplantation 73: 1269–1273, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Cardinal H, Hébert MJ, Rahme E, Houde I, Baran D, Masse M, Boucher A, Le Lorier J; Elderly Recipients Transplant Group : Modifiable factors predicting patient survival in elderly kidney transplant recipients. Kidney Int 68: 345–351, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Faravardeh A, Eickhoff M, Jackson S, Spong R, Kukla A, Issa N, Matas AJ, Ibrahim HN: Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation 96: 1089–1096, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Narayanan M, Pankewycz O, Shihab F, Wiland A, McCague K, Chan L: Long-term outcomes in African American kidney transplant recipients under contemporary immunosuppression: A four-yr analysis of the Mycophenolic acid Observational REnal transplant (MORE) study. Clin Transplant 28: 184–191, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Goodall C: A survey of smoothing techniques. In: Modern Methods of Data Analysis, edited by Long JS, Fox J, Newbury Park, CA, Sage, 1990, pp 126–176 [Google Scholar]
- 17.Austin PC, Platt RW: Survivor treatment bias, treatment selection bias, and propensity scores in observational research. J Clin Epidemiol 63: 136–138, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health), New York, Springer, 2001 [Google Scholar]
- 20.White IR, Carlin JB: Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat Med 29: 2920–2931, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Reese PP, Harhay MN, Abt PL, Levine MH, Halpern SD: New solutions to reduce discard of kidneys donated for transplantation. J Am Soc Nephrol 27: 973–980, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandolfini I, Buzio C, Zanelli P, Palmisano A, Cremaschi E, Vaglio A, Piotti G, Melfa L, La Manna G, Feliciangeli G, Cappuccilli M, Scolari MP, Capelli I, Panicali L, Baraldi O, Stefoni S, Buscaroli A, Ridolfi L, D’Errico A, Cappelli G, Bonucchi D, Rubbiani E, Albertazzi A, Mehrotra A, Cravedi P, Maggiore U: The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: Distribution and association with graft outcomes. Am J Transplant 14: 2515–2525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU: Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4: 1239–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.