Abstract
Background and objectives
Kidney disease is a critical concern in counseling patients with lupus considering pregnancy. This study sought to assess the risk of renal flares during pregnancy in women with previous lupus nephritis in partial or complete remission, particularly in those with antidouble-stranded DNA antibodies and low complement levels, and the risk of new-onset nephritis in patients with stable/mildly active SLE.
Design, setting, participants, & measurements
We assessed active nephritis (renal flares and de novo kidney disease) and associated predictors during pregnancy in patients with lupus with urine protein ≤1000 mg and serum creatinine <1.2 mg/dl at baseline; 373 patients (52% ethnic/racial minorities) enrolled between 2003 and 2012 were prospectively followed in the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus Study. Active nephritis was defined by proteinuria increase of >500 mg and/or red blood cell casts.
Results
Of 118 patients with previous kidney disease, 13 renal flares (11%) occurred (seven of 89 in complete remission and six of 29 in partial remission) compared with four with de novo kidney involvement (2%) in 255 patients without past kidney disease (P<0.001). Active nephritis was not associated with ethnicity, race, age, creatinine, BP, or antihypertensive and other medications. In multivariable logistic regression analyses, patients with past kidney disease in complete or partial remission more often experienced active nephritis (adjusted odds ratio, 6.88; 95% confidence interval, 1.84 to 25.71; P=0.004 and adjusted odds ratio, 20.98; 95% confidence interval, 4.69 to 93.98; P<0.001, respectively) than those without past kidney disease. Low C4 was associated with renal flares/de novo disease (adjusted odds ratio, 5.59; 95% confidence interval, 1.64 to 19.13; P<0.01) but not low C3 or positive anti-dsDNA alone.
Conclusions
De novo kidney involvement in SLE, even in ethnic/racial minorities, is uncommon during pregnancy. Past kidney disease and low C4 at baseline independently associate with higher risk of developing active nephritis. Antibodies to dsDNA alone should not raise concern, even in patients with past kidney disease, if in remission.
Keywords: systemic lupus erythematosus; Pregnancy Outcome; Antihypertensive Agents; Antiphospholipid Syndrome; Biomarkers; blood pressure; Counseling; creatinine; Erythrocytes; Female; Humans; Kidney Diseases; Logistic Models; Lupus Erythematosus, Systemic; lupus nephritis; Pregnancy; proteinuria; risk factors
Introduction
In counseling women with SLE regarding the risks to maternal and fetal health during pregnancy, a history of nephritis engenders apprehension. Patients with active nephritis or those in remission for <6 months after treatment induction are advised to postpone conception given the risk of adverse pregnancy outcomes (APOs), such as preterm birth and preeclampsia (1). A systematic literature review including 37 studies of pregnancy outcomes in women with SLE and a meta-analysis of the association of lupus nephritis with APOs found that hypertension occurred in 16%, that nephritis relapse or flare occurred in 16%, and that preeclampsia occurred in 8.6% (2). Even for patients in whom nephritis is clinically quiescent, past kidney involvement raises concern both with regard to renal flares and for overall pregnancy outcomes. The presence of serologic activity (positive antidouble-stranded DNA [anti-dsDNA] antibodies and low levels of complement) may negatively influence the decision regarding the optimal timing of conception, because these parameters often predict renal flare in nonpregnant patients (3,4).
Several studies in the last 6 years form the basis for recommendations regarding the management of kidney disease during pregnancy, but shortcomings include retrospective study design (5), limited numbers of patients (<60) with past kidney disease in remission (5–7), inclusion of predominantly or exclusively white women (7), and absence of focus or discussion regarding lupus serologic parameters as predictors (6,8,9). This study was undertaken to address the risk of renal flares in those with previous kidney disease in partial or complete remission in the first trimester, particularly if serologic activity as evidenced by anti-dsDNA antibodies and low complement levels was present. Additionally, the risk of first-time kidney involvement in a cohort of patients with stable/mildly active SLE was assessed. These objectives were approached by leveraging a large multicenter multiethnic/racial prospective observational study completed in 2013: the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Syndrome and Systemic Lupus Erythematosus (PROMISSE) Study (ClinicalTrials.gov identifier: NCT00198068).
Materials and Methods
Study Design and Patient Population
The PROMISSE Study is a multicenter, prospective observational study of pregnancies in women with SLE (four or more revised American College of Rheumatology [ACR] criteria) (10), women with SLE and antiphospholipid antibodies (aPLs), and women with aPLs alone (not included in this study). Pregnant study participants were enrolled between September of 2003 and December of 2012 at six United States sites and one Canadian site (11). Institutional review boards at sites approved the protocol and consent forms; written informed consent was obtained from all patients. Subsequent pregnancies occurring during the study period were not included in the PROMISSE Study.
At enrollment, patients met all inclusion criteria: a live singleton intrauterine pregnancy confirmed by ultrasound, age 18–45 years old, and hematocrit >26% as previously described (11). Because the overall goal of the PROMISSE Study was to identify mechanisms of APO that might be specifically actionable in lupus, other potential causes of APO were excluded: disease requiring prednisone >20 mg/d, urine protein-to-creatinine ratio (uPCR) >1000 mg/g on random sampling or 24-hour collection, erythrocyte casts on urinalysis, serum creatinine >1.2 mg/dl, type 1 or 2 diabetes mellitus, or BP≥140/90 mmHg at screening. These exclusions were consistent with current advice regarding timing of conception. Patients were enrolled before 12 weeks of gestation and followed by the site rheumatologist every trimester or when flaring, and bloods were obtained monthly. As previously described (11), APO in the PROMISSE Study included fetal or neonatal death; birth before 36 weeks due to placental insufficiency, hypertension, or preeclampsia; and small for gestational age neonate (birthweight below fifth percentile).
At first trimester enrollment in the PROMISSE Study, patients with previous kidney disease per ACR criteria (10,11) were considered to be in complete remission if proteinuria was <500 mg/d and partial remission if proteinuria was 500–1000 mg/d as assessed by 24-hour or random uPCR <0.5 or 0.5–1.0 or dipstick <2+.
Definition of SLE Disease Activity and Renal Flares during Pregnancy
Active nephritis representing either a renal flare or de novo kidney disease was defined as an increase of protein ≥500 mg/d with or without hematuria and/or red blood cell casts compared with the previous study visit. In the absence of an available uPCR, a change in the urine dipstick from 0 to 2+, from 1 to 3+, or from 2 to 4+ was considered active nephritis.
Measurements of C3, C4, and anti-dsDNA were carried out in local laboratories, with abnormal results defined as per the cutoffs of the laboratory.
Statistical Analyses
Bivariate associations between patient characteristics and occurrence of a renal flare/de novo kidney disease were evaluated with the Fisher exact and two-sample t tests for categorical and continuous variables, respectively. A multivariable logistic regression model was also fit to the data to evaluate the independent associations of C3 level at screening (low or normal), C4 at screening (low or normal), anti-dsDNA status (positive or negative), and history of kidney disease (never, past [in complete remission], or past [in partial remission]) with renal flare/de novo kidney involvement in the second or third trimester after adjusting for age and race/ethnicity. All statistical tests were two sided, and P values <0.05 were considered significant.
Results
Study Population and Kidney Outcomes
Of 976 consecutive pregnant women screened, 683 were enrolled in the PROMISSE Study as of December 31, 2012. Included were 385 with SLE who completed their pregnancy by December 31, 2012 (11). Of these, 12 had incomplete data (ten were missing complement, anti-dsDNA, or both, and two were missing baseline urine protein), leaving 373 patients for analysis. The ethnicity/race included 48% non-Hispanic white, 20% black, 17% Hispanic all races, 11% Asian, and 5% other. At the screening visit (first trimester), both anti-dsDNA and low complements were present in 21%, anti-dsDNA alone was present in 20%, low complement alone was present in 11% (low C3 and/or C4), and values for these analytes were in the normal range in 47% of the patients.
Two hundred fifty-five (68%) patients did not have a history of lupus nephritis. The remaining 118 (32%) had preexisting kidney disease as defined by ACR criteria (10). Of these, 85 (72%) had a previous kidney biopsy: 19 class III, 28 class IV, 20 class V, eight class III/IV, two class 4/5 IV/V, one class I (had persistent proteinuria by ACR criteria), and one class II. Kidney biopsy class was not available for six patients, two of whom had kidney transplants. At enrollment, 89 patients were in complete kidney remission, and 29 were in partial kidney remission. Of the 373 patients, 17 (4.6%) had a renal flare or de novo kidney disease: nine in the second trimester and eight in the third trimester. Renal flares/de novo disease were defined by quantitative measures for all but five patients, of whom three went from 0/trace to ≥3+ by dipstick. There were only two patients in whom a dipstick change of two levels was counted as a flare, and one of these flares was accompanied by hematuria. It is not always possible to differentiate between active lupus nephritis and preeclampsia, and in three of the 17 patients, the treating physicians classified them as having both nephritis and preeclampsia.
Bivariate Analyses and Renal Flares
Demographic and Past Medical History.
As summarized in Table 1, neither ethnicity nor race were associated with a renal flare or de novo disease. Age, creatinine, BP (systolic and diastolic), and current use of antihypertensive medications at screening also did not predict kidney outcome. However, patients with a history of kidney disease were more likely to experience an increase in proteinuria than those without a history of kidney disease. Specifically, of 118 with previous kidney disease, 13 (11.0%) had a renal flare compared with four (1.6%) of 225 without prior kidney involvement (P<0.001). The two patients with a kidney transplant did not experience a renal flare. Of patients with no history of kidney disease, 119 were ethnic/racial minorities, and their frequency of de novo disease was not different from non-Hispanic whites. Patients with past lupus nephritis in complete remission at baseline had fewer renal flares (seven of 89=7.9%) than those in partial remission (six of 29=20.7%), although the difference was not significant (P=0.08). Only three kidney flares in the 13 patients with previous kidney disease (one in complete remission at baseline and two in partial remission) required a change in therapy during pregnancy. A fourth patient in complete remission was treated postpartum.
Table 1.
Association of baseline patient characteristics and renal flare/de novo disease
| Patient Characteristics | Total (%) | No Kidney Flare/De Novo (%) | Kidney Flare/De Novo (%) | P Value |
|---|---|---|---|---|
| n | 373 | 356 | 17 | |
| Demographics | ||||
| Ethnicity/race | 0.62 | |||
| Non-Hispanic white | 179 (47.99) | 171 (48.03) | 8 (47.06) | |
| Hispanic all races | 62 (16.62) | 59 (16.57) | 3 (17.65) | |
| Black | 74 (19.84) | 71 (19.94) | 3 (17.65) | |
| Asian | 41 (10.99) | 40 (11.24) | 1 (5.88) | |
| Other | 17 (4.56) | 15 (4.21) | 2 (11.76) | |
| Mean age (SD), y | 30.9 (4.90) | 31.0 (4.87) | 29.6 (5.52) | 0.26 |
| Clinical history | ||||
| History of kidney disease | <0.001 | |||
| No kidney disease | 255 (68.36) | 251 (70.51) | 4 (23.53) | |
| Complete remission | 89 (23.86) | 82 (23.03) | 7 (41.18) | |
| Partial remission | 29 (7.77) | 23 (6.46) | 6 (35.29) | |
| aPL status | 0.14 | |||
| aPL+ | 50 (13.40) | 50 (14.04) | 0 (0.00) | |
| aPL− | 323 (86.60) | 306 (94.74) | 17 (100.00) | |
| Pregnancy history | 0.33 | |||
| Primigravida | 155 (41.55) | 150 (42.13) | 5 (29.41) | |
| Multigravida | 218 (58.45) | 206 (57.87) | 12 (70.59) | |
| Physical examination | ||||
| Mean systolic BP (SD), mmHg | 112.1 (12.96) | 111.8 (12.79) | 117.9 (15.40) | 0.13 |
| Mean diastolic BP (SD), mmHg | 67.7 (9.51) | 67.5 (9.48) | 70.0 (10.22) | 0.30 |
| Laboratory data | ||||
| Mean creatinine (SD), mg/dl | 0.64 (0.14) | 0.64 (0.14) | 0.67 (0.15) | 0.27 |
| C and anti-dsDNA antibody status | 0.01 | |||
| Positive anti-dsDNA and low C (low C3 and/or C4) | 80 (21.45) | 73 (20.51) | 7 (41.18) | |
| Positive anti-dsDNA only | 75 (20.11) | 74 (20.79) | 1 (5.88) | |
| Low C only (low C3 and/or C4) | 42 (11.26) | 37 (10.39) | 5 (29.41) | |
| Normal anti-dsDNA and normal C | 176 (47.18) | 172 (48.31) | 4 (23.53) | |
| C3 | 0.01 | |||
| Normal | 286 (79.89) | 277 (81.23) | 9 (52.94) | |
| Low | 72 (20.11) | 64 (18.77) | 8 (47.06) | |
| C4 | <0.001 | |||
| Normal | 265 (74.23) | 259 (76.18) | 6 (35.29) | |
| Low | 92 (25.77) | 81 (23.82) | 11 (64.71) | |
| Anti-dsDNA | 0.63 | |||
| Negative | 218 (58.45) | 209 (58.71) | 9(52.94) | |
| Positive | 155 (41.55) | 147 (41.29) | 8 (47.06) | |
| Medications | ||||
| Glucocorticoids | 0.13 | |||
| No | 224 (60.05) | 217 (60.96) | 7 (41.18) | |
| Yes | 149 (39.95) | 139 (39.04) | 10 (58.82) | |
| Hydroxychloroquine | 0.45 | |||
| No | 141 (37.80) | 133 (37.36) | 8 (47.06) | |
| Yes | 232 (62.20) | 223 (62.64) | 9 (52.94) | |
| Azathioprine | 0.51 | |||
| No | 308 (82.57) | 295 (82.87) | 13 (76.47) | |
| Yes | 65 (17.43) | 61 (17.13) | 4 (23.53) | |
| Heparin | 0.14 | |||
| No | 294 (78.82) | 278 (78.09) | 16 (94.12) | |
| Yes | 79 (21.18) | 78 (21.91) | 1 (5.88) | |
| Aspirin | 0.19 | |||
| No | 244 (65.42) | 230 (64.61) | 14 (82.35) | |
| Yes | 129 (34.58) | 126 (35.39) | 3 (17.65) | |
| Antihypertensives | >0.99 | |||
| No | 340 (91.15) | 324 (91.01) | 16 (94.12) | |
| Yes | 33 (8.85) | 32 (8.99) | 1 (5.88) |
Values are numbers (percentages) unless otherwise indicated. Baseline levels were measured at screening (first trimester). aPL, antiphospholipid antibody; dsDNA, double-stranded DNA.
Current use of glucocorticoids, hydroxychloroquine, azathioprine, aspirin, or heparin or past treatment with cyclophosphamide or methotrexate did not associate with a renal flare or de novo kidney disease.
Laboratory Values at Screening.
Rates of renal flare/de novo disease were significantly higher in those with both positive anti-dsDNA and low complement (seven of 80=8.6%) or low complement alone (i.e., low C3, low C4, or both; five of 42=11.9%) than in patients with no serologic activity at baseline (four of 176=2.3%; P<0.01). Specifically, of the patients with a low C3 level at baseline, 11.1% (eight of 72) developed active nephritis compared with 3.1% (nine of 286) in those with normal C3 values (P=0.01). Of those with low C4 at baseline, 12% (11 of 92) developed active nephritis compared with 2.3% (six of 265) of patients with normal C4 (P<0.001). Although serologic activity (low complement and/or anti-dsDNA) was present in 68 patients who were ethnic/racial minorities, only two patients developed de novo kidney disease. There were no renal flares/de novo disease in the 50 patients with SLE with aPLs compared with 17 renal flares/de novo disease in the 323 patients without aPLs (P=0.14). There was no significant difference in the percentage of aPL positivity between mothers with and without past kidney disease: 11 of 50 (22%) compared with 107 of 323 (33%; P=0.14). Likewise, there was no significant difference in the percentage of renal flares in patients with past kidney disease with or without the presence of aPLs; renal flares occurred in zero of 11 (0%) compared with 13 of 107 (12%) without aPLs (P=0.60).
Multivariable Analyses for Risk Factors of Kidney Outcome
In multivariable analyses (Table 2), previous history of kidney disease and serologic status at baseline were evaluated as independent predictors of renal flare/de novo disease in a logistic regression model that also adjusted for age and race/ethnicity. Compared with patients with no history of kidney disease, those in complete or partial remission were significantly more likely to experience an increase in proteinuria (adjusted odds ratio [ORadj], 6.88; 95% confidence interval [95% CI], 1.84 to 25.71; P=0.004 and ORadj, 20.98; 95% CI, 4.69 to 93; P<0.001, respectively). The difference in the risk of renal flare between patients in complete and partial remission was not significant (P=0.10). In addition, a low C4 at baseline was significantly associated with renal flare/de novo disease (ORadj, 5.59; 95% CI, 1.64 to 19.13; P<0.01) but not C3 (ORadj, 1.83; 95% CI, 0.50 to 6.64; P=0.36). Anti-dsDNA status did not predict kidney outcomes. Given the limited number of kidney flares/de novo kidney involvement, a sensitivity analysis was also performed by fitting a logistic regression model that included only the significant predictor variables, C4, and history of kidney disease. Results were similar: the estimated regression coefficients differed by <10% from the corresponding estimates in the full model.
Table 2.
Logistic regression model for predictors of kidney flare/de novo disease during the second or third trimester of pregnancy
| Predictor Variable | Adjusted Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age per 1 y | 0.92 | 0.82 to 1.03 | 0.16 |
| Non-Hispanic white | 1.94 | 0.59 to 6.37 | 0.28 |
| Low C3 | 1.83 | 0.50 to 6.64 | 0.36 |
| Low C4 | 5.59 | 1.64 to 19.13 | 0.01 |
| Positive anti-dsDNA | 0.57 | 0.18 to 1.78 | 0.33 |
| History of kidney disease | |||
| None | 1 | ||
| Complete | 6.88 | 1.84 to 25.71 | 0.004 |
| Partial | 20.98 | 4.69 to 93.98 | <0.001 |
95% CI, 95% confidence interval; dsDNA, double-stranded DNA.
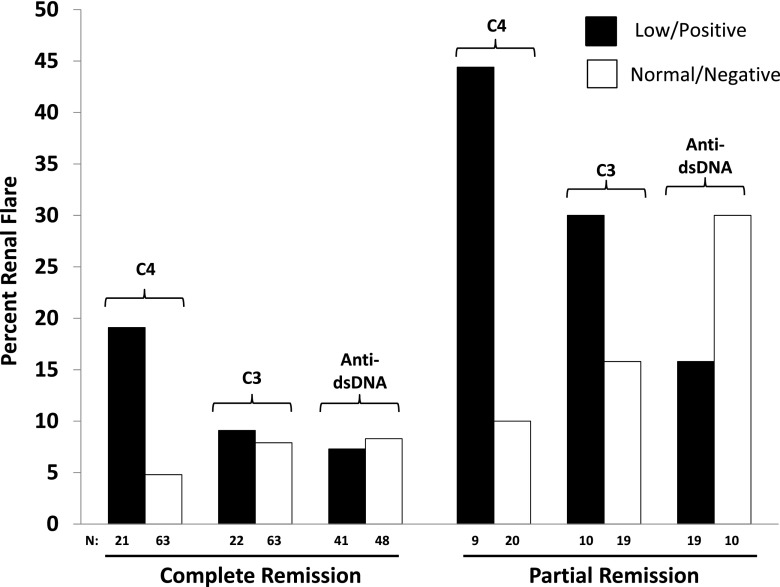
Figure 1 shows the percentage of renal flares in subgroups defined by C3, C4, anti-dsDNA, and remission status. These results further emphasize the finding that C4 accounts for the relationship between complement and flare in patients with a kidney history. Given that only four patients developed de novo kidney disease, results for patients with a past history of kidney disease are shown. Of 84 patients in complete remission, 19.1% (four of 21) of patients with a low C4 experienced a renal flare compared with 4.8% (three of 63) with a normal C4 at baseline (P=0.06). Although the numbers were smaller, low C4 at baseline as a predictor of renal flare was even more striking in the patients in partial remission. Of the 29 patients in partial remission, 44% (four of nine) with a low C4 had a renal flare compared with 10% (two of 20) with a normal C4 (P=0.06). Differences in renal flare rates between patients with low and normal C3 or negative and positive anti-dsDNA were not significant in either remission groups.
Figure 1.
Low C4 at baseline (but not anti-dsDNA or C3 alone) is a predictor of renal flare in patients with a past history of kidney disease. The bar graphs depict the percentage of renal flare in the 84 patients in complete remission and the 29 patients in partial remission. The black bars represent low C4 or C3 or positive anti-dsDNA, and the white bars represent normal C4 or C3 or negative anti-dsDNA.
With regard to kidney outcome, as per the PROMISSE Study protocol, patients were asked to return for a 3-month postpartum visit. Of the 17 patients with a renal flare/de novo kidney disease during pregnancy, 15 had repeat laboratory tests at 3 months postpartum; four of them had an increase in creatinine >0.2 mg/dl from the baseline obtained in the first trimester. In two of these four, the creatinine level was 1.4 mg/dl. Three patients had a resolution of proteinuria. One patient with no history of past kidney disease had a kidney biopsy after pregnancy that revealed class 4 nephritis.
Renal Flare and Pregnancy Outcome
Kidney flares and de novo involvement were not significantly associated with APOs. Specifically, among the 17 patients with kidney flares/de novo involvement, no APOs occurred in 12 (71%). However, five met the PROMISSE Study primary outcomes: three were induced prematurely and considered to have superimposed preeclampsia (one at 22.1 weeks with fetal death, one at 26.1 weeks with neonatal death, and one live birth at 32.6 weeks), and two had babies who were small for gestational age (<5% neonatal birth weight).
Discussion
In this largest prospective study of patients with inactive SLE or patients with stable mild/moderate SLE at conception, more than one half of whom were Hispanic and/or not white, renal flares occurred in 7.9% of those with past kidney disease in complete remission and 20.7% of those with past kidney disease in partial remission. Reassuringly, in patients without prior kidney involvement, new onset of kidney disease during pregnancy was extremely rare, occurring in <2%. Independent risk factors at enrollment for renal flare/de novo kidney involvement included past kidney disease, despite partial or complete remission, and a low C4. On the basis of multivariate analysis, anti-dsDNA and low C3 at baseline were not predictive.
The finding of decreased levels of C4 but not C3 as a predictor of developing active nephritis is of note. Both mouse and human data (12–14) suggest that classic and alternative pathways contribute to pregnancy complications, but the classic pathway is more strongly implicated in lupus nephritis. Interpretation of complement levels is confounded in pregnancy, because circulating complement reflects both synthesis (enhanced by estrogen) and consumption (15). Both C3 and C4 increase during pregnancy (16), and pregnancy itself is associated with systemic activation of complement (17). We have previously shown that, although mean levels of C3 and C4 remained in the normal range in patients with stable SLE as pregnancy progressed, a smaller of increase in C3 after the first trimester is associated with APOs (11), suggesting consumption of C3. It is not surprising that, given potential differences in the kinetics of synthesis and degradation of C3 and C4 in pregnant patients with lupus, the performance of C3 and C4 as predictors of nephritis differs. Furthermore, C4 null alleles may be contributory due to a limited capacity to increase synthesis in the face of increased consumption.
Although there are numerous publications on kidney disease during pregnancy in patients with lupus, those that are prospective are limited in number, focus largely on whites, include active nephritis at baseline, rarely focus on serology, and more often report on APOs and general lupus flares, not renal flares per se. A classic example is the highly cited retrospective study by Imbasciati et al. (18) that reported on renal flares in 81 white women with lupus nephritis. Of those in complete or partial kidney remission at baseline, 14% and 33%, respectively, experienced a renal flare (defined as an increase of >2000 mg protein per 24 hours). The rate of flare in our study was lower, 21%, for those in partial remission, despite the fact that our criteria for flare were less stringent and ethnic/racial minorities were included. Whether this is due to differences in treatment remains unknown. Although hypocomplementemia was significantly associated with overall adverse maternal and fetal outcomes in the study by Imbasciati et al. (18), low complement and/or anti-dsDNA was not specifically addressed with regard to kidney outcomes.
Two other studies prospectively followed pregnant patients with past nephritis. In a very recently published multicenter study reported by the Pregnancy Group of the Italian Society of Nephrology that included 71 pregnancies in 61 women (59 white and two Asian) with lupus nephritis (56 patients in remission and 15 patients active defined by >0.5 g proteinuria per 24 hours at the start of pregnancy), renal flares occurred in 20% (7). Low C3 and high anti-dsDNA antibodies predicted all renal flares (defined as increase in proteinuria >1000 mg/24 h and/or increase in creatinine by at least 30% over baseline), whereas low C4 predicted early flares. Fischer-Betz et al. (6) reported on 48 pregnancies in women with past nephritis who were either on continuous azathioprine or switched to azathioprine after mycophenolate mofetil, all with baseline proteinuria <1000 mg/24 h and remaining stable during pregnancy. Of note, low complement was present in 40%, and anti-dsDNA was present in 88%. Although not discussed by the authors, it is reassuring that nearly all patients had anti-dsDNA antibodies at baseline, but there were no renal flares (defined as an increase in proteinuria by 1000 mg/24 h). This is consistent with our finding of only one renal flare in the 75 patients who had anti-dsDNA antibodies alone.
The results reported herein differ somewhat from previous studies in that we include more extensive information on serologic activity. Clowse et al. (19), despite not focusing on kidney disease per se, reported that a positive anti-dsDNA at any time in pregnancy, but not low complement, was associated with proteinuria >500 mg/24 h. However, they did not specifically comment on kidney flares.
Several limitations of our study merit discussion. The study was underpowered to detect differences between patients with past kidney disease in partial remission and those in complete remission, but in accord with other published studies (6,20), complete remission should be the goal for patients before conception. Given the less stringent definition of renal flare/de novo disease defined by proteinuria exceeding a uPCR of 0.5 or dipstick change of two points, active nephritis may have been overestimated. The absence of initiation of new treatment in most of the patients with past kidney disease suggests that increases of proteinuria, despite being defined as a flare, were considered either clinically inconsequential or to be due to physiologic changes during pregnancy (e.g., increase in kidney blood flow by up to 80% above baseline, increase in GFR by about 50%, and decrease in tubular reabsorption of protein) by the treating physician or investigator (21).
The low rate of flares in this inactive/clinically stable group of patients was reassuring and reinforces the strong recommendation of disease quiescence before conception. Indeed, in patients with partial kidney remission, flare rates tended to be higher. Although anti-dsDNA was not measured in a central laboratory, most sites used an ELISA test, which is highly sensitive. However, in those patients evaluated for anti-dsDNA by Crithidia (a less sensitive test), only one who tested positive in the absence of a low C4 developed a renal flare. The low frequency of de novo disease in the patients without prior kidney involvement precludes conclusions regarding the role of serology in this group. Nevertheless, the main message in this subgroup of patients is that risk of de novo kidney disease is low, regardless of other patient characteristics. This study did not address kidney outcomes in patients with proteinuria of >1000 mg/d at conception, because these were excluded from enrollment. Finally, few studies address the difficulties inherent in differentiating lupus nephritis from preeclampsia. In fact, in some patients, both conditions may coexist as described in several patients in our study.
Data from this large multicenter, multiethnic prospective study should reassure physicians counseling patients with lupus with a history of kidney involvement. These findings are the first evidence that, in patients with past kidney disease in remission inclusive of Hispanics and blacks (in whom the prevalence of kidney disease is increased compared with whites) (22–24), serologic activity made up of only anti-dsDNA without accompanying low C4 does not warrant advising against pregnancy. Furthermore, de novo kidney disease during pregnancy is infrequent, even in these minority groups.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01 AR 49772 (to the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus Study, J.P.B., M.Y.K., M.M.G., E.R., M.P., C.A.L., L.R.S., D.W.B., T.F.P., A.S., J.T.M., E.C., and J.E.S.), NIH grant AR 43727 (to M.P.), and the Mary Kirkland Center for Lupus Research (to J.E.S.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11431116/-/DCSupplemental.
References
- 1.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JHM, Boletis J, Cervera R, Dorner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JP, Isenberg DA, Kallenberg CGM, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT; European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association : Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71: 1771–1782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, Garovic VD: A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 5: 2060–2068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linnik MD, Hu JZ, Heilbrunn KR, Strand V, Hurley FL, Joh T; LJP 394 Investigator Consortium : Relationship between anti-double-stranded DNA antibodies and exacerbation of renal disease in patients with systemic lupus erythematosus. Arthritis Rheum 52: 1129–1137, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Swaak AJ, Groenwold J, Bronsveld W: Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis 45: 359–366, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saavedra MA, Cruz-Reyes C, Vera-Lastra O, Romero GT, Cruz-Cruz P, Arias-Flores R, Jara LJ: Impact of previous lupus nephritis on maternal and fetal outcomes during pregnancy. Clin Rheumatol 31: 813–819, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Fischer-Betz R, Specker C, Brinks R, Aringer M, Schneider M: Low risk of renal flares and negative outcomes in women with lupus nephritis conceiving after switching from mycophenolate mofetil to azathioprine. Rheumatology (Oxford) 52: 1070–1076, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Moroni G, Doria A, Giglio E, Imbasciati E, Tani C, Zen M, Strigini F, Zaina B, Tincani A, Gatto M, de Liso F, Grossi C, Meroni PL, Cabiddu G, Messa P, Ravani P, Mosca M: Maternal outcome in pregnant women with lupus nephritis. A prospective multicenter study. J Autoimmun 74: 194–200, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Bramham K, Hunt BJ, Bewley S, Germain S, Calatayud I, Khamashta MA, Nelson-Piercy C: Pregnancy outcomes in systemic lupus erythematosus with and without previous nephritis. J Rheumatol 38: 1906–1913, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Gladman DD, Tandon A, Ibañez D, Urowitz MB: The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol 37: 754–758, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC: Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, Sammaritano L, Branch DW, Porter TF, Sawitzke A, Merrill JT, Stephenson MD, Cohn E, Garabet L, Salmon JE: Predictors of pregnancy outcomes in patients with lupus: A cohort study. Ann Intern Med 163: 153–163, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE: Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 112: 1644–1654, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch AM, Murphy JR, Gibbs RS, Levine RJ, Giclas PC, Salmon JE, Holers VM: The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG 117: 456–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamonki JM, Salmon JE, Hyjek E, Baergen RN. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol 196: 167.e1–167.e5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramson SB, Buyon JP: Activation of the complement pathway: Comparison of normal pregnancy, preeclampsia, and systemic lupus erythematosus during pregnancy. Am J Reprod Immunol 28: 183–187, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Johnson U, Gustavii B: Complement components in normal pregnancy. Acta Pathol Microbiol Immunol Scand C 95: 97–99, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M: Normal pregnancy is characterized by systemic activation of the complement system. J Matern Fetal Neonatal Med 17: 239–245, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imbasciati E, Tincani A, Gregorini G, Doria A, Moroni G, Cabiddu G, Marcelli D: Pregnancy in women with pre-existing lupus nephritis: Predictors of fetal and maternal outcome. Nephrol Dial Transplant 24: 519–525, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Clowse ME, Magder LS, Petri M: The clinical utility of measuring complement and anti-dsDNA antibodies during pregnancy in patients with systemic lupus erythematosus. J Rheumatol 38: 1012–1016, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Stanhope TJ, White WM, Moder KG, Smyth A, Garovic VD: Obstetric nephrology: Lupus and lupus nephritis in pregnancy. Clin J Am Soc Nephrol 7: 2089–2099, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Cheung KL, Lafayette RA: Renal physiology of pregnancy. Adv Chronic Kidney Dis 20: 209–214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanly JG, O’Keeffe AG, Su L, Urowitz MB, Romero-Diaz J, Gordon C, Bae SC, Bernatsky S, Clarke AE, Wallace DJ, Merrill JT, Isenberg DA, Rahman A, Ginzler EM, Fortin P, Gladman DD, Sanchez-Guerrero J, Petri M, Bruce IN, Dooley MA, Ramsey-Goldman R, Aranow C, Alarcón GS, Fessler BJ, Steinsson K, Nived O, Sturfelt GK, Manzi S, Khamashta MA, van Vollenhoven RF, Zoma AA, Ramos-Casals M, Ruiz-Irastorza G, Lim SS, Stoll T, Inanc M, Kalunian KC, Kamen DL, Maddison P, Peschken CA, Jacobsen S, Askanase A, Theriault C, Thompson K, Farewell V: The frequency and outcome of lupus nephritis: Results from an international inception cohort study. Rheumatology (Oxford) 55: 252–262, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C: The incidence and prevalence of systemic lupus erythematosus, 2002-2004: The Georgia Lupus Registry. Arthritis Rheumatol 66: 357–368, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP, Leisen J, Shaltis D, McCune WJ: Population-based incidence and prevalence of systemic lupus erythematosus: The Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 66: 369–378, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.