Abstract
Background and objectives
Although arteriovenous fistulas have been found to be the most cost-effective form of hemodialysis access, the relative benefits of placing an arteriovenous fistula versus an arteriovenous graft seem to be least certain for older adults and when placed preemptively. However, older adults’ life expectancy is heterogeneous, and most patients do not undergo permanent access creation until after dialysis initiation. We evaluated cost-effectiveness of arteriovenous fistula placement after dialysis initiation in older adults as a function of age and life expectancy.
Design, setting, participants, & measurements
Using a hypothetical cohort of patients on incident hemodialysis with central venous catheters, we constructed Markov models of three treatment options: (1) arteriovenous fistula placement, (2) arteriovenous graft placement, or (3) continued catheter use. Costs, utilities, and transitional probabilities were derived from existing literature. Probabilistic sensitivity analyses were performed by age group (65–69, 70–74, 75–79, 80–84, and 85–89 years old) and quartile of life expectancy. Costs, quality-adjusted life-months, and incremental cost-effectiveness ratios were evaluated for up to 5 years.
Results
The arteriovenous fistula option was cost effective compared with continued catheter use for all age and life expectancy groups, except for 85–89 year olds in the lowest life expectancy quartile. The arteriovenous fistula option was more cost effective than the arteriovenous graft option for all quartiles of life expectancy among the 65- to 69-year-old age group. For older age groups, differences in cost-effectiveness between the strategies were attenuated, and the arteriovenous fistula option tended to only be cost effective in patients with life expectancy >2 years. For groups for which the arteriovenous fistula option was not cost saving, the cost to gain one quality-adjusted life-month ranged from $2294 to $14,042.
Conclusions
Among older adults, the cost-effectiveness of an arteriovenous fistula placed within the first month of dialysis diminishes with increasing age and lower life expectancy and is not the most cost-effective option for those with the most limited life expectancy.
Keywords: frail elderly, vascular access, health care costs, geriatric nephrology; Medicare; dialysis; arteriovenous fistula; arteriovenous graft; life-expectancy; Arteriovenous Shunt, Surgical; Central Venous Catheters; Cost-Benefit Analysis; Humans; Probability; renal dialysis
Introduction
Policy makers, professional societies, and guideline makers endorse arteriovenous fistulas (AVFs) as the preferred and most cost-effective form of hemodialysis access on the basis of their lower mortality and complication rates compared with arteriovenous grafts (AVGs) and central venous catheters (CVCs) (1–5). However, there is growing concern that contemporary approaches to permanent access placement do not account for variations across individuals in characteristics that may shape the relative advantages of different forms of vascular access (6,7). In particular, there is evidence that fistula nonmaturation and patency issues may lower the relative benefits of AVF over AVG placement in older adults (3,8–11). Recent data also suggest that older adults may not experience the survival advantage associated with placing an AVF before dialysis initiation (12). Because survival tends to influence the cost-effectiveness of AVF placement relative to other treatment options (3,4), these data infer that preemptive AVF placement may not be the cost-effective approach in older adults. Meanwhile, initial permanent access placement after dialysis initiation is a far more common scenario (13); however, any cost-savings attributable to AVF placement may be modest in this scenario because of the costs associated with CVC infections that may occur while awaiting fistula maturation. The benefit of AVF placement in older adults is also questioned, because the time required before an AVF can be used may exceed an individual’s life expectancy.
Although life expectancy generally decreases with age, there is substantial heterogeneity in life expectancy among older adults of similar ages due to heterogeneity in functional status and burden of comorbidity (14,15). Therefore, we suspect that a one size fits all approach to vascular access may not be equally cost effective across the range of life expectancies in older adults initiating hemodialysis. Decision models for other conditions in older adults, such as when to stop cancer screening or initiate osteoporosis treatment, have determined that considering “health quartile” can identify thresholds at which a treatment option is no longer cost effective (16,17). Under this approach, decision making depends on whether an individual is especially healthy for his/her age (highest quartile of life expectancy), especially frail or with multiple comorbidities (lowest quartile), or of average health for his/her age (intermediate quartiles). We designed a study to assess how age and life expectancy modify the cost-effectiveness of AVF placement (compared with AVG placement and continued CVC use) within the first month after dialysis initiation within a population of older adults.
Materials and Methods
We used Markov modeling with Monte Carlo simulations to estimate costs per quality-adjusted life-month (QALM) for hypothetical cohorts of older adults initiating dialysis with a CVC at different starting ages and life expectancy quartiles.
Model Overview
Our base case analysis considered three treatment options for older patients who have initiated hemodialysis with a tunneled CVC as follows: (1) continue dialysis with a CVC, (2) undergo AVF surgery within 30 days after hemodialysis initiation, or (3) undergo AVG surgery within 30 days after hemodialysis initiation. Using TreeAge Pro software, Markov models for each treatment option were constructed for five age groups (65–69, 70–74, 75–79, 80–84, and 85–89 years old) and three life expectancy levels (25th [sickest], 50th [average health], and 75th [healthiest]) for a total of 15 age and life expectancy subgroups. We evaluated costs (from a health care system perspective) and effectiveness for each treatment option and subgroup over a 5-year time horizon.
Model Structure
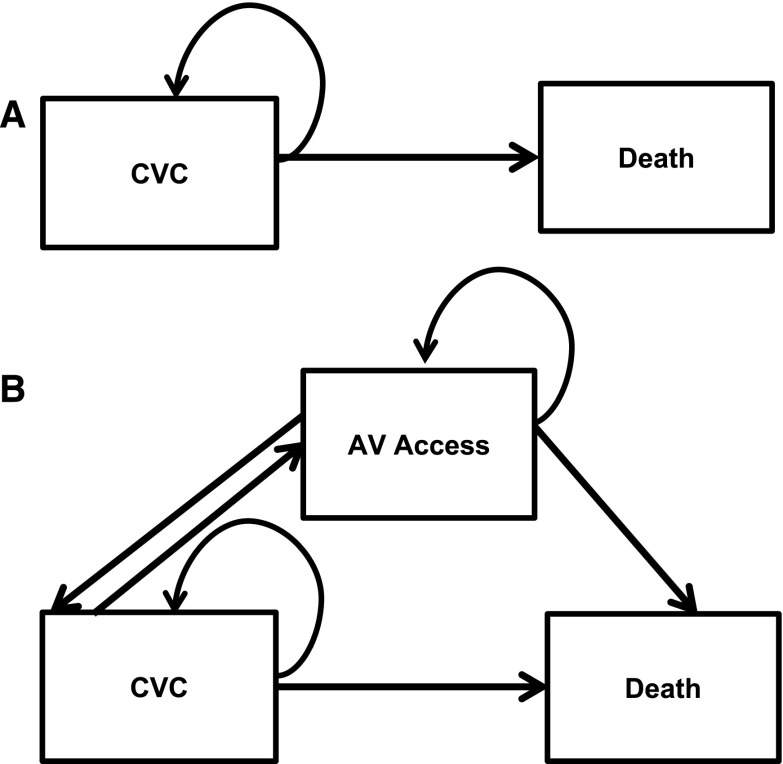
Each Markov model had monthly cycles in which patients could transition among different health states (Figure 1). Patients who did not receive a permanent vascular access could transition between two health states: (1) alive with CVC as dialysis access and (2) death. Patients who did undergo placement of permanent vascular access could transition between three health states: (1) alive with CVC as dialysis access, (2) alive with AVF (or AVG) as dialysis access, or (3) death. Here, patients could transition from CVC to AVF (or AVG) if (or when) the surgical access matured. If the surgical access did not mature in a prespecified time period felt to be reasonable for access maturation (4 months for AVF maturation and 1 month for AVG maturation) (18), patients remained in the CVC state and underwent one additional AVF (or AVG) surgical procedure. Patients with mature and functional AVFs (or AVGs) transitioned back to the CVC state if (or when) access infection or permanent loss of patency occurred. For each model, death was an absorbing state.
Figure 1.
Conceptual model demonstrates transitions among different health states. The decision is modeled as hemodialysis initiation with central venous catheter (CVC). Options included (A) continue hemodialysis with CVC as access or (B) undergo arteriovenous (AV) fistula or AV graft placement.
Assumptions were integrated into the Markov models for a simplified picture of possible pathways for access complications compared with what might be expected in real world settings. We limited salvage to one additional access surgery for the same access type. All vascular access infections required hospitalization with antibiotics for bacteremia. For vascular access procedures to re-establish an access with compromised patency, we assumed that 50% of interventions required hospitalization to account for older adults’ high prevalence of frailty (19).
Model Parameters
In the absence of randomized, controlled trial data on the efficacy and effectiveness of different hemodialysis access types in older adults, we obtained data for transitional probabilities through a comprehensive literature search. We searched MEDLINE for English language articles about vascular access outcomes in older adults. For the data search, we used medical subject headings terms (aged; arteriovenous shunt, surgical; renal insufficiency, chronic; and renal dialysis) and keywords (older adults, elderly, vascular access, and hemodialysis). We used data from cohort studies, clinical trials, and review articles that described outcomes in adults ≥65 years old. When data specific to older adults were not available, we used data from studies that included younger patients. Because the transitions in the model occurred on a monthly basis, published data were converted to transitional probabilities per month. To account for a range of values for each transitional probability, we incorporated probability distributions (where available) for infections, compromised patency, and access survival after intervention to restore patency (Table 1) (8,20–22). Because the probability of access maturation changes over time, we used maturation rate data from Xue et al. (4) to construct a distribution of probabilities for AVF and AVG maturation that peak after the fourth (13.8%) and second month (33.3%), respectively. Probability of death was derived from published literature on the probability of death by vascular access type and population-level data on the probability of death for age group–specific quartiles of life expectancy (23). These population-level data were derived from survival curves of patients with ESRD ages ≥65 years old between 2000 and 2004 (13).
Table 1.
Model input parameters for transitional probabilities
| Transitional Probability | Input Data | AVF | AVG | CVC | Distribution Typea |
|---|---|---|---|---|---|
| Probability of access infection | Monthly rate of bacteremia per 1000 patient-yr, mean (SD) (20) | 70.3 (23.5) | 127 (44.2) | 258.7 (97.2) | γ |
| Probability of compromised patency of access | Accesses requiring intervention over 12 mo (8,21), % | 35 | 34.3 | 47.5b | β |
| Probability of successful intervention to restore patency | Accesses with patency after intervention (22), % | 29 | 29 | N/A | β |
| Probability of access maturation | Mature accesses (of eligible accesses) (4), % | Cycle dependentc | Cycle dependentc | N/A | β |
| Probability of death | Death hazard ratio (95% CI) (23) | Reference | 1.39 (1.32 to 1.47) | 2.18 (2.11 to 2.26) | Log normal |
Data sources for input parameters are indicated by references. AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter; N/A, not applicable; 95% CI, 95% confidence interval.
For our Monte Carlo simulations, most input parameters were converted into probability distributions. The probability distribution types used were γ, β, and log normal. We converted infection rate into a γ-probability distribution by using mean (and SD) infection rates to calculate shape and rate parameter (α and β) values. We converted compromised patency, treatment of patency, and access maturation proportions into β-probability distributions by using the proportions as α (or β)-parameter values. We converted risk of death into a log-normal probability distribution by using the natural log of a hazard ratio and 95% CI to determine mean and SD parameter values. These parameter values were applied in specific formulas to create the probability distributions.
There is no distribution for probability of compromised CVC patency.
The probability of access maturation changed with each cycle of the Markov model.
From published literature, we also obtained data for costs and health-state utilities. Procedural costs (access placement, access removal, and percutaneous and surgical interventions) were derived from Centers for Medicare and Medicaid Services (CMS) expenditures (4). Because percutaneous procedures can be performed in either outpatient or inpatient settings, we estimated percutaneous procedures costs by averaging outpatient and inpatient hospital costs (5). Costs associated with bacteremia from vascular access infections included procedural, hospital, and physician costs (4). Costs associated with death were determined from CMS expenditures for patients at the end of life (24). We used the medical care component of the inflation-adjusted US Consumer Price Index to report costs in 2014 United States dollars (Table 2). Health-state utilities, representing the perceptions of quality of life in a given health state by patients on dialysis, for AVF, AVG, and CVC were 1.0, 0.95, and 0.91, respectively (4,25). Disutilities associated with compromised access patency and access-related infections were −0.08 and −0.6, respectively (4). We applied the disutility of the complication only to the cycle in which the event occurred.
Table 2.
Costs included in the Markov models
| Event | Cost, $ |
|---|---|
| AVF placement (4) | 888.37 |
| AVG placement (4) | 643.04 |
| CVC placement (4) | 318.90 |
| CVC removal (4) | 154.39 |
| Hospitalization for access infection (4) | 23,137.59 |
| Percutaneous procedure (4) | 508.04 |
| Adjusted hospital costs (5)a | 8601.19 |
| Death (24) | 10,917.36 |
Data sources for costs are indicated by references. AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter.
Adjusted hospital costs are defined as total cost =1/2 outpatient costs +1/2 inpatient costs.
Analyses
To account for uncertainty introduced through data synthesis, we performed Monte Carlo simulations (or probabilistic sensitivity analyses) in TreeAge Pro using 1000 samples and 1000 trials of microsimulation. These simulations generated estimates of accumulated costs, mean survival, and quality-adjusted survival. Quality-adjusted survival, expressed in QALMs (where one QALM represents a month of life in perfect health), accounts for health-state utilities (and disutilities) at each Markov model transition as a measure of effectiveness. Effectiveness and costs were compared across treatment options to determine whether AVF placement (compared with AVG placement or continued CVC use) showed cost-savings (described as less expensive and better quality-adjusted survival) or had an incremental cost to gain one additional QALM (incremental cost-effectiveness ratio [ICER]). We determined ICERs from the difference in costs divided by the difference in QALMs between AVF and each of its two comparators (AVG and CVC). We constructed cost-effectiveness acceptability curves to depict the probability that a treatment option is cost effective across a willingness to pay range from $0 to $100,000 per quality-adjusted life-year (QALY). Willingness to pay represents the amount of dollars that society is willing to pay for one QALY. Utilities and future costs were discounted at a 3% annual rate.
Results
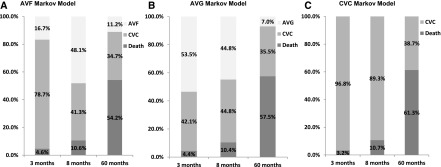
For the AVF and AVG Markov models, health-state probabilities changed from CVC to arteriovenous access or death over time. For example, among 65–69 year olds in the highest life expectancy quartile, the proportion with a functional AVF peaked at 48.1% by 8 months after dialysis initiation and declined thereafter (Figure 2). At that time, 41.3% of cohort patients had a CVC after failed attempts at AVF construction, and 10.6% had died. By 5 years after initiation, only 11.2% maintained a functional AVF, whereas more than one half (54.2%) had died. If an AVG is placed instead, the proportion of 65–69 year olds in the highest life expectancy quartile with a functional AVG peaked at 53.5% by 3 months after dialysis initiation. By 5 years after initiation, a smaller proportion (7.0%) had a functional AVG and a larger proportion (57.5%) had died compared with those who had AVF placement. In the same time period, the CVC Markov model estimated the largest probability of death (61.3%) compared with the AVF and AVG Markov models (Figure 2). For both AVF and AVG Markov models, the proportion of cohort members who achieved a functional access was lower in older age groups and lower life expectancy quartiles (Supplemental Tables 1 and 2). For AVF, AVG, and CVC Markov models, we estimated survival for each age and life expectancy group (Table 3). Mean survival was <4 years and longest among those who would have AVF placement followed by AVG placement and CVC use, respectively.
Figure 2.
Markov model health-state probabilities at three points in time over a 5-year horizon for all age and life expectancy groups: the probabilities of functional arteriovenous fistula (AVF) and functional arteriovenous graft (AVG) peaked at 8 and 3 months, respectively. Subsequently, probability of functional arteriovenous access decreased, whereas probability of central venous catheter (CVC) use or death increased. Health-state probabilities for functional arteriovenous access, CVC use, and death at three time points ([1] time at which there is the highest probability of functional AVG [3 months], [2] time at which there is the highest probability of functional AVF [8 months], and [3] the last month of the simulation) are depicted for (A) AVF and (B) AVG Markov models. Health-state probabilities for the (C) CVC Markov model show the probability of death or CVC use at the same three time points. The proportions shown are for the highest (75th percentile) quartile of the 65- to 69-year-old age group.
Table 3.
Mean survival in months for each treatment option by age and life expectancy groups
| Percentile | 65–69 yr | 70–74 yr | 75–79 yr | 80–84 yr | 85–89 yr |
|---|---|---|---|---|---|
| 75th | |||||
| AVF | 43.0 (0.7) | 38.4 (0.8) | 31.9 (0.9) | 24.1 (0.8) | 16.6 (0.8) |
| AVG | 40.8 (0.8) | 36.4 (0.9) | 30.2 (1.0) | 21.3 (1.1) | 14.2 (0.7) |
| CVC | 39.3 (1.0) | 34.5 (1.1) | 27.9 (1.2) | 20 (1.1) | 13.2 (0.8) |
| 50th | |||||
| AVF | 38.1 (0.8) | 31.5 (0.9) | 23.7 (0.8) | 15.3 (0.8) | 7.0 (0.4) |
| AVG | 36.2 (0.9) | 29.8 (1.0) | 20.8 (1.0) | 13.5 (0.6) | 6.4 (0.5) |
| CVC | 34.2 (1.1) | 27.5 (1.2) | 19.5 (1.1) | 12.3 (0.8) | 6.3 (0.4) |
| 25th | |||||
| AVF | 26.7 (0.8) | 19.9 (0.7) | 11.6 (0.5) | 7.2 (0.4) | 3.0 (0.2) |
| AVG | 24.7 (1.2) | 17.1 (0.8) | 10.5 (0.7) | 6.6 (0.5) | 2.8 (0.2) |
| CVC | 22.9 (1.2) | 16.0 (0.9) | 9.7 (0.6) | 6.5 (0.4) | 3.6 (0.2) |
Values shown are means (SD) in months. AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter.
Compared with the option to continue CVC use, the AVF option had the lowest cost and highest effectiveness (cost saving or cost effective) for each age and life expectancy group, with the exception of patients in the oldest age group in the lowest life expectancy quartile (Table 4, AVF Options Versus CVC Options). In this age and life expectancy group (mean survival =3.1 [SD=0.2] months [Table 3]), the AVF option’s QALM became lower than the QALM for continued CVC use (0.16 versus 0.20 QALMs). Although the AVF option’s costs were $524 less than continued CVC use for these patients, it would cost $14,042 to gain one additional QALM for this age and life expectancy group.
Table 4.
Cost-effectiveness of AVF compared with CVC and AVG options by life expectancy quartile and age
| Age, yr | AVF Options Versus CVC Options | AVF Options Versus AVG Options | ||||
|---|---|---|---|---|---|---|
| 75th Percentile | 50th Percentile | 25th Percentile | 75th Percentile | 50th Percentile | 25th Percentile | |
| 65–69 | Cost savinga | Cost saving | Cost saving | Cost saving | Cost saving | Cost saving |
| 70–74 | Cost saving | Cost saving | Cost saving | Cost saving | Cost saving | $3924/QALM |
| 75–79 | Cost saving | Cost saving | Cost saving | Cost saving | $2645/QALM | $2380/QALM |
| 80–84 | Cost saving | Cost saving | Cost saving | $2294/QALM | $2828/QALM | Cost saving |
| 85–89 | Cost saving | Cost saving | $14,042/QALM | $3860/QALM | Cost saving | $13,253/QALM |
AVF, arteriovenous fistula; CVC, central venous catheter; AVG, arteriovenous graft; QALM, quality-adjusted life-month. Data shown are incremental cost-effectiveness ratios defined as difference in costs divided by difference in effectiveness (QALM). The incremental cost-effectiveness ratio represents the cost required to gain one additional QALM.
Cost-savings is defined as a treatment option that has lower costs and greater effectiveness than the alternative treatment.
Compared with the option to place an AVG, the AVF option showed cost-savings for younger patients and those with longer life expectancy (Table 4, AVF Options Versus AVG Options). In older age groups, the AVF option’s cost-effectiveness was less consistent and varied with both age and life expectancy. For example, the AVF option was cost saving for those with highest life expectancy who were ages 65–79 years old but no longer cost saving for those ages 80–89 years old. There was a general trend that the AVF option was no longer cost saving for those ages ≥80 years old; however, there were two age and life expectancy groups in which cost-savings were identified: those ages 85–89 years old with intermediate life expectancy and those ages 80–85 years old with the lowest life expectancy. In these two groups, costs incurred from the AVF option were lower than AVG-related costs, likely attributable to higher infection rates (Table 1).
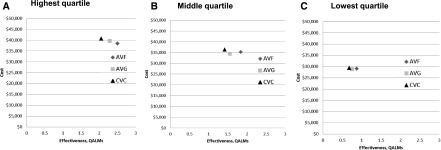
In the age and life expectancy groups that did not show cost-savings with the AVF option (compared with the AVG option), overall costs per QALM for AVF placement were <$15,000, and mean survival was <2 years. Figure 3 illustrates change in cost and effectiveness of AVF placement as a function of life expectancy in the 75- to 79-year-old age group. Unlike those within the highest life expectancy quartile, those within the intermediate and lowest life expectancy quartiles (mean survival =15.9 [SD=0.8] months) generated more costs over time with the AVF option. In these age/life expectancy groups, choosing AVF placement instead of AVG placement would cost $2380–$2645 to gain one additional QALM.
Figure 3.
Estimates of arteriovenous fistula (AVF) cost-effectiveness decrease as life-expectancy decreases. Estimated costs and quality-adjusted life-months (QALMs) for AVF placement, arteriovenous graft (AVG) placement, and continued central venous catheter (CVC) use for the 75- to 79-year-old age group are depicted by three life expectancy categories: (A) highest, (B) intermediate, and (C) lowest quartiles. The AVF option costs were higher with lower life expectancy.
Despite uncertainty from the model and its input parameters, the probability that AVF placement was the most cost-effective treatment option was 100% for 65–69 year olds in the highest life expectancy quartile for any willingness to pay threshold ≥$20,000 per QALY (Supplemental Figure 1). For 85–89 year olds in the lowest life expectancy quartile, AVF placement had a lower probability of cost-effectiveness than AVG placement and CVC use, and the probability of cost-effectiveness with CVC use was at least 95% for any willingness to pay threshold ≥$40,000 per QALY.
Discussion
Despite widespread endorsement among a wide range of stakeholders, there is growing reservation about the relative benefits of AVF over AVG placement, particularly in the predialysis period and older adults, arguing for greater flexibility in choice of vascular access (3,12,26,27). Our analyses of the cost-effectiveness of placement of AVF versus AVG after dialysis initiation among a population of older adults suggest that the benefits of AVFs are sensitive to differences in life expectancy. AVFs are no more cost effective than AVGs for those with a life expectancy of <2 years, and neither form of vascular access is more cost effective than catheters for patients with a life expectancy of <6 months. These results support AVG placement and in some instances, even continued CVC use after dialysis initiation as reasonable vascular access options for older adults with limited life expectancy.
Published cost-effectiveness studies provide evidence that the superior cost-effectiveness of AVFs (compared with other accesses) is sensitive to fistula maturation rates and several other patient characteristics (4). Xue et al. (4) and Rosas et al. (5) found that AVF placement is not superior to AVG placement if AVF maturation rates are <36%–41%. Drew et al. (3) further defined patient characteristics associated with a lower ICER of AVF placement, which include diabetes, women, and older age. These patient characteristics are associated with two important factors that determine whether there would be benefit of AVF placement: access maturation success and survival after ESRD onset, both of which tend to be less favorable among older adults (28,29).
Although prior research suggests that AVF placement is less cost effective in older adults (3), our study is novel, because its findings support an approach to decision making that acknowledges the substantial heterogeneity in life expectancy within the population of older adults. Specifically, we show that AVF cost-effectiveness can differ for individuals with the same age but differing life expectancy. For some subgroups with limited life expectancy, there was an increase in the incremental cost (ICER) of AVF compared with AVG. Although the ICERs were less than the commonly used willingness to pay threshold of $100,000 per QALY, the absence of AVF cost-savings combined with the short remaining lifetime suggests that initiatives that promote AVF placement, such as Fistula First Catheter Last, may not meet the intended goal of decreasing health care costs if AVFs are preferentially placed in older adults with limited life expectancy (30). At present, the CMS ESRD Quality Incentive Program uses the proportion of AVFs in use among all patients on hemodialysis to determine a dialysis facility’s payments (31). Considering that decrements in life expectancy can result from significant comorbidity, irrespective of age (32), our findings suggest that this policy may result in misalignment between facility-level incentives and treatment strategies that are most beneficial and least harmful for individual patients (33). Because our findings are derived from mathematical modeling, a prospective cohort study that evaluates access-related outcomes and mortality in a heterogeneous group of older adults would be an important next step for informing both policy and clinical decision making.
Although cost-effectiveness is not often the most important consideration for choosing vascular access for the individual patient, our findings have practical implications for incorporating life expectancy into clinical decision making. First, a nephrologist or access surgeon could explain that the benefits of AVF placement may or may not outweigh its potential harms, especially in patients with limited life expectancy. Second, our findings are consistent with an existing algorithm for choosing hemodialysis access that incorporates whether a patient’s life expectancy is <2 years into its decision tree (34). This algorithm requires providers to think about life expectancy in addition to age to determine the ideal access on an individual basis. As in other populations, there are tools for predicting life expectancy for older patients on dialysis (35,36). There is uncertainty in this prognostication; therefore, acknowledging uncertainty and integrating “best case-worst case” scenarios into discussions about access placement may help to support shared decision making (7,37–40). This approach to clinical decision making is particularly important for accommodating patients whose preferences change over the course of illness.
Our study’s strength is the incorporation of both age and life expectancy into our simulation models to assess cost-effectiveness. However, our results should be interpreted with consideration of the following limitations. First, the structure and assumptions of our Markov model may not represent all real world treatment practices. The variability in time allowed for access maturation, interventions to enhance access maturation, type and number of procedures after access failure (including switch from AVF to AVG), and treatment for access complications observed in the clinical setting (e.g., hospitalization, treatment regimen, and duration) could not be captured in our models. To address this variability, we developed a simple model structure reflecting a conservative approach to access management to prevent overestimation of costs. Thus, if anything, our results likely overestimate the cost-effectiveness of AVFs compared with other accesses. Second, data from our Markov models relied on existing literature, and therefore, we performed our analyses with probability distributions to gain insight into the robustness of our findings. Without cohort data, we were unable to model other relevant factors, such as sex, diabetes status, or surgical approach and training (3,41). For example, we used published survival data by access type; however, there is emerging evidence that the survival advantage often attributable to AVF may actually be driven by patient factors (42). Other factors, such as sex, could potentially influence transitional probabilities, which may yield different estimates of cost-effectiveness. We attempted to incorporate patient factors by evaluating 15 age/life expectancy groups; survival may be overestimated in our models, because we did not account for specific patient factors. Still, decreasing the AVF survival benefit would only further strengthen our conclusion that CVCs and AVGs are reasonable choices for frail or very elderly patients. Third, we did not account for facility fees from vascular access procedures, and therefore, our findings are conservative across all treatment options. Fourth, our analyses are likely not generalizable to other countries, especially countries with a larger proportion of patients on incident dialysis with AVFs. Fifth, our models only provide information on the cost-effectiveness of AVF placement within the first month of dialysis. Although this is a very common scenario in the clinical setting, our analyses do not address the cost-effectiveness of AVF placement earlier or later in the course of illness or account for changes in health or personal preferences that may occur over time.
In summary, the cost-effectiveness of AVFs is critically dependent on life expectancy. In older adults with the most limited life expectancy, AVFs were no more cost effective than AVGs and in some instances, CVCs. These findings call into question the one size fits all approach to vascular access placement codified in contemporary clinical practice guidelines and embedded in current quality initiatives.
Disclosures
None.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Romita Mukerjee, Weiping Sun, and Xun Tang for initial contributions to development of the study.
Support was provided through National Institute on Aging Claude D. Pepper Older Americans Independence Center grant P30 AG028716, GEMSSTAR Program grant R03 AG050834, and grant K24 AG049077-01A1; National Center for Advancing Translational Sciences grant UL1TR001117; and the T. Franklin Williams Scholarship Award provided by the American Society of Nephrology and the Alliance for Academic Internal Medicine.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Reassessing Recommendations for Choice of Vascular Access,” on pages 865–867.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11631116/-/DCSupplemental.
References
- 1.Huber TS, Carter JW, Carter RL, Seeger JM: Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: A systematic review. J Vasc Surg 38: 1005–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol 15: 477–486, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Drew DA, Lok CE, Cohen JT, Wagner M, Tangri N, Weiner DE: Vascular access choice in incident hemodialysis patients: A decision analysis. J Am Soc Nephrol 26: 183–191, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue H, Lacson E Jr., Wang W, Curhan GC, Brunelli SM: Choice of vascular access among incident hemodialysis patients: A decision and cost-utility analysis. Clin J Am Soc Nephrol 5: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosas SE, Feldman HI: Synthetic vascular hemodialysis access versus native arteriovenous fistula: A cost-utility analysis. Ann Surg 255: 181–186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wish JB: Catheter last, fistula not-so-first. J Am Soc Nephrol 26: 5–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hare AM: Vascular access for hemodialysis in older adults: A “patient first” approach. J Am Soc Nephrol 24: 1187–1190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN: A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg 45: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hod T, Desilva RN, Patibandla BK, Vin Y, Brown RS, Goldfarb-Rumyantzev AS: Factors predicting failure of AV “fistula first” policy in the elderly. Hemodial Int 18: 507–515, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, Mysore P, Lewin AM, Hiremath S, MacRae J, James MT, Miller L, Hemmelgarn BR, Moist LM, Garg AX, Chowdhury TT, Ravani P: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Renal Data System : USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 14.Tamura MK, Tan JC, O’Hare AM: Optimizing renal replacement therapy in older adults: A framework for making individualized decisions. Kidney Int 82: 261–269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grubbs V, Wasse H, Vittinghoff E, Grimes BA, Johansen KL: Health status as a potential mediator of the association between hemodialysis vascular access and mortality. Nephrol Dial Transplant 29: 892–898, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham AN, Datta SK, Weber TJ, Walter LC, Colón-Emeric CS: Cost-effectiveness of oral bisphosphonates for osteoporosis at different ages and levels of life expectancy. J Am Geriatr Soc 59: 1642–1649, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Breslau ES, Gorin SS, Edwards HM, Schonberg MA, Saiontz N, Walter LC: An individualized approach to cancer screening decisions in older adults: A multilevel framework. J Gen Intern Med 31: 539–547, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dember LM, Kaufman JS, Beck GJ, Dixon BS, Gassman JJ, Greene T, Himmelfarb J, Hunsicker LG, Kusek JW, Lawson JH, Middleton JP, Radeva M, Schwab SJ, Whiting JF, Feldman HI; DAC Study Group : Design of the Dialysis Access Consortium (DAC) clopidogrel prevention of early AV fistula thrombosis trial. Clin Trials 2: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 19.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, Walston JD, Segev DL: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 21.Little MA, O’Riordan A, Lucey B, Farrell M, Lee M, Conlon PJ, Walshe JJ: A prospective study of complications associated with cuffed, tunnelled haemodialysis catheters. Nephrol Dial Transplant 16: 2194–2200, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Swindlehurst N, Swindlehurst A, Lumgair H, Rebollo Mesa I, Mamode N, Cacciola R, Macdougall I: Vascular access for hemodialysis in the elderly. J Vasc Surg 53: 1039–1043, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Foley RN, Chen SC, Collins AJ: Hemodialysis access at initiation in the United States, 2005 to 2007: Still “catheter first”. Hemodial Int 13: 533–542, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH: Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol 1: 1248–1255, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Wasse H, Kutner N, Zhang R, Huang Y: Association of initial hemodialysis vascular access with patient-reported health status and quality of life. Clin J Am Soc Nephrol 2: 708–714, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalloo S, Blake PG, Wish J: A patient-centered approach to hemodialysis vascular access in the era of fistula first. Semin Dial 29: 148–157, 2016 [DOI] [PubMed] [Google Scholar]
- 27.DeSilva RN, Patibandla BK, Vin Y, Narra A, Chawla V, Brown RS, Goldfarb-Rumyantzev AS: Fistula first is not always the best strategy for the elderly. J Am Soc Nephrol 24: 1297–1304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K: Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis 42: 1000–1012, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kurella M, Covinsky KE, Collins AJ, Chertow GM: Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med 146: 177–183, 2007 [DOI] [PubMed] [Google Scholar]
- 30.ESRD National Coordinating Center: Fistula First Catheter Last. Available at: http://esrdncc.org/ffcl/about-us-fistula-first/. Accessed July 28, 2016
- 31.Center for Medicare and Medicaid Services: Technical Specifications for ESRD QIP Measures. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/PY-2017-Technical-Measure-Specifications.pdf. Accessed July 28, 2016
- 32.Davidson I, Gallieni M: Optimizing vascular access in the elderly: Words we use affect patient care. J Vasc Access 16: 437–438, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, Michael B, O’Hare AM, Schaefer HM, Shaffer RN, Trachtman H, Weiner DE, Falk AR; American Society of Nephrology Quality, and Patient Safety Task Force : Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Allon M, Lok CE: Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol 5: 2348–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H: Predicting early death among elderly dialysis patients: Development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis 66: 1024–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung KL, Montez-Rath ME, Chertow GM, Winkelmayer WC, Periyakoil VS, Kurella Tamura M: Prognostic stratification in older adults commencing dialysis. J Gerontol A Biol Sci Med Sci 69: 1033–1039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried TR: Shared decision making--Finding the sweet spot. N Engl J Med 374: 104–106, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Blake PG, Quinn RR, Oliver MJ: The risks of vascular access. Kidney Int 82: 623–625, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Casey JR, Hanson CS, Winkelmayer WC, Craig JC, Palmer S, Strippoli GF, Tong A: Patients’ perspectives on hemodialysis vascular access: A systematic review of qualitative studies. Am J Kidney Dis 64: 937–953, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Schell JO, Cohen RA: A communication framework for dialysis decision-making for frail elderly patients. Clin J Am Soc Nephrol 9: 2014–2021, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo K, Lok CE: New insights into dialysis vascular access: What is the optimal vascular access type and timing of access creation in CKD and dialysis patients? Clin J Am Soc Nephrol 11: 1487–1494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS: The survival benefit of “fistula first, catheter last” in hemodialysis is primarily due to patient factors. J Am Soc Nephrol 28: 645–652, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.