Abstract
Background and objectives
The role of albuminuria as an indicator of progression has not been investigated in children with CKD in the absence of diabetes.
Design, setting, participants, & measurements
Children were enrolled from 49 centers of the CKD in Children study between January of 2005 and March of 2014. Cross-sectional multivariable linear regression (n=647) was used to examine the relationship between urine protein-to-creatinine (UP/C [milligrams per milligram]) and albumin-to-creatinine (ACR [milligrams per gram]) with eGFR (milliliters per minute per 1.73 m2). Parametric time-to-event analysis (n=751) was used to assess the association of UP/C, ACR, and urine nonalbumin-to-creatinine (Unon-alb/cr [milligrams per gram]) on the time to the composite endpoint of initiation of RRT or 50% decline in eGFR.
Results
The median follow-up time was 3.4 years and 202 individuals experienced the event. Participants with a UP/C≥0.2 mg/mg and ACR≥30 mg/g had a mean eGFR that was 16 ml/min per 1.73 m2 lower than those with a UP/C<0.2 mg/mg and ACR<30 mg/g. Individuals with ACR<30 mg/g, but a UP/C≥0.2 mg/mg, had a mean eGFR that was 9.3 ml/min per 1.73 m2 lower than those with a UP/C<0.2 mg/mg and ACR<30 mg/g. When categories of ACR and Unon-alb/cr were created on the basis of clinically meaningful cutoff values of UP/C with the same sample sizes for comparison, the relative times (RTs) to the composite end-point were almost identical when comparing the middle (RT=0.31 for UP/C [0.2–2.0 mg/mg], RT=0.38 for ACR [56–1333 mg/g], RT=0.31 for Unon-alb/cr [118–715 mg/g]) and the highest (RT=0.08 for UP/C [>2.0 mg/mg], RT=0.09 for ACR [>1333 mg/g], RT=0.07 for Unon-alb/cr [>715 mg/g]) levels to the lowest levels. A similar trend was seen when categories were created on the basis of clinically meaningful cutoff values of ACR (<30, 30–300, >300 mg/g).
Conclusions
In children with CKD without diabetes, the utility of an initial UP/C, ACR, and Unon-alb/cr for characterizing progression is similar.
Keywords: albuminuria; pediatrics; progression of chronic renal failure; renal function decline; creatinine; Cross-Sectional Studies; diabetes mellitus; Disease Progression; Follow-Up Studies; glomerular filtration rate; Humans; kidney; Linear Models; proteinuria; Renal Insufficiency, Chronic; Renal Replacement Therapy; Sample Size
Introduction
Proteinuria is a major prognostic indicator of renal progression in both children and adults with CKD (1–4). The Chronic Kidney Disease in Children (CKiD) study reported that total urine protein-to-creatinine ratios (UP/C) >2.0 mg/mg in children with glomerular CKD were associated with a 94% reduction in the time to either a 50% decline in eGFR or the initiation of RRT (1). Children with nonglomerular CKD and a UP/C>2.0 mg/mg had a 79% reduction in the time to this same endpoint. Indeed, compared with hypoalbuminemia, elevated BP, dyslipidemia, and anemia, nephrotic range proteinuria was the strongest risk factor for renal progression in children with CKD, regardless of glomerular or nonglomerular cause (1). In 2009, the randomized, prospective The Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) trial in children with CKD demonstrated that higher levels of proteinuria were associated with a more rapid decline in GFR (3).
The 2012 Kidney Disease Improving Global Outcomes guidelines for staging and predicting the progression of CKD designate a urine albumin-to-creatinine ratio (ACR) of <30 mg/g as “normal-to-mildly increased,” 30–300 mg/g as “moderately increased,” and >300 mg/g as “severely increased.” (5) Traditionally, in studies of adults with CKD, albuminuria, rather than overall proteinuria, has been utilized in studies of progression (2, 6–10). Although the association of normal or mildly increased levels of albuminuria on worsening renal function has been explored in the adult CKD population, the association of smaller amounts of albuminuria (alternatively termed “microalbuminuria”) and kidney disease progression in pediatric patients has largely been confined to studies of patients with diabetes mellitus (8, 11). Because of this difference in urine protein quantification methods in adult versus pediatric studies, we wanted to determine whether albuminuria, specifically, has particular importance compared with general proteinuria as an indicator of renal progression in children without diabetes mellitus.
Evaluation for albuminuria is currently not part of the routine care of children with CKD without diabetes. Likewise, investigating the amount of nonalbumin proteins in the urine, whose increased presence indicates tubular dysfunction, is not routinely done in the evaluation of pediatric patients with CKD (12–14). This study sought to: (1) quantify the cross-sectional relationship between both UP/C and ACR with eGFR, (2) determine the cross-sectional relationships between UP/C, ACR, and urine nonalbumin-to-creatinine (Unon-alb/cr), and (3) compare the association of each of UP/C, ACR, and Unon-alb/cr with time to RRT or >50% decline in eGFR.
Materials and Methods
Study Population
The CKiD study is a multicenter, prospective cohort study of children with mild-to-moderate CKD conducted at 49 nephrology centers across North America. The study design and conduct were approved by an observational study-monitoring board appointed by the National Institute of Diabetes and Digestive and Kidney Diseases and by the internal review boards of each participating center. Each participating family provided informed consent. The demographic and clinical characteristics of the cohort as a whole have been published elsewhere (15). Study participants were seen at annual follow-up visits after their initial baseline visit which occurred between January of 2005 and March of 2014. Beginning in June of 2008, urine albumin was added to the urine panel of tests as part of each annual visit. Of the 891 participants enrolled, 757 had at least one visit with ACR measured; the visit with the first ACR measurement was defined as the index visit. Our time-to-event analyses were restricted to the 751 participants who had follow-up data after the index visit to determine time to first of >50% decline in eGFR or RRT. eGFR (milliliters per minute per 1.73 m2) was calculated at each visit using: 0.413×(height [centimeters]/serum creatinine [milligrams per deciliter]) (16).
Protein Measures
Participants provided a random urine collection on the morning of the study. Urine albumin was measured using an immunoturbidimetric assay whereby anti-albumin antibodies react with the urine sample to form antigen-antibody complexes. After agglutination, the antigen-antibody complexes were measured turbidimetrically by quantifying the loss of intensity of a light beam placed through the solution. The quantity of albumin (milligrams) was then divided by the quantity of creatinine (grams) in the urine in order to obtain the ratio, ACR. The total urine protein content of the urine was also determined by a turbidimetric method. The quantity of protein (milligrams) was divided by the quantity of creatinine (milligrams) in the urine to obtain the ratio, UP/C. Unon-alb/cr was defined as urine total protein (milligrams) minus urine albumin (milligrams) divided by creatinine (grams). Coefficients of variation on the Roche Hitachi Cobas modular analyzer for within-run and between-run assays were 1.5% and 3.5%, respectively, for urine albumin, and 1.5% and 1.5% for urine protein.
Statistical Analyses
Linear regression was used to examine the cross-sectional relationship between mean eGFR level and both UP/C and ACR in study participants in whom either proteinuria level was elevated in comparison to the reference group (UP/C<0.2 mg/mg and ACR<30 mg/g) using the following categories: UP/C<0.2 mg/mg and ACR≥30 mg/g; UP/C≥0.2 mg/mg and ACR<30 mg/g; UP/C≥0.2 mg/mg and ACR≥30 mg/g (5, 17–19), overall, and also among those not reporting an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at the index visit. On the basis of previous studies showing significant associations with eGFR, the linear regression model was adjusted for: age, sex, race (white versus nonwhite), CKD cause (glomerular versus nonglomerular), hypertension status (systolic or diastolic BP ≥age-sex-height–specific 95th percentile, versus systolic and diastolic BP <age-sex-height–specific 95th percentile), age-sex–specific body mass index (≥95th percentile versus <95th percentile), and uric acid values (1, 20–22). Pearson correlation coefficients were used to quantify the strength of the cross-sectional linear relationship between log(UP/C) and log(ACR), log(UP/C) and log(Unon-alb/cr), and log(ACR) and log(Unon-alb/cr) at the index visit.
Parametric failure time models assuming a generalized gamma (GG) distribution (23) of event times were used to assess the independent association of index visit values of UP/C, ACR, and Unon-alb/cr on the time to the composite end-point of either >50% decline in eGFR (compared with eGFR at the index visit) or initiation of RRT. Two sets of analyses were performed: one on the basis of clinically meaningful UP/C cutoffs and a second set on the basis of clinically meaningful ACR cutoffs. Because proteinuria is considered clinically relevant when the UP/C is ≥0.2 mg/mg and nephrotic range proteinuria is typically defined as a UP/C>2.0 mg/mg, these values were used as cutoffs in determining categories for UP/C (<0.2, 0.2–2.0, and >2.0 mg/mg) (19, 24). We then examined the distribution of ACR and Unon-alb/cr and chose cutoffs for these variables (<56, 56–1333, and >1333 mg/g for ACR; <118, 118–715, and >715 mg/g for Unon-alb/cr) that would yield the same sample sizes (287, 363, and 101) for the three different categories across the three sets of analyses. Similarly, because ACR is considered moderately increased when ≥30 mg/g and severely increased when >300 mg/g, these values were used as cutoffs for ACR categories (<30, 30–300, and >300 mg/g). Cutoffs for UP/C (<0.139, 0.139–0.630, and >0.630 mg/mg) and for Unon-alb/cr (<86, 86–273, and >273 mg/g) were chosen to yield the same sample sizes (220, 271, and 260) for the three different categories across the three sets of analyses.
Relative times (RTs) were used to quantify the strength of the relationship between each of these variables and the composite event. Specifically, RTs represent the time it takes for P% (P can be any percentage between 0% and 100%) of the “exposed group” to develop the composite event divided by the time it takes for the same P% of the “unexposed group” to develop the composite event. We chose to use RTs as opposed to relative hazards because time is the unit of measurement of the dependent variable, and to quantify the association of UP/C, ACR, and Unon-alb/cr on CKD progression in terms of how higher levels of each exposure shorten the time to the event (i.e., RT<1). We utilized regression models under the assumption of proportional times (i.e., the RTs did not depend on “P”). The appropriateness of the GG distribution was assessed by comparing each estimated survival curve on the basis of the GG parameter estimates with the corresponding nonparametric Kaplan–Meier survival curve. All analyses used a 0.05 level of significance.
Results
Characteristics at the index visit of the 751 CKiD participants included in our time-to-event analyses are shown in Table 1. The median age was 12.4 years, 15% of participants had stage 1 hypertension or greater, and the median eGFR was 54.6 ml/min per 1.73 m2. A glomerular cause for CKD occurred in 30% of the participants. At the index visit, 56% of participants were on an ACE inhibitor or ARB.
Table 1.
Characteristics at the index visit of the 751 CKiD participants
| Characteristics | Median (Interquartile Range) or % (n) |
|---|---|
| Age, yr | 12.4 (8.6–15.7) |
| Men | 61 (457) |
| White race | 65 (491) |
| Systolic or diastolic BP ≥95th percentilea | 15 (108) |
| Current ACE or ARB use | 56 (417) |
| eGFRb, ml/min per 1.73 m2 | 54.6 (39.5–71.8) |
| Glomerular CKD cause | 30 (228) |
| Age-sex–specific body mass index ≥95th percentilec | 17 (129) |
| Urine protein-to-creatinine, mg/mg | 0.32 (0.12–1.04) |
| Urine albumin-to-creatinine, mg/g | 112.0 (20.9–615.0) |
| Urine nonalbumin-to-creatinine, mg/g | 167.6 (75.0–400.7) |
| Urine albumin-to-protein, mg/mg | 0.44 (0.15–0.65) |
n, number of participants.
BP percentiles were determined using age/sex/height–specific values; missing for 33 participants.
eGFR=0.413×(height [centimeters]/serum creatinine [milligrams per deciliter]).
Body mass index percentiles were determined using age/sex–specific values; missing for 12 participants.
Cross-Sectional Analyses
Table 2 shows the results of a multivariable linear regression analysis to quantify the relationship between mean eGFR level and both UP/C and ACR at the index visit. The mean eGFR in the individuals with albuminuria (ACR≥30 mg/g) in the absence of overt proteinuria (UP/C<0.2 mg/mg) did not differ significantly from those of the reference group (UP/C<0.2 mg/mg and ACR<30 mg/g). In contrast, those with both albuminuria and overt proteinuria (UP/C≥0.2 mg/mg and ACR≥30 mg/g) had a mean eGFR that was 16.0 ml/min per 1.73 m2 lower (95% confidence interval [95% CI], −19.7 to −12.4) than that of the reference group. Importantly, participants with overt proteinuria (UP/C≥0.2 mg/mg), but ACR<30 mg/g, had a mean eGFR that was 9.3 ml/min per 1.73 m2 lower (95% CI, −17.8 to −0.7) than those with UP/C<0.2 mg/mg and ACR<30 mg/g.
Table 2.
Multivariablea linear regression analysis of index visit values of eGFR on urine protein-to-creatinine (milligrams per milligram) and urine albumin-to-creatinine (milligrams per gram), n=647
| Exposureb | No. | Mean eGFR (millilitre per minute per 1.73 m2) Relative to Reference | 95% Confidence Interval |
|---|---|---|---|
| Urine protein-to-creatinine <0.2 and urine albumin-to-creatinine <30 | 170 | 0 (reference) | |
| Urine protein-to-creatinine <0.2 and urine albumin-to-creatinine ≥30 | 86 | −3.1 | −8.1 to 1.9 |
| Urine protein-to-creatinine ≥0.2 and urine albumin-to-creatinine <30 | 21 | −9.3 | −17.8 to −0.7 |
| Urine protein-to-creatinine ≥0.2 and urine albumin-to-creatinine ≥30 | 370 | −16.0 | −19.7 to −12.4 |
Adjusted for age, sex, race, CKD cause, hypertension status, body mass index, and uric acid.
For urine protein-to-creatinine the units are mg/mg; for urine albumin-to-creatinine the units are mg/g.
We repeated the cross-sectional multivariable linear regression including only the 289 patients not on an ACE and/or ARB at the index visit (Supplemental Table 1). Similar results were obtained to the analysis where patients on an ACE and/or ARB were included; individuals with albuminuria (ACR≥30 mg/g) in the absence of overt proteinuria (UP/C<0.2 mg/mg) had a mean eGFR that was not significantly different from the reference group. In comparing the analyses with and without the restriction of ACE inhibitor/ARB use, the magnitude of the difference in means was greater when comparing those with UP/C≥0.2 mg/mg and ACR≥30 mg/g to those with UP/C<0.2 mg/mg and ACR<30 mg/g when the analysis was restricted to those not on an ACE or ARB.
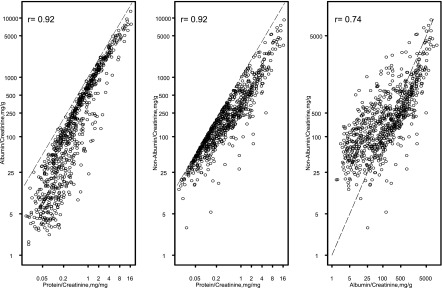
Figure 1 shows the strong collinearity of ACR and UP/C (r=0.92), Unon-alb/cr and UP/C (r=0.92), and Unon-alb/cr and ACR (r=0.74) at the index visit.
Figure 1.
Collinearity between the three methods to quantify proteinuria at the index study visit, n=751. The dashed line shown in each of the panels in Figure 1 is the line on which all of the data would fall if there were perfect agreement between the two variables.
Time-to-Event Analyses
The median follow-up time from the index visit to the composite event was 3.4 years (interquartile range, 2.2–5.0) and 27% of the study population (n=202) were observed to develop the composite event (>50% decline in eGFR or initiation of RRT). Seventy-seven children had a >50% decline in eGFR before undergoing dialysis or transplant and 37 had a >50% decline in eGFR without subsequent dialysis or transplant. Eighty-eight children went onto RRT without first exhibiting a >50% decline in eGFR. As shown in Table 3, the times to the composite event were 92% shorter for those with UP/C>2.0 mg/mg when compared with participants with a UP/C<0.2 mg/mg (RT=0.08; 95% CI, 0.04 to 0.13). For albuminuria, the times to the composite event were 84% shorter for participants with ACR>300 mg/g when compared with those with ACR<30 mg/g (RT=0.16; 95% CI, 0.10 to 0.26). Similarly, the times to the composite event were 86% shorter for participants with a Unon-alb/cr≥300 mg/g when compared with Unon-alb/cr<100 mg/g (RT=0.14; 95% CI, 0.08 to 0.24).
Table 3.
Relationship between indicated exposure at index visit and subsequent decline of >50% in GFR or initiation of RRT, n=751
| Exposure | No. | No. (%) with Event | Regression Modela | Relative Time | 95% Confidence Interval |
|---|---|---|---|---|---|
| Urine protein-to-creatinine, mg/mg | |||||
| <0.2 | 287 | 25 (9) | GG (3.553,1.086,0.690) | 1 (reference) | |
| 0.2–2.0 | 363 | 112 (31) | GG (2.393,1.086,0.690) | 0.31 | 0.21 to 0.47 |
| >2.0 | 101 | 65 (64) | GG (0.974,1.086,0.690) | 0.08 | 0.04 to 0.13 |
| Urine albumin-to-creatinineb, mg/g | |||||
| <56 | 287 | 31 (11) | GG (3.391,1.024,0.784) | 1 (reference) | |
| 56–1333 | 363 | 104 (29) | GG (2.428,1.024,0.784) | 0.38 | 0.26 to 0.55 |
| >1333 | 101 | 67 (66) | GG (1.031,1.024,0.784) | 0.09 | 0.06 to 0.15 |
| Urine nonalbumin-to-creatinineb, mg/g | |||||
| <118 | 287 | 25 (9) | GG (3.593,1.233,0.507) | 1 (reference) | |
| 118–715 | 363 | 112 (31) | GG (2.411,1.233,0.507) | 0.31 | 0.20 to 0.46 |
| >715 | 101 | 65 (64) | GG (0.936,1.233,0.507) | 0.07 | 0.04 to 0.12 |
GG, generalized gamma.
Includes the location, scale, and shape parameters of the regression model.
Cutoffs for urine albumin-to-creatinine and urine nonalbumin-to-creatinine were chosen to yield same sample sizes as determined by urine protein-to-creatinine cutoffs of 0.2 and 2.0.
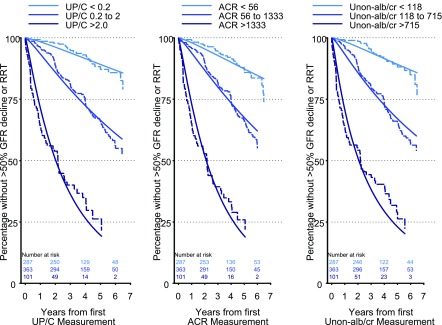
In order to compare the three methods to quantify proteinuria, UP/C, ACR, and Unon-alb/cr were stratified into categories on the basis of levels of UP/C (<0.2 mg/mg [n=287], 0.2–2.0 mg/mg [n=363], and >2.0 mg/mg [n=101]) as shown (Figure 2, Table 3). Categories of ACR and Unon-alb/cr were chosen on the basis of where 0.2 and 2.0 fell in the distribution of UP/C so that the lowest levels of UP/C, ACR, and Unon-alb/cr would have the same sample sizes but not necessarily the same participants. Similarly, the middle categories (as well as the highest categories) of UP/C, ACR, and Unon-alb/cr would have the same number of participants. The lowest category of each measure of proteinuria was designated as a reference group (i.e., UP/C<0.2 mg/mg, ACR<56 mg/g, and Unon-alb/cr<118 mg/g). The RTs to a >50% decline in eGFR or the initiation of RRT were almost identical for each of the three measures of proteinuria when comparing the middle category to the reference group (0.31 for UP/C, 0.38 for ACR, 0.31 for Unon-alb/cr) and highest category to the reference group (0.08 for UP/C, 0.09 for ACR, 0.07 for Unon-alb/cr).
Figure 2.
Similar prognostic ability for each of the three methods to quantify proteinuria for characterizing a >50% decline in GFR or need for RRT based on clinically meaningful cutoffs of UP/C (Kaplan–Meier, dashed lines and Generalized Gamma, solid lines). Left panel: UP/C categorized as <0.2, 0.2–2.0, and >2.0; middle panel: ACR categorized as <56, 56 to <1333, and >1333; right panel: Unon-alb/cr categorized as <118, 118 to <715, and >715. n=751. ACR, urine albumin-to-creatinine ratio; Unon-alb/cr, urine nonalbumin-to-creatinine ratio; UP/C, urine protein-to-creatinine ratio.
In order to further explore the subgroup of study participants with UP/C<0.2 mg/mg (n=287), the association between ACR (treated as a continuous variable) and the composite event was examined. Each log higher in ACR was not associated with any greater risk of reaching the composite endpoint (P=0.96).
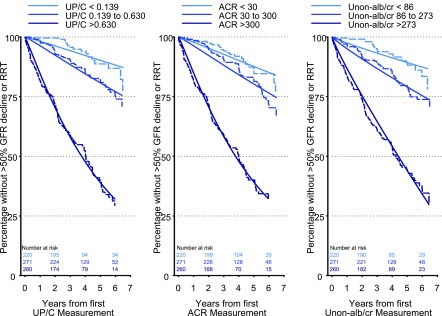
When UP/C, ACR, and Unon-alb/cr were compared on the basis of the levels of ACR (<30 mg/g [n=220], 30–300 mg/g [n=271], and >300 mg/g [n=260]), the RTs to the event were very similar when comparing the middle and highest categories of each of the three measures of proteinuria to the reference group (Figure 3, Table 4).
Figure 3.
Similar prognostic ability for each of the three methods to quantify proteinuria for characterizing a >50% decline in GFR or need for RRT based on clinically meaningful cutoffs of ACR (Kaplan–Meier, dashed lines and Generalized Gamma, solid lines). Left panel: UP/C categorized as <0.139, 0.139–0.630, and >0.630; middle panel: ACR categorized as <30, 30–300, and >300; right panel: Unon-alb/cr categorized as <86, 86–273, and >273. n=751. ACR, urine albumin-to-creatinine ratio; Unon-alb/cr, urine nonalbumin-to-creatinine ratio; UP/C, urine protein-to-creatinine ratio.
Table 4.
Relationship between indicated exposure at index visit and subsequent decline of >50% in GFR or initiation of RRT, n=751
| Exposure | No. | No. (%) with Event | Regression Modela | Relative Time | 95% Confidence Interval |
|---|---|---|---|---|---|
| Urine protein-to-creatinineb, mg/mg | |||||
| <0.139 | 220 | 17 (8) | GG (3.721,0.935,0.991) | 1 (reference) | |
| 0.139–0.630 | 271 | 46 (17) | GG (3.043,0.935,0.991) | 0.51 | 0.30 to 0.86 |
| >0.630 | 260 | 139 (53) | GG (1.662,0.935,0.991) | 0.13 | 0.07 to 0.22 |
| Urine albumin-to-creatinine, mg/g | |||||
| <30 | 220 | 23 (10) | GG (3.469,0.932,0.977) | 1 (reference) | |
| 30–300 | 271 | 46 (17) | GG (3.001,0.932,0.977) | 0.63 | 0.40 to 1.00 |
| >300 | 260 | 133 (51) | GG (1.663,0.932,0.977) | 0.16 | 0.10 to 0.26 |
| Urine nonalbumin-to-creatinineb, mg/g | |||||
| <86 | 220 | 18 (8) | GG (3.913,0.336,3.328) | 1 (reference) | |
| 86–273 | 271 | 49 (18) | GG (3.166,0.336,3.328) | 0.47 | 0.27 to 0.84 |
| >273 | 260 | 135 (52) | GG (2.065,0.336,3.328) | 0.16 | 0.09 to 0.28 |
GG, generalized gamma.
Includes the location, scale, and shape parameters of the regression model.
Cutoffs for urine protein-to-creatinine and urine nonalbumin-to-creatinine were chosen to yield same sample sizes as determined by urine albumin-to-creatinine cutoffs of 30 and 300.
Discussion
In this large study of North American children with CKD without diabetes, it was found that UP/C, ACR, and Unon-alb/cr had a similar ability to characterize progression to a composite event of a >50% decline in eGFR or initiation of RRT. The times to the event were significantly shorter for increasing levels of UP/C, ACR, and Unon-alb/cr. Consistent with previous studies, higher UP/C values were significantly associated with lower eGFR values (1–4). Not unexpectedly, when the UP/C was ≥0.2 mg/mg, there was a significant association with lower mean eGFR values for individuals with ACR≥30 mg/g (i.e., those with UP/C≥0.2 mg/mg and ACR≥30 mg/g had significantly lower eGFR compared with those with UP/C<0.2 mg/mg and ACR<30 mg/g). Importantly, however, a significant association with lower eGFR was also seen in participants with a UP/C≥0.2 mg/mg and ACR<30 mg/g.
To our knowledge, this study is the first to examine the association of albuminuria and renal progression in a pediatric CKD population in the absence of diabetes. The results of this analysis indicate that ACR and UP/C are very well correlated (r=0.92) and the discrepancy in their correlation does not yield differential inferences with respect to characterizing the time to >50% decline in eGFR or initiation of RRT in the population studied. The congruence of the results for the analyses whereby methods of protein quantification were compared on the basis of UP/C as well as ACR categories indicates an equivalent prognostic ability for each of these markers in terms of characterizing CKD progression.
It can be argued that screening for albuminuria, which is more sensitive than the assay for proteinuria, may detect patients with CKD at an earlier point in their chronic disease. Prior investigations have supported the belief that microalbuminuria in patients with diabetes provides an ominous connotation regarding the progression of the diabetes (25–27). However, more recent studies have shown that microalbuminuria may be a transient finding in children and adults with diabetes (28, 29) and is not consistently a strong predictor of renal disease progression in this patient population (30–32). The cost of measuring urine albumin is more than the cost of measuring total protein. Thus, there may be a cost-saving benefit to avoiding additional urine protein studies when UP/C is already known. However, future cost effectiveness studies are required to make any determinations of the cost-saving advantage of one method of urine protein quantification over another in the pediatric CKD population.
Although our study demonstrates that albuminuria is not superior to proteinuria in monitoring renal progression in children with CKD, there may be utility in quantifying both a urine protein and albumin in certain patient groups. Prior studies in both children and adults with nephrotic syndrome have shown a significant association of the selectivity of the proteinuria and prognosis and response to therapy (33–36). In order to further explore the role of urine albumin in this study cohort, UP/C, ACR, and Unon-alb/cr were dichotomized on the basis of a urine albumin-to-protein ratio (APR [milligrams per milligram] of <0.4 and ≥0.4). The 0.4 cutoff value was on the basis of prior study results showing the utility of an APR for identifying tubular interstitial disorders in adults (13). The RTs to a >50% decline or initiation of RRT comparing participants with the highest APR (>0.4 mg/mg), highest UP/C (>0.28 mg/mg), highest ACR(>90 mg/g), and highest Unon-alb/cr (>148 mg/g) compared with those with the lowest were 0.44, 0.20, 0.23, and 0.20, respectively, suggesting that obtaining an APR may provide somewhat different information regarding progression when compared with UP/C, ACR, and Unon-alb/Cr. Further studies are needed to determine if APR is a useful measurement in children with CKD.
The study does have limitations. A single random urine specimen was obtained on each participant, not accounting for potential intraindividual variability in urine protein content. Additionally, although urine collections were obtained in the morning of the study visit, specimens were not consistently first morning voids, providing the potential for orthostatic proteinuria. Nonalbumin protein was calculated indirectly using urine total protein and albumin measurements. We did not directly measure specific larger–molecular weight tubular proteins, which may have added utility in predicting CKD progression in children. In adults with primary GN, the fractional excretion of both IgG and albumin have been found to be significant predictors for the progression to stage 5 CKD or death (33). Notably, albuminuria has been shown in multiple studies to be an independent indicator of increased cardiovascular disease risk (37–40), an outcome not explored in this study. The exact timing of ACE inhibitor and/or ARB medication initiation could not be determined from the study data. Given that these medications are common among the CKD population and can result in a stabilization or decline in the degree of proteinuria and albuminuria (18, 41), patients not on an ACE inhibitor or ARB at the index study visit were included in a cross-sectional multivariable linear regression with similar results to the entire patient cohort.
In conclusion, in children with CKD without diabetes, initial UP/C, ACR, and Unon-alb/cr determinations are well correlated and provide a similar characterization of CKD progression.
Disclosures
G.J.S. is a consultant for Tricida. The remaining coauthors have nothing to disclose.
Supplementary Material
Acknowledgments
The authors are grateful to GE Healthcare for providing the Omnipaque 300 for the iohexol GFR studies and to Paula Maier for accurate data entry.
The Chronic Kidney Disease in children prospective cohort (CKiD) study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Schriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK82194, U01-DK-66143, U01-DK-66174, and U01-DK-66116). The CKID website is located at http://www.statepi.jhsph.edu/ckid.
Data in this manuscript were collected by the CKiD study with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady) and The Children’s Hospital of Philadelphia (Susan Furth), data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz), and the Central Biochemistry Laboratory at the University of Rochester (George J. Schwartz).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11971116/-/DCSupplemental.
References
- 1.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambers Heerspink HJ, Gansevoort RT, Brenner BM, Cooper ME, Parving HH, Shahinfar S, de Zeeuw D: Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol 21: 1355–1360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F; ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL: CKiD (CKD in children) prospective cohort study: A review of current findings. Am J Kidney Dis 60: 1002–1011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin A, Stevens PE: Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49–61, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M; International Diabetic Nephopathy Study Group : The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171, 2005 [DOI] [PubMed] [Google Scholar]
- 9.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Mogensen CE, Christensen CK: Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 311: 89–93, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Dunger DB, Schwarze CP, Cooper JD, Widmer B, Neil HA, Shield J, Edge JA, Jones TW, Daneman D, Dalton RN: Can we identify adolescents at high risk for nephropathy before the development of microalbuminuria? Diabet Med 24: 131–136, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Peterson PA, Evrin PE, Berggård I: Differentiation of glomerular, tubular, and normal proteinuria: Determinations of urinary excretion of beta-2-macroglobulin, albumin, and total protein. J Clin Invest 48: 1189–1198, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG: The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients. Nephrol Dial Transplant 27: 1534–1541, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Abitbol CL, Chandar J, Onder AM, Nwobi O, Montané B, Zilleruelo G: Profiling proteinuria in pediatric patients. Pediatr Nephrol 21: 995–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA: Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg JM, Chang BS, Matarese RA, Garella S: Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309: 1543–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Ruggenenti P, Gaspari F, Perna A, Remuzzi G: Cross sectional longitudinal study of spot morning urine protein:creatinine ratio, 24 hour urine protein excretion rate, glomerular filtration rate, and end stage renal failure in chronic renal disease in patients without diabetes. BMJ 316: 504–509, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houser M: Assessment of proteinuria using random urine samples. J Pediatr 104: 845–848, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA; Chronic Kidney Disease in Children Study Group : Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension 52: 631–637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlow SE; Expert Committee : Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 120[Suppl 4]: S164–S192, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Rodenbach KE, Schneider MF, Furth SL, Moxey-Mims MM, Mitsnefes MM, Weaver DJ, Warady BA, Schwartz GJ: Hyperuricemia and progression of CKD in children and adolescents: The chronic kidney disease in children (CKiD) cohort study. Am J Kidney Dis 66: 984–992, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox C, Chu H, Schneider MF, Muñoz A: Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med 26: 4352–4374, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Houser MT, Jahn MF, Kobayashi A, Walburn J: Assessment of urinary protein excretion in the adolescent: Effect of body position and exercise. J Pediatr 109: 556–561, 1986 [DOI] [PubMed] [Google Scholar]
- 25.Coonrod BA, Ellis D, Becker DJ, Bunker CH, Kelsey SF, Lloyd CE, Drash AL, Kuller LH, Orchard TJ; Pittsburgh Epidemiology of Diabetes Complications Study : Predictors of microalbuminuria in individuals with IDDM. Diabetes Care 16: 1376–1383, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH: Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 332: 1251–1255, 1995 [DOI] [PubMed] [Google Scholar]
- 27.The Microalbuminuria Collaborative Study Group : Predictors of the development of microalbuminuria in patients with type 1 diabetes mellitus: A seven-year prospective study. Diabet Med 16: 918–925, 1999 [PubMed] [Google Scholar]
- 28.Salardi S, Balsamo C, Zucchini S, Maltoni G, Scipione M, Rollo A, Gualandi S, Cicognani A: High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril: The influence of HDL cholesterol. Diabetes Care 34: 424–429, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alleyn CR, Volkening LK, Wolfson J, Rodriguez-Ventura A, Wood JR, Laffel LM: Occurrence of microalbuminuria in young people with Type 1 diabetes: Importance of age and diabetes duration. Diabet Med 27: 532–537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348: 2285–2293, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Giorgino F, Laviola L, Cavallo Perin P, Solnica B, Fuller J, Chaturvedi N: Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: The EURODIAB Prospective Complications Study. Diabetologia 47: 1020–1028, 2004 [DOI] [PubMed] [Google Scholar]
- 32.de Boer IH, Afkarian M, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group : Renal outcomes in patients with type 1 diabetes and macroalbuminuria. J Am Soc Nephrol 25: 2342–2350, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuarrie EP, Shakerdi L, Jardine AG, Fox JG, Mackinnon B: Fractional excretions of albumin and IgG are the best predictors of progression in primary glomerulonephritis. Nephrol Dial Transplant 26: 1563–1569, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Cameron JS, White RH: Selectivity of proteinuria in children with the nephrotic syndrome. Lancet 1: 463–465, 1965 [DOI] [PubMed] [Google Scholar]
- 35.Cameron JS, Blandford G: The simple assessment of selectivity in heavy proteinuria. Lancet 2: 242–247, 1966 [DOI] [PubMed] [Google Scholar]
- 36.White RH, Glasgow EF, Mills RJ: Clinicopathological study of nephrotic syndrome in childhood. Lancet 1: 1353–1359, 1970 [DOI] [PubMed] [Google Scholar]
- 37.Dinneen SF, Gerstein HC: The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med 157: 1413–1418, 1997 [PubMed] [Google Scholar]
- 38.Jager A, Kostense PJ, Ruhé HG, Heine RJ, Nijpels G, Dekker JM, Bouter LM, Stehouwer CD: Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: Five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol 19: 617–624, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators : Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Yuyun MF, Dinneen SF, Edwards OM, Wood E, Wareham NJ: Absolute level and rate of change of albuminuria over 1 year independently predict mortality and cardiovascular events in patients with diabetic nephropathy. Diabet Med 20: 277–282, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.