Abstract
Background and objectives
People receiving hemodialysis to treat kidney failure need a vascular access (a fistula, a graft, or a central venous catheter) to connect to the blood purification machine. Higher rates of access complications are considered the mechanism responsible for the excess mortality observed among catheter or graft users versus fistula users. We tested this hypothesis using mediation analysis.
Design, setting, participants, & measurements
We studied incident patients who started hemodialysis therapy from North America, Europe, and Australasia (the Dialysis Outcomes and Practice Patterns Study; 1996–2011). We evaluated the association between access type and time to noninfectious (e.g., thrombosis) and infectious complications of the access (mediator model) and the relationship between access type and time-dependent access complications with 6-month mortality from the creation of the first permanent access (outcome model). In mediation analysis, we formally tested whether access complications explain the association between access type and mortality.
Results
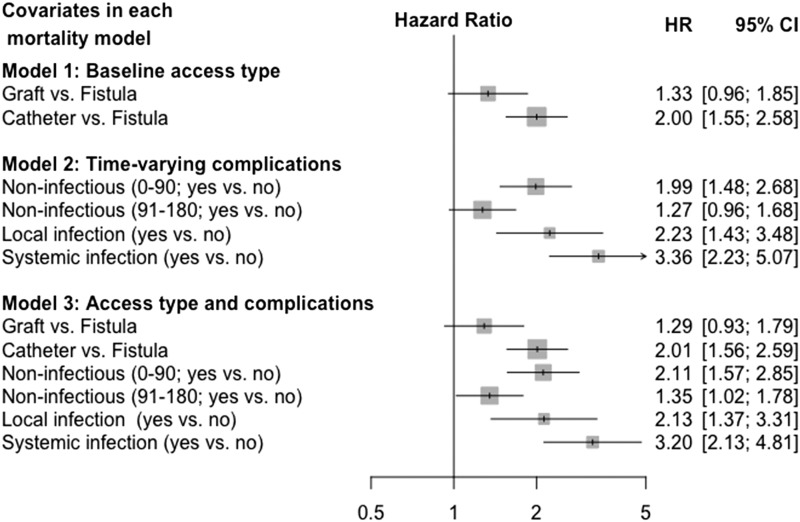
Of the 6119 adults that we studied (mean age =64 [SD=15] years old; 58% men; 47% patients with diabetes), 50% had a permanent catheter for vascular access, 37% had a fistula, and 13% had a graft. During the 6-month study follow-up, 2084 participants (34%) developed a noninfectious complication of the access, 542 (8.9%) developed an infectious complication, and 526 (8.6%) died. Access type predicted the occurrence of access complications; both access type and complications predicted mortality. The associations between access type and mortality were nearly identical in models excluding and including access complications (hazard ratio, 2.00; 95% confidence interval, 1.55 to 2.58 versus hazard ratio, 2.01; 95% confidence interval, 1.56 to 2.59 for catheter versus fistula, respectively). In mediation analysis, higher mortality with catheters or grafts versus fistulas was not the result of increased rates of access complications.
Conclusions
Hemodialysis access complications do not seem to explain the association between access type and mortality. Clinical trials are needed to clarify whether these associations are causal or reflect confounding by underlying disease severity.
Keywords: Arteriovenous Shunt, Surgical; Australasia; Central Venous Catheters; diabetes mellitus; Europe; Fistula; Follow-Up Studies; Humans; Male; North America; renal dialysis; Renal Insufficiency; thrombosis
Introduction
About 70% of people with end stage kidney failure (approximately 3 million people globally) are treated with hemodialysis (1,2). Although effective for sustaining life, hemodialysis therapy is associated with poor outcomes (one in six people die each year), and it is extremely costly. People receiving hemodialysis make up <1% of the Centers for Medicare and Medicaid Services (CMS) beneficiaries but account for 6%–7% of health care spending (3). A large fraction of this cost is related to establishing and maintaining a vascular access (a fistula, a graft, or a tunneled catheter) that allows access to the bloodstream for the delivery of hemodialysis therapy (4,5). Complications of this hemodialysis access (i.e., clotting or infection) are common and lead to access-related procedures or medical interventions, which are considered a key driver of morbidity and mortality in this patient population (4,6,7).
Clinical practice guidelines (8–10) recommend the fistula as the best form of access, because many observational studies have shown that fistula users have fewer access complications (11) and better prognosis (12) than graft or catheter users. Recognizing the potential to improve patient outcomes and lower the costs of providing care, the CMS developed a national initiative to increase the use of fistulas and reduce the use of catheters (13,14). The CMS also incorporated vascular access quality outcomes into Quality Incentive Program metrics and tied funding to the attainment of fistula utilization targets (15). Although these initiatives are well intentioned to improve outcomes, the quality of the evidence on which they are based is low when assessed against recommended standards (16). There has never been a randomized comparison of different access strategies with mortality or other hard end points as the outcome, and the observational literature supporting the superiority of fistulas over grafts or catheters has important limitations (12). However, vascular access planning is important to patients. In a research priorities setting study, patients have identified decision making about the vascular access among the top ten uncertainties (17). Qualitative data indicate that patients profoundly fear access complications and related procedures (18). For patients, a vascular access and its complications evoke an agonizing sense of vulnerability and mechanization of the body, disrupting their identity and lifestyle (18).
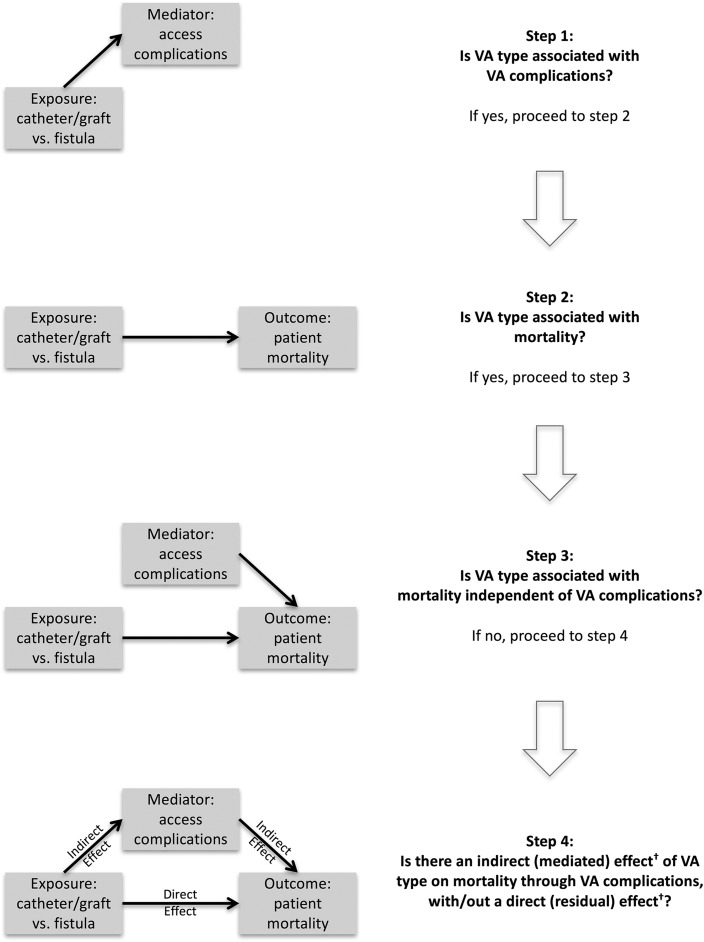
Vascular access complications are considered the mechanism responsible for the increased risk of death in patients treated with catheters or grafts versus fistulas (9,14,19–21). We tested this hypothesis using data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) (11,22). We used mediation analysis (Figure 1), a multistep procedure that assesses the extent to which an intermediate variable or event (access complication) explains the relationship between an intervention or exposure (vascular access type) and a more distant outcome (mortality) (23).
Figure 1.
Mediation analysis: associations between vascular access type and vascular access complications with mortality. Mediation analysis is a multistep procedure that seeks to identify a mechanism underlying an observed relationship between an intervention or exposure and an outcome via a third variable, known as mediator, mediating variable, intermediary variable, or intervening variable. Using this approach, we sought to assess the extent to which the association between vascular access (VA) type and mortality is mediated via access complications. In the first steps, we assessed the association between VA type and VA complications (step 1) and between VA type and mortality (step 2). Next, we assessed whether the association between VA type and mortality was affected by the inclusion of VA complications, the hypothesized mediator, in the mortality model: a change in the association between VA type and mortality would suggest that part of the effect of VA type on mortality is mediated by VA complications, and no change in this association would suggest independent effect (step 3). In other words, VA type would be associated with mortality through mechanisms that do not involve complications. In step 4, the indirect or mediated effect of VA type on mortality via VA complications is quantified, separated from the residual direct effect, and formally tested for statistical significance. †Given the observational design of this study and the potential for residual confounding, these effects are interpreted as measures of association.
Materials and Methods
Details about ethics approval and the DOPPS design are reported in Supplemental Material.
Data Source and Participants
We assembled an incident cohort of patients who received hemodialysis for 180 days or less at the DOPPS entry and never received other renal replacement therapies. We included in main analyses people whose first permanent access (a fistula, a graft, or a tunneled catheter [referred to as catheter]) was placed between 15 days before and 180 days after the DOPPS entry. We included in sensitivity analyses people whose first permanent access was placed between 15 and 180 days before the DOPPS entry.
Study Objective and Design
We tested whether access complications are the mechanism that mediates fully or in part the association between access type and mortality. We used survival analyses with the date of the first permanent access placement as time zero and a time-varying (time-updated) covariate approach for complications (mediator) (24). When a complication occurred, all time before the complication was considered complication free; all time after was considered exposed to that complication. In sensitivity analyses, we used the first 30 days of follow-up to define the mediator (occurrence of access complications) and the subsequent 5 months to study mortality in 30-day survivors (sample restriction as an alternative method to address immortal time bias) (24).
Outcome
The main outcome measure was all-cause mortality up to 180 days from access placement. We focused on the first 180 days, because during this time, the risks of access complications (11) and mortality (22,25) are highest and because we wanted to study the effect of the type of permanent access strategy that was initially prescribed. Observations with a survival time >180 days were censored at day 180. Reasons for earlier censoring included switch to peritoneal dialysis, kidney transplant, transfer out of a DOPPS facility, recovery of kidney function, and study end date (December 31, 2011) (22).
Exposure
The first permanent vascular access attempted was the exposure of interest. We used this intention to treat approach to minimize collider stratification bias due to time-varying confounding affected by previous treatment, because the occurrence of an access complication may affect the probability of a change in the access and vice versa, and both may affect mortality (26). In sensitivity analyses, we censored observations at 30 days after a change in the access in those who received a second access (e.g., for a complication of the first access or because another access was planned), assuming that, during these 30 days, the effect on mortality of access type and its complications could still be attributed to the initial access.
Mediator
Noninfectious complications included access dysfunction or failure events due to any noninfectious-related cause (access stenosis or thrombosis, fibrin within/around a catheter, and catheter migration) requiring a revision procedure to maintain patency or improve access performance or requiring creation of a new access. Infectious complications were defined as any infection of the access requiring medical intervention or bacteremia or sepsis that was potentially access related (Supplemental Material) (11). For each participant, we considered only the first complication of each type in main analyses. If the same person experienced both a local and a systemic infection, we considered the systemic infection only for that person. In descriptive analyses, participants who experienced both complications were classified as having an infectious complication; in survival analyses, we used all time-varying information for the first occurrence of both types of complication.
Covariates
In all analyses, we used the following information at the DOPPS entry as covariates: country region, demographic variables (age, sex, and race), whether the first permanent access was created before or within 30 days after the start of hemodialysis therapy versus 31–180 days after (i.e., a measure of exposure to nontunneled temporary catheters), duration of dialysis therapy at the DOPPS entry (30 days or less versus 31–180 days), and clinical characteristics (smoking status, diabetes, history of cancer, hypertension, peripheral vascular disease, cerebrovascular disease, coronary artery disease, heart failure and other cardiovascular diseases, and chronic lung or systemic diseases). In all analyses, we also tested the interactions between a noninfectious or infectious complication and type of access, sex, age, study time (before 2000 versus 2000 or later), and region.
Statistical Analyses
In mediation analysis (Figure 1), we used Cox regression and Weibull regression to model the marginal distributions of time to access complications (mediator) as a function of access type (exposure), censoring at death (step 1) and adjusting for the covariates and interaction terms listed above. We stratified these models by complication type (early noninfectious complications [within 90 days], late noninfectious complications [90–180 days], local infectious complications, and systemic infectious complications). We used robust variance estimation to account for data correlation induced by the occurrence of repeated complications in the same person (27). Next, we used Cox regression and Weibull regression to study the relationship between access type (exposure) and mortality (step 2) and study 180-day mortality from access creation as a function of both baseline access type and time-varying access complications (step 3), adjusting for the same covariates and interactions as in the previous step. To formally test for mediation effects (step 4), we used the package mediation in R using Weibull models as inputs (the model of the mediator as a function of the exposure and the model of the outcome as a function of both the mediator and the exposure) (28). Methods for descriptive statistics, multiple imputation, model building and verification, sensitivity analyses, and power analyses are described in Supplemental Material.
Results
Participants
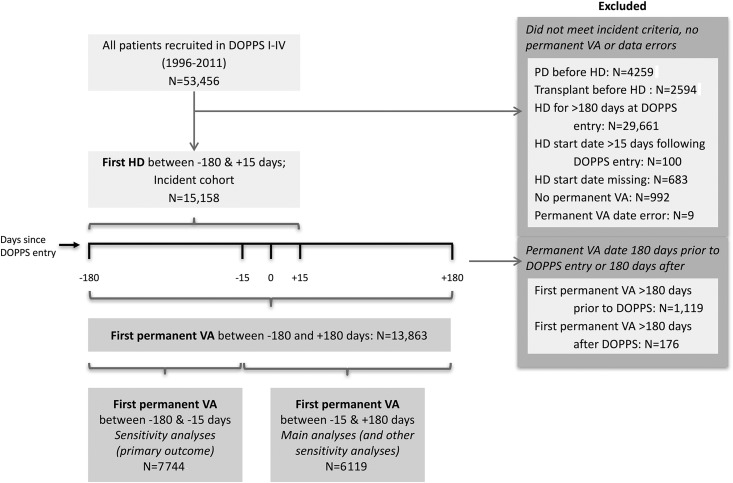
In the DOPPS I–IV, 6119 met the study eligibility criteria for the main analyses, and 7744 met the study eligibility criteria for additional sensitivity analyses, all with complete information on access type (Figure 2). These two cohorts had similar clinical characteristics (Supplemental Tables 1–3, Table 1). However, in the former cohort, more people had been treated with hemodialysis for 30 days or less when they entered the DOPPS (78% versus 48%), fewer received their initial permanent access before or within 30 days of hemodialysis start (68% versus 84%), and more had a catheter for initial permanent access (50% versus 18%).
Figure 2.
Study cohort derivation. In the Dialysis Outcomes and Practice Patterns Study (DOPPS) I–IV, 15,158 participants received hemodialysis (HD) for 180 days or less at study entry (days since DOPPS entry =0) and did not receive peritoneal dialysis (PD) or a kidney transplant before HD. Of these, 6119 met our eligibility criteria of having received their first permanent vascular access (VA; a fistula, a graft, or a tunneled catheter) between 15 days before DOPPS entry and 180 days after DOPPS entry. We established these criteria to minimize survivorship bias and misclassification bias (rates of recorded complications were lower when the access was placed earlier relative to DOPPS entry). Although we excluded from main analyses the people who received their first permanent VA between 15 and 180 days before DOPPS entry (n=7744), we included them in sensitivity analyses.
Table 1.
Clinical characteristics of main study participants (n=6119)
| Clinical Characteristics | All, n=6119 | Missing Data, % | Complications during the First 180 d from the First Permanent Access Placement | P Value | ||
|---|---|---|---|---|---|---|
| Neither, n=3493 | Noninfectious Only, n=2084 | Any Infectious, n=542 | ||||
| Age, yr | 63.7 (14.9) | 30 (0.49) | 64.0 (14.9) | 63.7 (14.9) | 62.0 (14.6) | 0.02 |
| Men | 3540 (57.9) | 14 (0.23) | 2036 (58.3) | 1212 (58.2) | 292 (53.9) | 0.40 |
| Smoking | ||||||
| Never | 2475 (40.5) | 214 (3.49) | 1406 (40.3) | 838 (40.2) | 231 (42.6) | 0.03 |
| Current | 1122 (18.3) | 653 (18.7) | 365 (17.5) | 104 (19.2) | ||
| Previous | 1172 (19.2) | 626 (17.9) | 433 (20.8) | 113 (20.9) | ||
| Unknown | 1136 (18.6) | 687 (19.7) | 372 (17.9) | 77 (14.2) | ||
| Race | ||||||
| White | 3951 (64.6) | 0 | 2130 (61.0) | 1428 (68.5) | 393 (72.5) | <0.001 |
| Black | 919 (15.0) | 441 (12.6) | 382 (18.3) | 96 (17.7) | ||
| Asian | 1002 (16.4) | 781 (22.4) | 200 (9.60) | 21 (3.87) | ||
| Other | 247 (4.04) | 141 (4.04) | 74 (3.55) | 32 (5.90) | ||
| Access type | ||||||
| Fistula | 2263 (37.0) | 0 | 1419 (40.6) | 719 (34.5) | 125 (23.1) | <0.001 |
| Graft | 796 (13.0) | 333 (9.53) | 389 (18.7) | 74 (13.7) | ||
| Catheter | 3060 (50.0) | 1741 (49.8) | 976 (46.8) | 343 (63.3) | ||
| Regiona | ||||||
| North America | 3422 (55.9) | 0 | 1753 (50.2) | 1337 (64.2) | 332 (61.3) | <0.001 |
| Europe | 1682 (27.5) | 963 (27.6) | 545 (26.2) | 174 (32.1) | ||
| Australasia | 1015 (16.6) | 777 (22.2) | 202 (9.7) | 36 (6.7) | ||
| Dialysis durationb | 4809 (78.6) | 0 | 2579 (73.8) | 1789 (85.8) | 441 (81.4) | <0.001 |
| Timing of access insertionc | 4186 (68.4) | 0 | 2301 (65.9) | 1500 (72) | 385 (71) | <0.001 |
| Complications within 30 d | 1293 (21.1) | 0 | — | 1012 (48.6) | 281 (51.9) | <0.001 |
| Coronary artery disease | 2676 (43.7) | 25 (0.41) | 1445 (41.4) | 974 (46.7) | 257 (47.4) | <0.001 |
| Congestive heart failure | 2537 (41.5) | 75 (1.23) | 1371 (39.2) | 919 (44.1) | 247 (45.6) | <0.001 |
| Other cardiovascular disease | 1846 (30.2) | 59 (0.96) | 982 (28.1) | 691 (33.2) | 173 (31.9) | <0.001 |
| Hypertension | 4985 (81.5) | 63 (1.03) | 2780 (79.6) | 1740 (83.5) | 465 (85.8) | <0.001 |
| Cerebrovascular disease | 1015 (16.6) | 69 (1.13) | 568 (16.3) | 351 (16.8) | 96 (17.7) | 0.80 |
| Peripheral vascular disease | 1554 (25.4) | 67 (1.09) | 840 (24.1) | 565 (27.1) | 149 (27.5) | 0.90 |
| Diabetes | 2880 (47.1) | 72 (1.18) | 1604 (45.9) | 982 (47.1) | 294 (54.2) | <0.01 |
| Cancer | 827 (13.5) | 101 (1.65) | 447 (12.8) | 316 (15.2) | 64 (11.8) | 0.06 |
Summary measures: Mean (SD) is used to summarize age (in years); absolute N and relative (percentage) frequencies are used for all categorical variables. —, not applicable.
Region: North America includes Canada and the United States (49%); Europe includes Sweden, Belgium, France, Spain, Germany, Italy, and the United Kingdom; and Australasia includes Japan, Australia, and New Zealand.
Dialysis duration ≤30 versus 31–180 d at the time that participants entered the Dialysis Outcomes and Practice Patterns Study.
Permanent access insertion before or within 30 d versus at >30 d of hemodialysis therapy. Clinical characteristics after multiple imputation for missing data are reported in Supplemental Table 1.
Access Complications
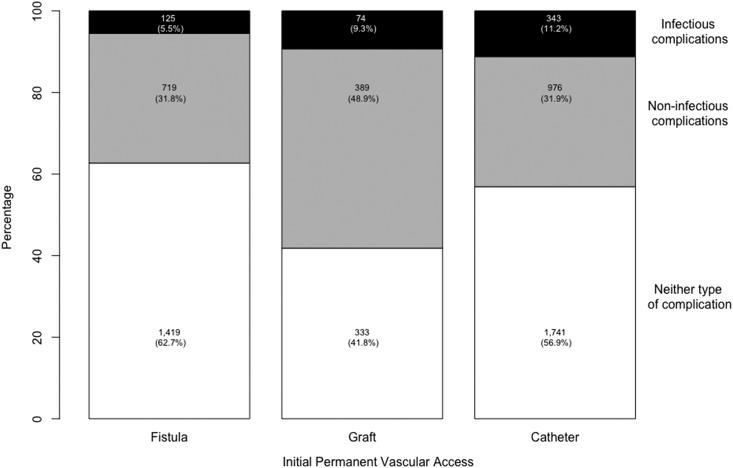
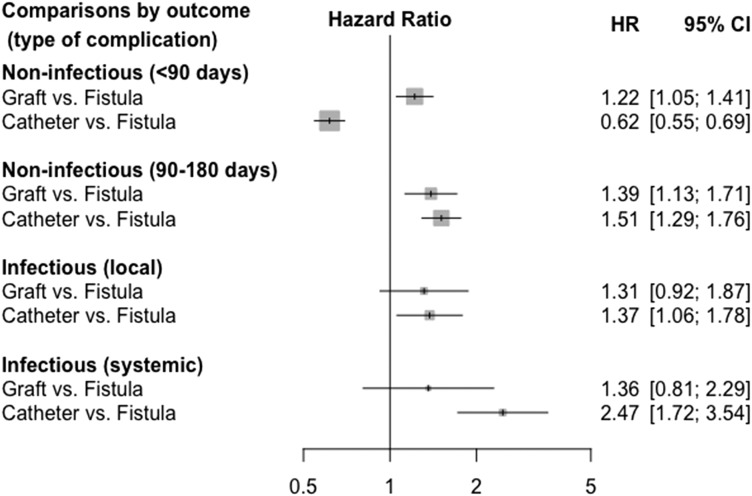
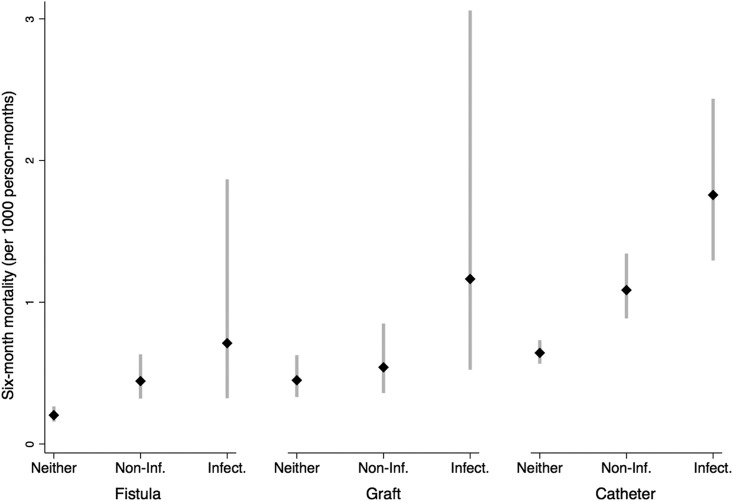
The 6-month risk of access complications was high in this cohort: 2084 participants experienced at least one noninfectious complication but did not have any infectious complications (34%) (Figure 1, step 1). Of the 542 participants who experienced at least one infectious complication (9%), 325 also experienced a noninfectious complication (Supplemental Table 4). Noninfectious complications were largely due to thrombosis (n=1772; 85%), whereas infectious complications consisted of local access infections (n=331; 61%) and sepsis or bacteremia, referred to as systemic infections (n=211; 39%). Most infections occurred after the placement of another access (Supplemental Tables 5 and 6). Noninfectious complications were most common among people who received a graft (49%), whereas those who received a catheter or fistula had a similar frequency of noninfectious complications (32%) (Figure 3). Infectious complications were more common among those who received a catheter (11%) or graft (9.3%) than among those who received a fistula (5.5%). Compared with people who received a fistula, people with a catheter had higher adjusted risks of late noninfectious complications and both local and systemic infections but a lower risk of early noninfectious complications; those with a graft had higher risks for both early and late noninfectious complications (Figure 4).
Figure 3.
Distribution of access complications by initial permanent access type. Access complications include noninfectious complications only (n=2084) and any infectious complications (n=542). In this plot, people who experienced both complications (n=325) were included in the infectious complication group only.
Figure 4.
Associations between type of access and access complications. Cox model stratified by complication type and adjusted for all clinical characteristics described in Table 1 (analysis with multiply imputed data; n=6119). The model accounts for the correlation among repeated complications of different types in the same person (325 participants). The corresponding model including only participants with complete data (n=5722), showing similar results, is reported in Supplemental Figure 2; other sensitivity analyses are reported in Supplemental Figures 3–5. The parenthetical indications <90 and 90–180 days refer to noninfectious complications occurring during the first 90 days since access creation and after, respectively. 95% CI, 95% confidence interval; HR, hazard ratio.
Patient Mortality
During the 180-day follow-up, 526 people died (78 in the first 30 days) (Figure 1, steps 2 and 3). Crude mortality displayed similar patterns of association with access type by access complications and access complications by access type (Figure 5). In separate models, adjusted risks were highest in people using catheters (Figure 6, model 1) and those with early noninfectious complications or infections (Figure 6, model 2). When the access type and access complications were included in the same model, the estimates of these associations remained nearly identical (Figure 6, model 3), suggesting that these two relationships (access type with mortality and access complications with mortality) do not share any disease mechanism. We found no interaction between access complications and access type, age, sex, study time, and study region.
Figure 5.
Crude mortality rates by initial permanent access and type of access complication. Access complications include noninfectious (Non-Inf.) complications only (n=2084) and any infectious (Infect.) complications (n=542). In this plot, people who experienced both complications (n=325) were included in the Infect. complication group only.
Figure 6.
Adjusted mortality from access insertion. Cox models of mortality were adjusted for all clinical characteristics described in Table 1 (analysis with multiply imputed data; n=6119). The corresponding models including only participants with complete data (n=5722), showing similar results, are reported in Supplemental Figure 6; other sensitivity analyses are reported in Supplemental Figures 7–11. Model 1 includes the baseline vascular access type and does not include time-varying vascular access complications. Model 2 includes time-varying vascular access complications and does not include the baseline vascular access type, and model 3 includes both baseline vascular access type and time-varying access complications. The parenthetical indications 0–90 and 91–180 refer to noninfectious complications occurring during the first 90 days since access creation (early noninfectious complications) and after (late noninfectious complications), respectively. 95% CI, 95% confidence interval; HR, hazard ratio.
Other Analyses
Estimates of indirect effects (step 4) and all sensitivity analyses are reported in Supplemental Figures 1–11. Findings from these analyses were similar to those of main analyses, including analyses restricted to complete cases, analyses accounting for the competing risks of death, and analyses that censored observations with a second access. We also found similar results in people who received their first permanent vascular access from 15 to 180 days before the DOPPS entry (n=7744). Compared with the cohort included in all other analyses, in this cohort, fewer events occurred: 1184 people had a noninfectious complication, 153 had an infectious complication, 121 had both types of complications, and 256 died.
Discussion
In this international cohort of people starting hemodialysis therapy, vascular access complications were more common in graft and catheter users than in fistula users and predicted mortality. However, we found that access complications did not mediate the differences in mortality that we observed with different access types. This unexpected finding suggests that both access type and access complications may be independent markers of health status and therefore, patient prognosis. Patients with a poorer prognosis tend to have more complications and be treated with catheters or grafts, which may explain the observed differences in outcomes. Our study is consistent with recent studies showing that patient characteristics account for a large fraction of the excess mortality associated with catheters (29,30) and underlines the need to realign current recommendations and policies on the basis of emerging evidence.
Data on the association between access type and access complications abound (11,19). To the best of our knowledge, however, this is the first study assessing whether access complications explain the relationship between access type and mortality. Access-related chronic inflammation or infectious complications are the hypothesized link between access type and patient outcomes (12,20). However, if the increased risk of death observed in people with grafts or catheters compared with fistulas was due to access complications, the association between access type and mortality would be at least partially explained by the occurrence of access complications. Our study could not confirm this hypothesis.
This study was designed on the basis of three hypotheses: that there is an association between type of hemodialysis access and mortality, that there is an association between access type and access complications, and that access complications are on the causal pathway between access type and mortality if these relationships are causal. Our study supports the existence of the two former associations but does not support the hypothesis that access complications explain the increased risk of death in patients using catheters or grafts compared with those using fistulas. These findings require careful interpretation, including consideration of the study limitations. Although based on a large sample size, international data capture, and prospective design, our study remains an observational study and as such, can only support inference about associations, because unmeasured or residual confounding cannot be excluded. Ideally, only a clinical trial randomly assigning both access type and an intervention to prevent access complications (31) can clarify whether the relationship between access type and mortality is causal and whether access complications are on this causal pathway (i.e., have a mediation effect) (28,32).
Failure of access complications to explain the relationship between access type and mortality in our study may be due to bias, chance, or absence of effect. Although we tried to minimize survivorship bias, immortal time bias (24), and collider stratification bias (26) in our study design, many other forms of bias exist. Examples include misclassification of access complications or death and bias in the ascertainment of severity of complications. However, the DOPPS includes strategies designed to minimize misclassification bias during data collection. To minimize bias in the ascertainment of the severity of complications, we considered separately local infection and systemic infections as well as early and late noninfectious complications. We did many sensitivity analyses, in which we defined the mediator in different ways and obtained consistent results. We also found similar results in people who received their first permanent access between 15 and 180 days before the DOPPS entry (i.e., earlier relative to their hemodialysis start date). Although we cannot exclude the possibility that reduced dialysis efficiency and chronic inflammation (20,21) as opposed to complications requiring an intervention as defined in our study may explain the observed association between access type and mortality, such a hypothesis remains to be proven. False negative results can be due to chance, as in any study. Our study may have failed to detect the mediation effect that we hypothesized or may be underpowered to detect a mediation effect smaller than we anticipated (32). However, results were consistent across many sensitivity analyses. These considered, for example, more powerful parametric modeling, the role of the competing risk of death in the relationship between access type and access complications, and the potential change in mortality associated with the placement of a new access that we addressed by censoring observations 30 days after placement of a new access instead of continuing follow-up for 180 days. Finally, results from our study could be truly negative (i.e., access complications and access type are not on the same causal pathway leading to death). This would be the case, for example, if both access type and access complications were markers of general health and if mortality was unrelated to the access type. Consistent with this hypothesis, in a recent study from our group, only 2.3% of deaths in incident patients on hemodialysis were adjudicated as being access related (30). The mechanisms through which access type affects patient outcomes, if it does, remain to be determined.
Our study has policy, clinical, and research implications. Notwithstanding current policy, achieving a suitable fistula may take months and is difficult or impossible in many people who are usually older and sicker. These people will often receive extensive interventions to promote fistula maturation and continue using catheters or grafts, because they have poor vasculature and thus, cannot have a fistula. In North America, only 20% of people use a fistula when they start hemodialysis, and one in three who use a catheter at hemodialysis start will still use a catheter after 1 year (6). Consistent with these data, we found a higher risk of early noninfectious complications in people who received a fistula for initial hemodialysis access compared with those who received a catheter. In our study, early noninfectious complications were associated with worse outcomes than late noninfectious complications. Resource-intensive programs implemented to maximize fistula use may have inadvertently led to increased rates of unsuccessful fistula attempts and complications, possibly resulting in smaller incremental benefits than anticipated and increased potential harms (33). Our study highlights the importance of allowing more room for clinical judgement to better match the type of vascular access to individual health conditions (34) and personal preferences and values (35). Existing studies may have overstated the benefits of fistulas and underestimated the potential harms of practices and policies established to achieve them by comparing people who attained a mature fistula with all catheter users, including people in whom a fistula never matured (16). When patients are advised to have a fistula for vascular access, they make choices assuming that the fistula represents a clearly superior path. Patients may express different preferences if they knew that current evidence supporting fistulas does not clarify whether the observed differences in outcomes depend on the type of access or the patients who use them (12). Adoption of health care practices with insufficient evidence for safety and efficacy may lead to unintended consequences for patients or the health care system. Prior examples include the widespread adoption of erythropoiesis-stimulating agents in patients with CKD to normalize hemoglobin levels until randomized trials showed that such interventions were harmful (36). Only a randomized trial can characterize the benefits and harms of different access strategies. A pilot study is currently underway in Canada to test the feasibility and safety of randomizing people starting hemodialysis with catheters to either continue use of a catheter or receive a fistula (37).
In summary, vascular access complications are very common and associated with increased mortality. Although access complications more commonly affect people using a graft or a catheter than people using fistulas, they do not seem to explain the observed association of access type with mortality. Clinical trials are needed to clarify whether these associations are causal or confounded by other prognostically important factors. Our findings underline the need to realign current recommendations and policies on the basis of the strength of the evidence and compare different access strategies in a randomized trial. Finally, in vascular access counseling, patients should come before the type of access. In the absence of evidence from randomized, controlled trials supporting a clearly superior hemodialysis access strategy, patient health conditions and their preferences and values should play a major role in vascular access planning.
Disclosures
The authors have nothing to disclose in relation to this study.
Supplementary Material
Acknowledgments
The authors thank Mr. Brian Bieber from Arbor Research Collaborative for Health (Ann Arbor, MI) for assistance with preparation of this study. The authors also thank Dr. Bommae Kim and Mr. Clay Ford for helpful discussion about mediation analysis on the basis of Dr. Kim’s work (http://data.library.virginia.edu/introduction-to-mediation-analysis/). The authors thank Dr. Douglas Schaubel from the Department of Biostatistics, School of Public Health, University of Michigan for providing feedback on sensitivity analyses.
This work was supported by Canadian Institute of Health Research grants FRN 111223 (to P.R.) and FRN 119366 (to P.R.).
Amgen, Kyowa Hakko Kirin, Abb Vie, Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma support the Dialysis Outcomes and Practice Patterns Study (DOPPS) Program. Additional support for specific projects and countries is provided by Keryx Biopharmaceuticals, Merck Sharp & Dohme, Proteon Therapeutics, Relypsa, and F. Hoffmann-LaRoche. Additional support in Canada is by Amgen, BHC Medical, Janssen, Takeda, and the Kidney Foundation of Canada (logistics support). Additional support in Germany is by Hexal, DGfN, Shire, and the WiNe Institute, and additional support for Peritoneal DOPPS in Japan is by the Japanese Society for Peritoneal Dialysis. All support is provided without restrictions on publications. Grants are made to Arbor Research Collaborative for Health and are not made to individual investigators.
The results of this study have not been published previously and are not under consideration for publication elsewhere.
The funding organizations played no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Reassessing Recommendations for Choice of Vascular Access,” on pages 865–867.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12181116/-/DCSupplemental.
References
- 1.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Thomas B, Wulf S, Bikbov B, Perico N, Cortinovis M, Courville de Vaccaro K, Flaxman A, Peterson H, Delossantos A, Haring D, Mehrotra R, Himmelfarb J, Remuzzi G, Murray C, Naghavi M: Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol 26: 2621–2633, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Renal Data System: 2015 USRDS Annual Data Report: Medicare Expenditures for Persons with ESRD. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015
- 4.Manns B, Tonelli M, Yilmaz S, Lee H, Laupland K, Klarenbach S, Radkevich V, Murphy B: Establishment and maintenance of vascular access in incident hemodialysis patients: A prospective cost analysis. J Am Soc Nephrol 16: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Allon M, Dinwiddie L, Lacson E Jr., Latos DL, Lok CE, Steinman T, Weiner DE: Medicare reimbursement policies and hemodialysis vascular access outcomes: A need for change. J Am Soc Nephrol 22: 426–430, 2011 [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System: 2014 USRDS annual data report: Vascular access use during the first year of hemodialysis by time since initiation of ESRD treatment. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014
- 7.Besarab A: Resolved: Fistulas are preferred to grafts as initial vascular access for dialysis. Pro. J Am Soc Nephrol 19: 1629–1631, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF; Canadian Society of Nephrology Committee for Clinical Practice Guidelines : Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Polkinghorne K; Caring for Australians with Renal Impairment (CARI) : The CARI guidelines. Vascular access surveillance. Nephrology (Carlton) 13[Suppl 2]: S1–S11, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ravani P, Gillespie BW, Quinn RR, MacRae J, Manns B, Mendelssohn D, Tonelli M, Hemmelgarn B, James M, Pannu N, Robinson BM, Zhang X, Pisoni R: Temporal risk profile for infectious and noninfectious complications of hemodialysis access. J Am Soc Nephrol 24: 1668–1677, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, Pannu NI, Thomas C, Hemmelgarn BR, Craig JC, Manns B, Tonelli M, Strippoli GF, James MT: Associations between hemodialysis access type and clinical outcomes: A systematic review. J Am Soc Nephrol 24: 465–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters VJ, Clemons G, Augustine B: “Fistula first” as a CMS breakthrough initiative: Improving vascular access through collaboration. Nephrol Nurs J 32: 686–687, 2005 [PubMed] [Google Scholar]
- 14.Vassalotti JA, Jennings WC, Beathard GA, Neumann M, Caponi S, Fox CH, Spergel LM; Fistula First Breakthrough Initiative Community Education Committee : Fistula first breakthrough initiative: Targeting catheter last in fistula first. Semin Dial 25: 303–310, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services: Medicare program; ESRD quality incentive program. Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/QIP-Details-PY15.pdf. Accessed September 20, 2015
- 16.Quinn RR, Ravani P: Fistula-first and catheter-last: Fading certainties and growing doubts. Nephrol Dial Transplant 29: 727–730, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Manns B, Hemmelgarn B, Lillie E, Dip SC, Cyr A, Gladish M, Large C, Silverman H, Toth B, Wolfs W, Laupacis A: Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol 9: 1813–1821, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casey JR, Hanson CS, Winkelmayer WC, Craig JC, Palmer S, Strippoli GF, Tong A: Patients’ perspectives on hemodialysis vascular access: A systematic review of qualitative studies. Am J Kidney Dis 64: 937–953, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Jones SM, Ravani P, Hemmelgarn BR, Muruve D, Macrae JM: Morphometric and biological characterization of biofilm in tunneled hemodialysis catheters. Am J Kidney Dis 57: 449–455, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Sachdeva M, Hung A, Kovalchuk O, Bitzer M, Mokrzycki MH: The initial vascular access type contributes to inflammation in incident hemodialysis patients. Int J Nephrol 2012: 917465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson BM, Zhang J, Morgenstern H, Bradbury BD, Ng LJ, McCullough KP, Gillespie BW, Hakim R, Rayner H, Fort J, Akizawa T, Tentori F, Pisoni RL: Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int 85: 158–165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51: 1173–1182, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z, Rahme E, Abrahamowicz M, Pilote L: Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: A comparison of methods. Am J Epidemiol 162: 1016–1023, 2005 [DOI] [PubMed] [Google Scholar]
- 25.United States Renal Data System: 2012 USRDS annual data report: Mortality during the first year of ESRD. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 . [Google Scholar]
- 26.Cole SR, Hernán MA: Fallibility in estimating direct effects. Int J Epidemiol 31: 163–165, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lin DY, Wei LJ: The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84: 1074–1078, 1989 [Google Scholar]
- 28.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K: Mediation: R package for causal mediation analysis. J Stat Softw 59: 1–38, 2014. 26917999 [Google Scholar]
- 29.Brown RS, Patibandla BK, Goldfarb-Rumyantzev AS: The survival benefit of “fistula first, catheter last” in hemodialysis is primarily due to patient factors. J Am Soc Nephrol 28: 645–652, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn RR, Oliver MJ, Devoe D, Poinen K, Kabani R, Kamar F, Mysore P, Lewin AM, Hiremath S, MacRae J, James MT, Miller L, Hemmelgarn BR, Moist LM, Garg AX, Chowdhury TT, Ravani P: The effect of predialysis fistula attempt on risk of all-cause and access-related death. J Am Soc Nephrol 28: 613–620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravani P, Quinn RR, Oliver MJ, Karsanji DJ, James MT, MacRae JM, Palmer SC, Strippoli GF: Preemptive correction of arteriovenous access stenosis: A systematic review and meta-analysis of randomized controlled trials. Am J Kidney Dis 67: 446–460, 2016 [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele TJ: Causal mediation analysis with survival data. Epidemiology 22: 582–585, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalloo S, Blake PG, Wish J: A patient-centered approach to hemodialysis vascular access in the era of fistula first. Semin Dial 29: 148–157, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Mehrotra R, Cheung AK, Meyer T, Nath KA: Vascular access for hemodialysis and value-based purchasing for ESRD. J Am Soc Nephrol 28: 395–397, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barry MJ, Edgman-Levitan S: Shared decision making--Pinnacle of patient-centered care. N Engl J Med 366: 780–781, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G, McGee R, Nicolucci A, Tognoni G, Strippoli GF: Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 153: 23–33, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Quinn R, Ravani P; ACCESS HD Investigators: ACCESS HD pilot: A randomised feasibility trial Comparing Catheters with fistulas in Elderly patientS Starting haemodialysis. BMJ Open 6: e013081, 2016 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.