Abstract
Context:
Pheochromocytomas and paragangliomas (PPGLs) in children are often hereditary and may present with different characteristics compared with adults. Hereditary PPGLs can be separated into cluster 1 and cluster 2 tumors due to mutations impacting hypoxia and kinase receptor signaling pathways, respectively.
Objective:
To identify differences in presentation of PPGLs between children and adults.
Design:
A retrospective cross-sectional clinical study.
Setting:
Seven tertiary medical centers.
Patients:
The study included 748 patients with PPGLs, including 95 with a first presentation during childhood. Genetic testing was available in 611 patients. Other data included locations of primary tumors, presence of recurrent or metastatic disease, and plasma concentrations of metanephrines and 3-methoxytyramine.
Results:
Children showed higher (P < 0.0001) prevalence than adults of hereditary (80.4% vs 52.6%), extra-adrenal (66.3% vs 35.1%), multifocal (32.6% vs 13.5%), metastatic (49.5% vs 29.1%), and recurrent (29.5% vs 14.2%) PPGLs. Tumors due to cluster 1 mutations were more prevalent among children than adults (76.1% vs 39.3%; P < 0.0001), and this paralleled a higher prevalence of noradrenergic tumors, characterized by relative lack of increased plasma metanephrine, in children than in adults (93.2% vs 57.3%; P < 0.0001).
Conclusions:
The higher prevalence of hereditary, extra-adrenal, multifocal, and metastatic PPGLs in children than adults represents interrelated features that, in part, reflect the lower age of disease presentation of noradrenergic cluster 1 than adrenergic cluster 2 tumors. The differences in disease presentation are important to consider in children at risk for PPGLs due to a known mutation or previous history of tumor.
This study establishes the link between extraadrenal, multifocal, metastatic, reccurent, hereditary PPGLs to a higher prevalence of noradrenergic and cluster 1 tumors in pediatric than adults.
Pheochromocytomas and paragangliomas (PPGLs) are neuroendocrine tumors derived from chromaffin cells of the adrenal medulla or associated with paravertebral autonomic ganglia. The tumors account for up to 0.6% of cases of adult hypertension and 1% of pediatric hypertension (1). Although rare, with a reported annual incidence of two to five cases per million, of which only 10% occur in children (2–4), PPGLs are potentially lethal.
At least 30% of PPGLs have a hereditary background, reflecting mutations in at least 14 tumor susceptibility genes (5, 6). These susceptibility genes can be divided into two cluster groups based on transcriptomic profiles revealed by gene expression microarray analyses (7–9). Cluster 1 tumors include those due to mutations of genes encoding the von Hippel-Lindau (VHL) suppressor, the four subunits of the succinate dehydrogenase complex (SDHA, SDHB, SDHC, and SDHD), and less commonly, the enzyme responsible for flavination of the SDHA subunit (SDHAF2), fumarate hydratase, malate dehydrogenase 2, and prolyl hydroxylases 1 and 2. Additional activating mutations of hypoxia-inducible factor 2 alpha (HIF2α) have also been identified, but these are mainly somatic in nature (10). All cluster 1 mutations result in stabilization of hypoxia-inducible factors and activation of the hypoxia signaling pathways (11).
The second cluster group includes tumors due to mutations of the neurofibromatosis type 1 (NF1) tumor suppressor gene, the rearranged during transfection (RET) proto-oncogene, genes encoding transmembrane protein 127 (TMEM127), and MYC-associated factor X (MAX). Mutations of the Harvey rat sarcoma viral oncogene homolog (HRAS) also belong to this cluster 2 group, but similar to HIF2α, appear to be somatic in nature (12). Mutations of cluster group 2 genes result in activation of kinase receptor signaling pathways, translation initiation, protein synthesis, and pathways involved in maintenance of neural/neuroendocrine identity (13).
As shown in several publications and most recently by Qin et al. (14), cluster 1 PPGLs are characterized by absence of epinephrine production (noradrenergic phenotype), whereas cluster 2 tumors produce epinephrine (adrenergic phenotype). These differences reflect absence vs presence of the enzyme phenylethanolamine N-methyltransferase, responsible for conversion of norepinephrine to epinephrine.
Existing data suggest a higher prevalence of hereditary disease in children that in adults (1, 5, 15) and phenotypic presentations of childhood PPGLs characterized by bilateral, multiple, and extra-adrenal tumors (16–19). However, the reported prevalence of hereditary disease in the aforementioned pediatric series varied considerably from 22% to 70%. The most recent published study from Bausch et al. (20) reported an even higher 80% frequency of germline mutations among pediatric cases. Despite the large pediatric cohort in that series, there was no direct comparison with an adult population.
Based on findings of a younger age of disease presentation in patients with noradrenergic than adrenergic PPGLs (21), we hypothesized that the higher prevalence of hereditary, metastatic, and extra-adrenal PPGLs in children than adults might reflect a predisposition of children to cluster 1 noradrenergic tumors. The present retrospective study, the first, to our knowledge, to explore differences in disease presentation in a large series of both children and adults, was undertaken to examine that hypothesis.
Materials and Methods
Subjects
This study involved retrospective analysis of data from 748 patients with PPGLs enrolled into clinical protocols at seven tertiary clinical care centers: (1) National Institutes of Health, Bethesda, Maryland; (2) University Hospital Carl Gustav Carus, Dresden, Germany; (3) Institute of Cardiology, Warsaw, Poland; (4) University Hospital of Munich, Munich, Germany; (5) Radboud University Medical Centre, Nijmegen, The Netherlands; (6) University Hospital of Wuerzburg, Wuerzburg, Germany; and (7) University Medical Center Schleswig-Holstein, Luebeck, Germany. Patients were investigated under an intramural review board or ethics committee–approved protocols at each center, with European centers covered under a single multicenter protocol (https://pmt-study.pressor.org/) distinct from that at the National Institutes of Health. Informed consent was provided by all patients, including written parental consent for those enrolled as children.
Recruitment into clinical protocols over 20 years up until 2016 was based on clinical suspicion or increased risk of PPGLs according to four main criteria: (1) presence of signs and symptoms (45.2% of patients); (2) incidental finding of an adrenal or abdominal mass during imaging studies for an unrelated condition (15.8% of patients); (3) previous history of PPGLs (22.1% of patients); or (4) presence of a hereditary syndrome or mutation of a tumor susceptibility gene (18.4% of patients).
Patients with PPGLs were divided into pediatric and adult groups based on an age of ≤18 years or >18 years at first diagnosis of tumors. Based on observations that biochemically positive PPGLs are often present 12 years or more before they are diagnosed (22), it can be expected that many PPGLs in younger adults develop in childhood. Thus, in an additional analysis, adult patients were divided into two subgroups based on age of less than or more than 35 years at first diagnosis of tumors. Confirmation of PPGLs required histopathological examination of surgically resected or biopsied tumor tissue or a diagnosis of inoperable malignant disease based on functional imaging evidence of metastatic lesions. Locations of tumors were determined based on results of imaging studies and surgical and pathological records. Retrieved data included assessment of the history or presence of multifocal and recurrent disease. Malignancy was defined by the presence of metastases at sites distant from the primary tumor where chromaffin tissue is normally absent, including lungs, bones, liver, and lymph nodes, with diagnosis at the latter sites dependent on histopathology.
Genetic testing
Mutation testing, using leukocyte DNA, was carried out in 611 patients. Testing for germline mutations of VHL, RET, SDHB, SDHD, SDHC, MAX, and TMEM127 genes was mainly by bidirectional Sanger sequencing and multiplex ligation probe amplification, the latter to test for deletions of selected genes (e.g., VHL and SDHx). For NF1, the diagnosis was based mainly on clinical manifestations according to established criteria (23). After 2014, testing included next-generation sequencing, which was directed primarily to testing of tumor tissue (n = 257) for somatic mutations of RET, VHL, SDHB, SDHD, SDHC, SDHA, MAX, TMEM127, NF1, HRAS, and HIF2α genes, with germline testing subsequently restricted to positive cases to establish the origin of the mutation.
Blood sample collections and laboratory analysis
Measurements of plasma-free normetanephrine, metanephrine, and 3-methoxytyramine concentrations, available from 661 patients at time of PPGL diagnosis, were performed using liquid chromatography with electrochemical detection (24) or by liquid chromatography with tandem mass spectrometry (25). For these measurements, heparinized blood was collected with instructions that patients should have fasted overnight and rested supine for at least 20 minutes before samples were drawn. Blood samples were kept on ice until plasma was separated and stored frozen at –80°C before analyses.
Catecholamine biochemical phenotypes
Designation of catecholamine biochemical phenotypes was based on relative tumor-derived increases in plasma concentrations of normetanephrine, metanephrine, and 3-methoxytyramine according to established methods (Supplemental Methods (681.6KB, docx) ). Briefly, tumor-derived increments were calculated by subtracting the concentration of each metabolite in each patient with a PPGL from mean concentrations of normetanephrine (52 pg/mL), metanephrine (26 pg/mL), and 3-methoxytyramine (5 pg/mL) in a previously described reference group (25, 26). Epinephrine-producing adrenergic tumors were defined as those in patients who showed both an increase in plasma metanephrine above the upper cutoff of reference intervals (62 pg/mL) and a tumor-derived increment of metanephrine larger than 5% of combined increments of all O-methylated metabolites. All other tumors were defined as nonadrenergic, including both noradrenergic and the less common dopaminergic tumors.
Statistical analysis
Statistical analyses used the JMP statistics software package (SAS Institute, Cary, NC). Overall differences in continuous parameters between groups were assessed using the Kruskal-Wallis test. χ2 and Fisher’s exact tests were used to compare frequencies of adrenergic, hereditary, malignant, multifocal, recurrent, and extra-adrenal presentation among different groups.
Results
Demographic and tumor characteristics
Of the 748 patients with PPGLs, 12.7% first occurred during childhood (Table 1). Compared with adults, children presented less frequently with adrenal tumors (36.8% vs 65.4%; P < 0.0001), but more frequently with extra-adrenal tumors (66.3% vs 35.1%; P < 0.0001). Although there was no difference in the presentation of bilateral adrenal tumors between adults and children, the prevalence of multifocal extra-adrenal tumors, including combined adrenal and extra-adrenal tumors, was 2.4-fold higher (P < 0.0001) in children than adults. In contrast, the presentation of solitary adrenal tumors was 2.4-fold higher in adults than in children (P < 0.0001).
Table 1.
Demographic and Tumor Characteristics of Pediatric and Adult Patients With PPGLs
| Characteristics | Pediatric | Adult | P Value |
|---|---|---|---|
| N | 95 | 653 | |
| Age at initial diagnosisa | 13.3 ± 3.5 | 44.7 ± 14.4 | |
| Male | 55.8% (53/95) | 48.1% (314/653) | 0.0980 |
| Primary tumor locations | |||
| Solitary adrenal | 22.1% (21/95) | 56.2% (367/653) | <0.0001 |
| Solitary extra-adrenal | 33.7% (32/95) | 21.6% (141/653) | <0.0001 |
| Bilateral adrenal | 11.6% (11/95) | 8.7% (57/653) | 0.2020 |
| Multifocalb | 32.6% (31/95) | 13.5% (88/653) | <0.0001 |
| Hereditary casesc | 80.4% (74/92) | 52.6% (273/519) | <0.0001 |
| Recurrent primary tumorsd | 29.5% (28/95) | 14.2% (93/653) | <0.0001 |
| Metastatic disease | 49.5% (47/95) | 29.1% (190/653) | <0.0001 |
| No. N/D phenotype | 93.2% (68/73) | 57.3% (337/588) | <0.0001 |
Abbreviations: N/D, noradrenergic/dopaminergic.
Age is shown as mean ± standard deviation.
Multifocal locations indicate multiple extra-adrenal tumors or extra-adrenal and adrenal tumors but exclude bilateral adrenal tumors unless accompanied by one or more extra-adrenal tumors.
Results were retrieved from 611 patients who underwent genetic testing.
Recurrent primary tumors are defined as recurrences at an original site of tumor resection as well as new primary tumors at other locations a year or more after diagnosis of the first primary tumor.
Among the 611 patients who underwent genetic testing, 80.4% (74/92) of all children and 52.6% (273/519) of all adults were identified with germline mutations of tumor susceptibility genes, confirming a higher (P < 0.0001) prevalence of hereditary PPGLs in children than adults (Table 1). Among children, the prevalence of recurrent primary tumors, excluding metastases, at the sites of previously resected tumors or at new locations was 2.1-fold higher (P < 0.0001) than among adults. The prevalence of metastatic disease was also 1.7-fold higher (P < 0.0001) in children than in adults (Table 1).
Biochemical characteristics
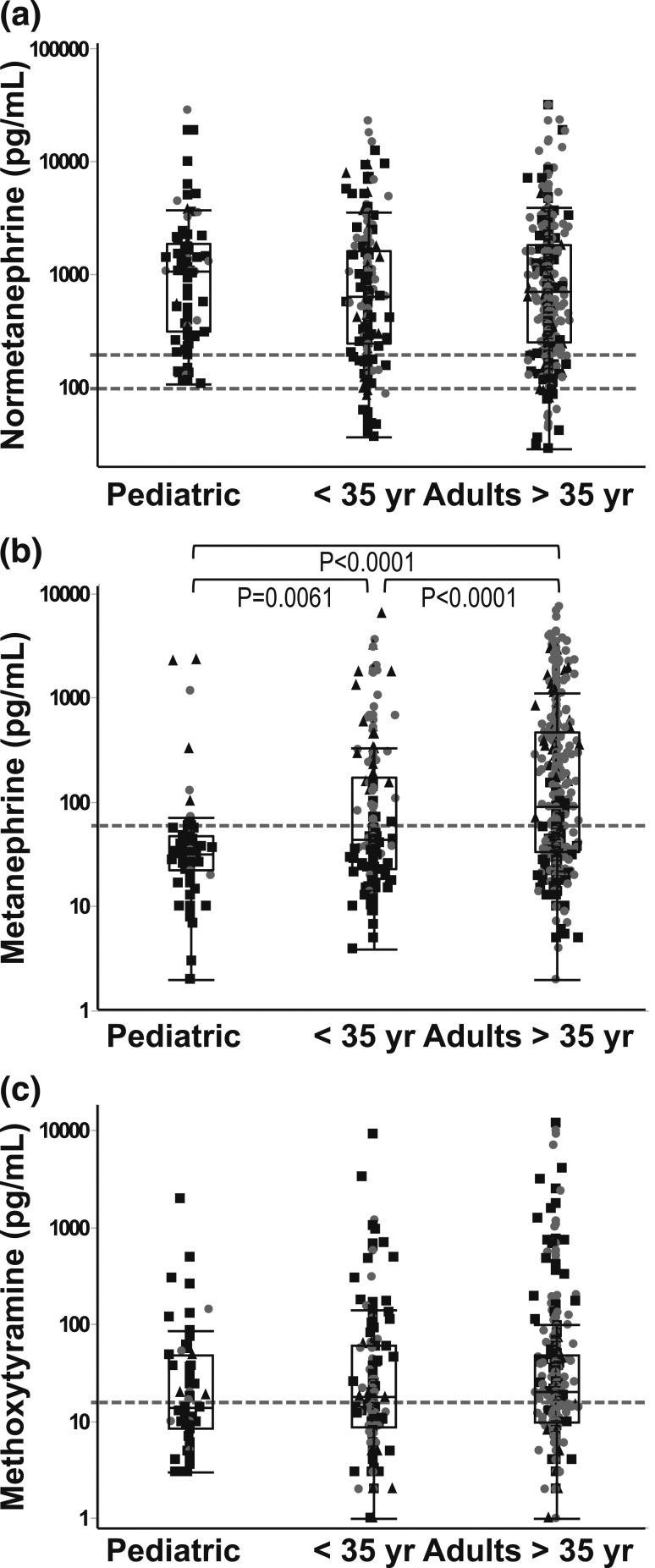
Results for plasma concentrations of normetanephrine, metanephrine, and 3-methoxytyramine, available among the 661 patients at the time of tumor diagnosis, indicated lower (P < 0.0001) plasma concentrations of metanephrine in children than adults, with no differences for normetanephrine and methoxytyramine (Fig. 1).
Figure 1.
Plasma concentrations of (a) normetanephrine, (b) metanephrine, and (c) methoxytyramine in children and young (<35 years old) and old (>35 years old) adult patients.
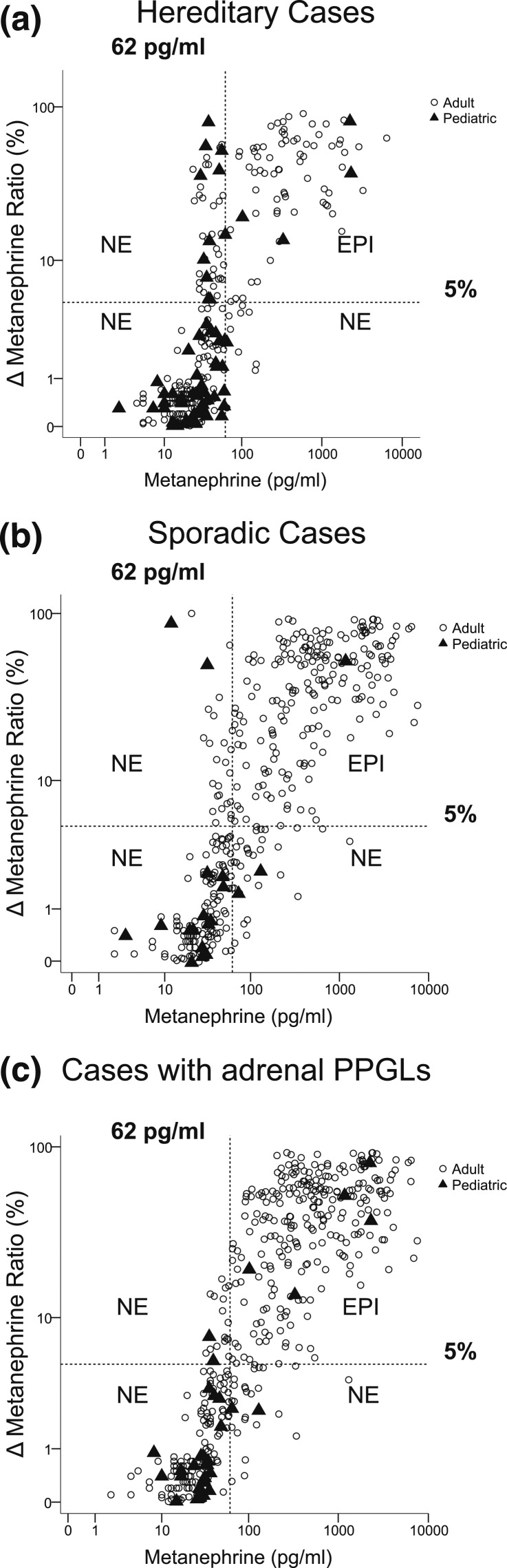
Only 4 of 56 (7.1%) pediatric patients with hereditary pheochromocytoma, all diagnosed with cluster 2 mutations, presented with both elevated plasma concentrations of metanephrine and increases of metanephrine larger than 5% of the summed total increases of all three metabolites [Fig. 2(a)]. This contrasted with a 4.2-fold higher (P = 0.0003) proportion (67/226) of adult hereditary cases who presented with both elevated plasma concentrations of metanephrine and increases of metanephrine larger than 5% of the summed total increases of all three metabolites. By the criteria outlined in the Materials and Methods section, these patients were all designated with epinephrine-producing adrenergic tumors.
Figure 2.
Biochemical phenotypes of (a) pediatric and adult sporadic, (b) hereditary, and (c) adrenal-located PPGLs. Characterization of PPGLs with and without appreciable epinephrine (EPI) production [adrenergic (EPI) vs noradrenergic (NE) phenotypes] was established according to the scatterplot relationship of plasma concentrations of metanephrine vs increases of plasma metanephrine as a percentage of increases of the summed total of all O-methylated metabolites above reference (Δ metanephrine ratio). The dashed vertical line depicts the upper reference limit (62 mg/mL) used to establish increased vs normal plasma concentrations of metanephrine. The dashed horizontal line depicts the cutoff at 5% used to establish appreciable metanephrine (epinephrine) production according to total production of all O-methylated metabolites.
Among patients with sporadic PPGLs, only one child among 17 (5.9%) presented with an adrenergic tumor, a proportion much lower (P = 0.0002) than the 51.0% (185/363) of adult cases of sporadic PPGLs with adrenergic tumors [Fig. 2(b)].
All five children with adrenergic tumors, including the four with hereditary tumors [Fig. 2(a)] and the single case with sporadic disease [Fig. 2(b)], presented with adrenal pheochromocytomas [Fig. 1(c)]. Among patients with adrenal pheochromocytomas, the prevalence of tumors with a noradrenergic or dopaminergic phenotype was twofold higher (P < 0.0001) in children relative to adults (88.0% vs 44.4%). Among all 661 patients with PPGLs in whom measurements of metanephrines were available at the time of diagnosis, the prevalence of tumors with a noradrenergic or dopaminergic phenotype was 1.6-fold higher (P < 0.0001) among children compared with adults (Table 1).
Genetics
Among the 611 patients who underwent genetic testing, both children and adults showed a higher prevalence of germline mutations of the cluster 1 than of the cluster 2 group of genes (Table 2). However, the prevalence of cluster 1 mutations involving VHL and SDHx genes was 1.9-fold higher (P < 0.0001) in children than in adults. In contrast, the prevalence of cluster 2 mutations involving RET, NF1, TMEM127, and MAX was threefold higher (P = 0.0008) in adults than children (13.3% vs 4.3%; P < 0.0001), clarifying that the overall higher prevalence of hereditary PPGLs in children than adults is restricted to PPGLs due to cluster 1 mutations.
Table 2.
Gene-Specific Characteristics of Pediatric and Adult Patients With PPGLs
| Pediatric | Adult | P Value | |
|---|---|---|---|
| Cluster 1 mutations | |||
| VHL | 27.2% (25/92) | 10.2% (53/519) | <0.0001 |
| Metastatic | 12.0% (3/25) | 5.7% (3/53) | 0.3070 |
| Recurrent | 18.5% (5/25) | 5.7% (3/53) | 0.0730 |
| SDHBa | 39.1% (36/92) | 17.3% (90/519) | <0.0001 |
| Metastatic | 86% (31/36) | 68.9% (62/90) | 0.0350 |
| Recurrent | 19.4% (7/36) | 20.0% (18/90) | 0.5780 |
| SDHDa | 9.8% (9/92) | 10.6% (55/519) | 0.4660 |
| Metastatic | 33.3% (3/9) | 30.9% (17/55) | 0.5680 |
| Recurrent | 66.7% (6/9) | 58.2% (32/55) | 0.4380 |
| SDHA/Ca | 0% (0/92) | 1.2% (6/519) | — |
| Metastatic | — | 16.7% (1/6) | — |
| Recurrent | — | 0.0% (0/6) | — |
| Total for cluster 1 | 76.1% (70/92) | 39.3% (204/519) | <0.0001 |
| Metastatic | 52.9% (37/70) | 40.7% (83/204) | 0.0820 |
| Recurrent | 25.7% (18/70) | 26.0% (53/204) | 0.4340 |
| Cluster 2 mutations | |||
| RETa | 3.3% (3/92) | 8.9% (46/519) | 0.0270 |
| Metastatic | 0.0% (0/3) | 4.4% (2/46) | — |
| Recurrent | 66.7% (2/3) | 17.4% (8/46) | 0.0910 |
| NF1 | 1.1% (1/92) | 3.7% (19/519) | 0.1750 |
| Metastatic | 0.0% (0/1) | 5.2% (1/19) | — |
| Recurrent | 0.0% (0/1) | 10.5% (2/19) | — |
| MAX/TMEM127a | 0% (0/92) | 0.7% (4/519) | — |
| Metastatic | — | 25.0% (1/4) | — |
| Recurrent | — | 50.0% (2/4) | — |
| Total for cluster 2 | 4.3% (4/92) | 13.3% (69/519) | 0.0008 |
| Metastatic | 0% (0/4) | 5.8% (4/69) | — |
| Recurrent | 50.0% (2/4) | 17.4% (12/69) | 0.1420 |
Percentage is shown according to the total number of patients tested for germline mutations. For NF1, diagnosis was based on clinical manifestations and, for SDHA, TMEM127, and MAX, was restricted to limited patient numbers as described in Materials and Methods. Percentages of metastatic and recurrent disease are shown according to the particular mutated gene.
Among our cohort, 19 patients were identified with somatic mutations. In particular, four children were identified with HIF2α mutations and two with VHL mutations in tumor tissue but not in the germline. Among adult patients, five were identified with HIF2α somatic mutations, two with HRAS mutations, three with RET mutations, and two with VHL somatic mutations. One additional adult patient, as described by us elsewhere (26), was found to have methylation of the SDHC promoter. These somatic mutations or epigenetic variants thus showed a similar pattern to findings with germline mutations with proportionally more cluster 1 mutations among the pediatric than the adult cohort and a reverse pattern for cluster 2 mutations. With additional inclusion of the aforementioned somatic mutations and epigenetic variants, 80.0% (76/95) of all pediatric patients had cluster 1 mutations compared with only 4.2% (4/95) with cluster 2 mutations, completely different (P < 0.0001) proportions from the 32.5% (212/653) and 11.3% (74/653) of adult patients with respective cluster 1 and 2 mutations.
Metastatic and recurrent disease
For both adults and children, metastatic disease was more prevalent (P < 0.0001) in patients with cluster 1 than cluster 2 mutations (52.9% and 40.7% vs 0% and 5.8%), largely reflecting the high rate of metastatic disease in patients with SDHB mutations (Table 2). Although the overall prevalence of metastatic disease was higher (P < 0.0001) in children than in adults (Table 1), there were no differences when examined separately for cluster 1 or cluster 2 mutations (Table 2). Nevertheless, the percentages of children with metastatic disease remained higher compared with adults for both hereditary (50.0% vs 31.8%; P < 0.0001) and sporadic groups (47.6% vs 27.2%; P < 0.0001).
Compared with adults, children showed a higher (P = 0.0019) prevalence of nonsynchronous metastatic disease (i.e., metastases diagnosed a year or more after initial diagnosis of a primary tumor) than metastatic disease diagnosed synchronously at first diagnosis of PPGLs (Table 3). Locations of metastases overall did not differ except for those of the liver, which showed a higher (P = 0.0230) prevalence in adults than children (Table 3).
Table 3.
Metastatic Disease in Pediatric and Adult Patients With PPGLs
| Characteristics | Pediatric | Adult | P Value |
|---|---|---|---|
| Metastatic diseasea | |||
| Synchronousa | 25.5% (12/47) | 43.2% (82/190) | 0.0019 |
| Nonsynchronousa | 74.5% (35/47) | 56.8% (108/190) | |
| Location of metastases | |||
| Bones | 73.9% (34/46) | 69.9% (128/183) | 0.3690 |
| Lungs | 39.1% (18/46) | 25.7% (47/183) | 0.0540 |
| Liver | 23.9% (11/46) | 40.9% (75/183) | 0.0230 |
| Lymph nodes | 50.0% (23/46) | 45.6% (84/184) | 0.3690 |
Metastatic disease is defined as either synchronous or nonsynchronous depending on findings of metastases (lungs, liver, bones, lymph nodes, or other sites where chromaffin or autonomic progenitors are normally absent) at the time of first diagnosis of disease or at one or more years after first diagnosis, respectively.
There were no significant differences in rates of recurrent disease between patients with cluster 1 and cluster 2 mutations (Table 2). Although the rate of recurrent disease did not differ among hereditary pediatric and adult cases, children with sporadic PPGLs presented more often with recurrent disease (38% vs 7%; P < 0.0001) compared with adults.
Younger vs older adults
Age-related changes in disease presentation
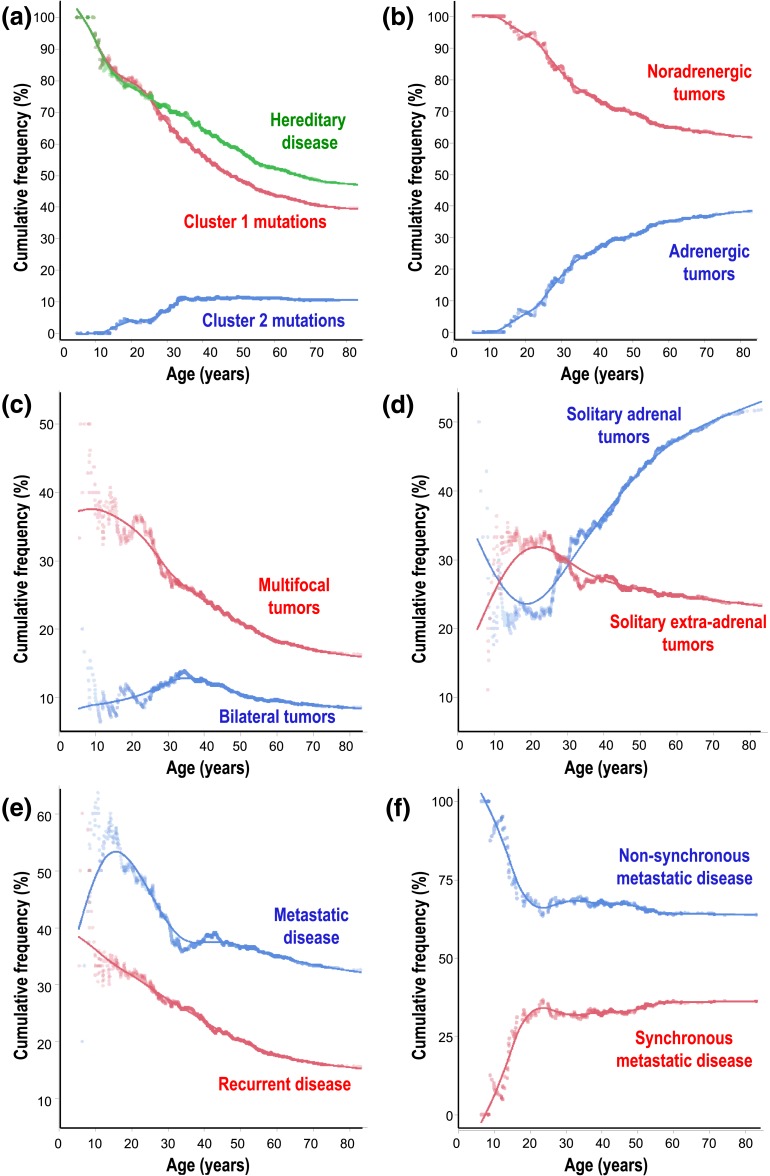
Differences in presentation of disease in children compared with adults for some manifestations also extended into adulthood as reflected by changes in age-related cumulative frequencies of hereditary disease, different tumoral biochemical phenotypes, locations of primary tumors, and development of recurrent or metastatic disease (Fig. 3). Consequently, compared with older adults, younger adults showed many but not all of the same differences in disease presentation observed between children and adults (Supplemental Table 1 (17.9KB, docx) ). In particular, higher prevalence of hereditary disease and noradrenergic, multifocal tumors in children compared with adults and lower prevalence of adrenergic and adrenal tumors in adults compared with children were repeated in younger adults compared with older adults. Age-related differences in cumulative frequencies of adrenergic tumors were also reflected in higher (P < 0.0001) plasma concentrations of plasma metanephrine in older adults than both younger adults and children and higher (P < 0.0061) concentrations of metanephrine in younger adults than children (Fig. 1). In contrast, the higher prevalence of metastatic disease, particularly nonsynchronous metastatic disease, was confined to children. Also, although the prevalence of bilateral tumors showed no overall difference between adults and children, this presentation peaked in young adults, who showed a higher prevalence of bilateral tumors than older adults.
Figure 3.
Cumulative frequencies as a function of age in hereditary disease, including (a) tumors due to cluster 1 vs cluster 2 mutations, (b) noradrenergic vs adrenergic tumors, (c) multifocal vs bilateral adrenal tumors, (d) solitary adrenal vs extra-adrenal tumors, (e) metastatic disease vs recurrent nonmetastatic disease, and (f) nonsynchronous vs synchronous metastatic disease.
Discussion
This study involving a large cohort of pediatric and adult patients with PPGLs outlines a higher prevalence of noradrenergic PPGLs in children than in adults. Furthermore, we establish that the childhood predominance of noradrenergic PPGLs reflects in part the higher prevalence of tumors due to cluster 1 mutations among pediatric compared with adult cases. These observations may reflect different developmental origins of cluster 1 and 2 tumors and are of relevance for personalized management and surveillance programs for both genetically at-risk children and those requiring long-term follow-up after development of PPGLs during childhood.
In a report from 1960, Hume (1) noted that most PPGLs in childhood seemed to secrete predominantly norepinephrine, an observation that until this study has not been confirmed by measurements of metanephrines to establish catecholamine phenotypic features more accurately. In that classic report of Hume (1), other findings of more often extra-adrenal and multifocal locations of PPGLs among pediatric than adult patients have been supported by other series, some of which additionally reported a high prevalence of hereditary and metastatic disease in pediatric patients (4, 15, 17, 20, 27, 28). However, only three of these studies involved comparisons with adult cohorts, and both involved limited patient numbers (4, 15, 17).
The present comparative study involving both adults and children not only confirms a higher prevalence of extra-adrenal, multifocal, metastatic, recurrent, and hereditary PPGLs in children than adults but establishes the link between these phenotypic features to a higher prevalence of noradrenergic and related cluster 1 hereditary tumors in pediatric than adult patients.
Earlier age of presentation of multifocal tumors among patients with cluster 1 gene mutations has been suggested previously to reflect second-hit mutations in embryogenesis before neural crest-derived cells migrate to their final locations (29). Support for the concept of embryological origins of some PPGLs has since been provided by findings of mosaicism involving activating HIF2α somatic mutations in patients with PPGLs and polycythemia (30, 31) as well as identical HIF2α mutations in different tumors from the same patient (32). Furthermore, mosaicism has been reported for VHL somatic mutations (33), again indicating that cluster 1–type mutations can occur early in embryonic development.
In line with the aforementioned concepts, other reports have highlighted the importance of HIF2α in neural crest development; cluster 1 gene mutations, which stabilize HIFs, have thereby been proposed to create an environment favoring survival of chromaffin progenitor cells that lack epinephrine production (8, 34, 35). This proposed developmental pathway for cluster 1 PPGLs thereby offers an explanation for the high prevalence of hereditary and largely noradrenergic cluster 1–type PPGLs in children compared with adults.
Our findings of higher prevalence of metastatic PPGLs in children than adults can also be traced to the higher risk of metastatic disease associated with cluster 1 mutations, particularly SDHB mutations more prevalent in children than adults. Although high prevalence of metastatic disease and SDHB mutations in childhood PPGLs is in agreement with the study of King et al. (28), this finding is not consistent with other studies reporting rates of malignant PPGLs among children from 2.4% to 19% (16, 17, 36, 37). This discrepancy may in part reflect referral bias involving high numbers of patients with metastatic disease at the specialist centers participating in the current study. However, lower rates of childhood metastatic PPGLs reported in other studies may also be partly explained by insufficient patient follow-up (38). As indicated by our series, and particularly important for pediatric cases of PPGLs, occurrence of metastatic disease was most often not synchronous with presentation of primary tumors. Most often, metastatic disease was diagnosed in adulthood after initial presentation of the primary tumor in childhood at outside centers, with usually little or no periodic follow-up to check for disease recurrence. Without such follow-up, recurrent or metastatic progression may be underestimated.
Based on findings of a 12-year lag in positive biochemical results indicative of PPGLs to actual diagnosis of the tumors (22), it seems likely that many cases of childhood PPGLs are not detected until adulthood. Our observations that young adults showed similar presentations of PPGLs as children further suggests that the 12.7% prevalence of childhood PPGLs in the current study likely underestimates the true prevalence. Thus, in addition to a need for improved follow-up of childhood PPGLs, there is also likely a need for improved recognition of the tumors by pediatricians.
Our study, like all previous studies on pediatric PPGLs, has limitations associated with potential for referral bias, retrospective nature, and incomplete follow-up and genetic testing. Referral bias might be expected to lead to higher proportions of hereditary, metastatic, and recurrent disease, but this should not compromise comparisons of adult and pediatric populations. In contrast, incomplete follow-up and genetic testing would be expected to result in lower proportions of hereditary, metastatic, and recurrent disease. Although fully prospective studies could minimize these limitations, such studies would require a long time frame (>10 years) and considerable flexibility in meeting demands of accelerating scientific advances (e.g., new mutations and technologies).
Despite the aforementioned limitations, our study not only supports previous findings, but also builds on these to advance patient management. The higher prevalence of hereditary, malignant, and recurrent disease and the differences in the sites of primary tumors and metastases in children compared with adults, in particular, highlights the importance in pediatric medicine of following Endocrine Society clinical practice guidelines recommending that patients with PPGLs be managed by multidisciplinary teams at specialist centers with appropriate expertise (39). All children with PPGLs should undergo genetic testing with choice and interpretation of testing dictated by family history or presence of syndromic and clinical features. Such features include the biochemical phenotype of the tumor, which can also indicate adrenal vs extra-adrenal locations. Although computed tomography is recommended by Endocrine Society guidelines as the method of first choice for locating PPGLs (39), high–signal intensity T2-weighted magnetic resonance imaging is the more appropriate modality for the pediatric population with focus on the detection of extra-adrenal tumors, especially those in unusual locations.
For postoperative care, the high risk of recurrent or metastatic disease associated with childhood PPGLs mandates not only diligent follow-up, but also appropriate transition from pediatric into adult care. Periodic biochemical surveillance for PPGLs is recommended for all mutation carriers, regardless of disease history, but is particularly important starting at an early age (i.e., five years) in those children harboring cluster 1 mutations. Surveillance programs for PPGLs should be tailored according to the mutation and, where indicated, should include screening for other tumors associated with mutations (e.g., retinal hemiangioblastomas in VHL mutation carriers), again highlighting the importance of multidisciplinary team approaches. For children with mutations of SDHx genes, biochemical testing should include measurements of plasma methoxytyramine, also important as a biomarker for extra-adrenal and metastatic disease (39, 40); this is particularly important for children carrying SDHB gene mutations, who carry a high risk of malignancy. Because large tumor size is a risk factor for metastatic disease, earlier detection through regular surveillance programs may offer the best means to avoid subsequent development of metastatic disease.
Conclusion
The higher prevalence of hereditary, extra-adrenal, and metastatic PPGLs in children than adults represent interrelated features that in part reflect the lower age of disease presentation of noradrenergic cluster 1 PPGLs than adrenergic cluster 2 tumors. The differences in disease presentation are important to consider in both presurgical and postsurgical management of childhood PPGLs as well as during routine surveillance of at-risk children.
Acknowledgments
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (EI855/1/2; to C.P., M.P., and G.E), the European Union Seventh Framework Program (FP7/2007-2013) under Grant 259735 (ENSAT CANCER; to M.P., F.B., H.J.L.M.T., M.R., R.O.S., and G.E.), the Fondo de Investigaciones Sanitarias (PI14/00240; to M.R.), and the National Institutes of Health (to B.H., M.L., C.A.S., and K.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- HIF2α
- hypoxia-inducible factor 2 alpha
- HRAS
- Harvey rat sarcoma viral oncogene homolog
- MAX
- MYC-associated factor X
- NF1
- neurofibromatosis type 1
- PPGL
- paraganglioma
- RET
- rearranged during transfection
- TMEM127
- transmembrane protein 127
- VHL
- von Hippel-Lindau.
References
- 1.Hume DM. Pheochromocytoma in the adult and in the child. Am J Surg. 1960;99:458–496. [DOI] [PubMed] [Google Scholar]
- 2.Barontini M, Levin G, Sanso G. Characteristics of pheochromocytoma in a 4- to 20-year-old population. Ann N Y Acad Sci. 2006;1073:30–37. [DOI] [PubMed] [Google Scholar]
- 3.Robles JF, Mercado Asis LB, Pacak K. Pheochromocytoma: unmasking the chameleon. In: Koch CA, Chrousos GP, eds. Endocrine Hypertension: Underlying Mechanisms and Therapy. New York, NY: Humana Press; 2012:123–148. [Google Scholar]
- 4.Fonkalsrud EW. Pheochromocytoma in childhood. Prog Pediatr Surg. 1991;26:103–111. [DOI] [PubMed] [Google Scholar]
- 5.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peçzkowska M, Szmigielski C, Eng C; Freiburg-Warsaw-Columbus Pheochromocytoma Study Group . Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459–1466. [DOI] [PubMed] [Google Scholar]
- 6.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44(5):328–333. [DOI] [PubMed] [Google Scholar]
- 7.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nosé V, Li C, Stiles CDA. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Jiménez E, Gómez-López G, Leandro-García LJ, Muñoz I, Schiavi F, Montero-Conde C, de Cubas AA, Ramires R, Landa I, Leskelä S, Maliszewska A, Inglada-Pérez L, de la Vega L, Rodríguez-Antona C, Letón R, Bernal C, de Campos JM, Diez-Tascón C, Fraga MF, Boullosa C, Pisano DG, Opocher G, Robledo M, Cascón A. Research resource: transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24(12):2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favier J, Gimenez-Roqueplo AP. Pheochromocytomas: the (pseudo)-hypoxia hypothesis. Best Pract Res Clin Endocrinol Metab. 2010;24(6):957–968. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(10):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crona J, Delgado Verdugo A, Maharjan R, Stålberg P, Granberg D, Hellman P, Björklund P. Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J Clin Endocrinol Metab. 2013;98(7):E1266–E1271. [DOI] [PubMed] [Google Scholar]
- 13.Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108–119. [DOI] [PubMed] [Google Scholar]
- 14.Qin N, de Cubas AA, Garcia-Martin R, Richter S, Peitzsch M, Menschikowski M, Lenders JW, Timmers HJ, Mannelli M, Opocher G, Economopoulou M, Siegert G, Chavakis T, Pacak K, Robledo M, Eisenhofer G. Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: insights from MYC-associated factor X. Int J Cancer. 2014;135(9):2054–2064. [DOI] [PubMed] [Google Scholar]
- 15.Cascón A, Inglada-Pérez L, Comino-Méndez I, de Cubas AA, Letón R, Mora J, Marazuela M, Galofré JC, Quesada-Charneco M, Robledo M. Genetics of pheochromocytoma and paraganglioma in Spanish pediatric patients. Endocr Relat Cancer. 2013;20(3):L1–L6. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman BH, Telander RL, van Heerden JA, Zimmerman D, Sheps SG, Dawson B. Pheochromocytoma in the pediatric age group: current status. J Pediatr Surg. 1983;18(6):879–884. [DOI] [PubMed] [Google Scholar]
- 17.Stackpole RH, Melicow MM, Uson AC. Pheochromocytoma in children. J Pediatr. 1963;63:315–330. [DOI] [PubMed] [Google Scholar]
- 18.Caty MG, Coran AG, Geagen M, Thompson NW. Current diagnosis and treatment of pheochromocytoma in children. Experience with 22 consecutive tumors in 14 patients. Arch Surg. 1990;125(8):978–981. [DOI] [PubMed] [Google Scholar]
- 19.Ciftci AO, Tanyel FC, Senocak ME, Büyükpamukçu N. Pheochromocytoma in children. J Pediatr Surg. 2001;36(3):447–452. [DOI] [PubMed] [Google Scholar]
- 20.Bausch B, Wellner U, Bausch D, Schiavi F, Barontini M, Sanso G, Walz MK, Peczkowska M, Weryha G, Dall’igna P, Cecchetto G, Bisogno G, Moeller LC, Bockenhauer D, Patocs A, Rácz K, Zabolotnyi D, Yaremchuk S, Dzivite-Krisane I, Castinetti F, Taieb D, Malinoc A, von Dobschuetz E, Roessler J, Schmid KW, Opocher G, Eng C, Neumann HP. Long-term prognosis of patients with pediatric pheochromocytoma. Endocr Relat Cancer. 2013;21(1):17–25. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhofer G, Timmers HJ, Lenders JW, Bornstein SR, Tiebel O, Mannelli M, King KS, Vocke CD, Linehan WM, Bratslavsky G, Pacak K. Age at diagnosis of pheochromocytoma differs according to catecholamine phenotype and tumor location. J Clin Endocrinol Metab. 2011;96(2):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson SW, Yoon S, Baker T, Prince LK, Oliver D, Abbott KC. Longitudinal plasma metanephrines preceding pheochromocytoma diagnosis: a retrospective case-control serum repository study. Eur J Endocrinol. 2015;174(3):289–295. [DOI] [PubMed] [Google Scholar]
- 23.Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13(1):2–7. [DOI] [PubMed] [Google Scholar]
- 24.Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1993;39(1):97–103. [PubMed] [Google Scholar]
- 25.Peitzsch M, Prejbisz A, Kroiß M, Beuschlein F, Arlt W, Januszewicz A, Siegert G, Eisenhofer G. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann Clin Biochem. 2013;50(Pt 2):147–155. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, Adams KT, Pacak K. Biochemical and clinical manifestations of dopamine-producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90(4):2068–2075. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan J, Groshong T, Tobias JD. Presenting signs and symptoms of pheochromocytoma in pediatric-aged patients. Clin Pediatr (Phila). 2005;44(8):715–719. [DOI] [PubMed] [Google Scholar]
- 28.King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, Zacharin M, Wesley R, Lodish M, Raygada M, Gimenez-Roqueplo AP, McCormack S, Eisenhofer G, Milosevic D, Kebebew E, Stratakis CA, Pacak K. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29(31):4137–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter S, Klink B, Nacke B, de Cubas AA, Mangelis A, Rapizzi E, Meinhardt M, Skondra C, Mannelli M, Robledo M, Menschikowski M, Eisenhofer G. Epigenetic mutation of the succinate dehydrogenase C promoter in a patient with two paragangliomas. J Clin Endocrinol Metab. 2016;101(2):359–363. [DOI] [PubMed] [Google Scholar]
- 30.Buffet A, Smati S, Mansuy L, Ménara M, Lebras M, Heymann MF, Simian C, Favier J, Murat A, Cariou B, Gimenez-Roqueplo AP. Mosaicism in HIF2A-related polycythemia-paraganglioma syndrome. J Clin Endocrinol Metab. 2014;99(2):E369–E373. [DOI] [PubMed] [Google Scholar]
- 31.Yang C, Hong CS, Prchal JT, Balint MT, Pacak K, Zhuang Z. Somatic mosaicism of EPAS1 mutations in the syndrome of paraganglioma and somatostatinoma associated with polycythemia. Hum Gen Var. 2015;10(2):15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murgia A, Martella M, Vinanzi C, Polli R, Perilongo G, Opocher G. Somatic mosaicism in von Hippel-Lindau Disease. Hum Mutat. 2000;15(1):114. [DOI] [PubMed] [Google Scholar]
- 33.Comino-Méndez I, de Cubas AA, Bernal C, Álvarez-Escolá C, Sánchez-Malo C, Ramírez-Tortosa CL, Pedrinaci S, Rapizzi E, Ercolino T, Bernini G, Bacca A, Letón R, Pita G, Alonso MR, Leandro-García LJ, Gómez-Graña A, Inglada-Pérez L, Mancikova V, Rodríguez-Antona C, Mannelli M, Robledo M, Cascón A. Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum Mol Genet. 2013;22(11):2169–2176. [DOI] [PubMed] [Google Scholar]
- 34. doi: 10.1016/B978-0-12-411512-5.00014-2. Richter S, Qin N, Pacak K, Eisenhofer G. Role of hypoxia and HIF2αG. Role of hypoxia and sympathoadrenal cell lineage and chromaffin cell tumors with distinct catecholamine phenotypic features. Adv Pharmacol. 2013;68:285–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnichon N, Vescovo L, Amar L, Libé R, de Reynies A, Venisse A, Jouanno E, Laurendeau I, Parfait B, Bertherat J, Plouin PF, Jeunemaitre X, Favier J, Gimenez-Roqueplo AP. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–3985. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RE, O’Neill JA Jr, Holcomb GW III, Morgan WM III, Neblett WW III, Oates JA, Brown N, Nadeau J, Smith B, Page DL, Abumrad NN, Scott HW Jr. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy VS, O’Neill JA Jr, Holcomb GW III, Neblett WW III, Pietsch JB, Morgan WM III, Goldstein RE. Twenty-five-year surgical experience with pheochromocytoma in children. Am Surg. 2000;66(12):1085–1092. [PubMed] [Google Scholar]
- 38.Amar L, Lussey-Lepoutre C, Lenders JW, Djadi-Prat J, Plouin PF, Steichen O. Management of endocrine disease: Recurrence or new tumors after complete resection of pheochromocytomas and paragangliomas: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(4):R135–R145. [DOI] [PubMed] [Google Scholar]
- 39.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society . Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, Mannelli M, Linehan WM, Adams K, Timmers HJ, Pacak K. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]