Abstract
Context:
Approximately half of patients with primary aldosteronism (PA) have clinically evident disease according to clinical (hypertension) and/or laboratory (aldosterone and renin levels) findings but do not have nodules detectable in routine cross-sectional imaging. However, the detailed histopathologic, steroidogenic, and pathobiological features of cross-sectional image–negative PA are controversial.
Objective:
To examine histopathology, steroidogenic enzyme expression, and aldosterone-driver gene somatic mutation status in cross-sectional image–negative hyperaldosteronism.
Methods:
Twenty-five cross-sectional image–negative cases were retrospectively reviewed. In situ adrenal aldosterone production capacity was determined using immunohistochemistry (IHC) of steroidogenic enzymes. Aldosterone-driver gene somatic mutation status (ATP1A1, ATP2B3, CACNA1D, and KCNJ5) was determined in the CYP11B2 immunopositive areas [n = 35; micronodule, n = 32; zona glomerulosa (ZG), n = 3] using next-generation sequencing after macrodissection.
Results:
Cases were classified as multiple adrenocortical micronodules (MN; n = 13) or diffuse hyperplasia (DH) of ZG (n = 12) based upon histopathological evaluation and CYP11B2 IHC. Aldosterone-driver gene somatic mutations were detected in 21 of 26 (81%) of CYP11B2-positive cortical micronodules in MN; 17 (65%) mutations were in CACNA1D, 2 (8%) in KCNJ5, and 1 each (4% each) in ATP1A1 and ATP2B. One of 6 (17%) of nodules in DH harbored somatic aldosterone-driver gene mutations (CACNA1D); however, no mutations were detected in CYP11B2-positive nonnodular DH areas.
Conclusion:
Morphologic evaluation and CYP11B2 IHC enabled the classification of cross-sectional image–negative hyperaldosteronism into MN and DH. Somatic mutations driving aldosterone overproduction are common in micronodules of MN, suggesting a histological entity possibly related to aldosterone-producing cell cluster development.
We reviewed CYP11B2 immunolocalization and aldosterone-driver gene somatic mutation status of cross-sectional image–negative hyperaldosteronism and developed histological classification.
Primary aldosteronism (PA) is a major cause of secondary hypertension, responsible for 5% to 10% of all hypertension cases (1–3). PA has been classified into aldosterone-producing adenoma (APA) and idiopathic hyperaldosteronism (IHA) on the basis of histopathological findings (1). PA has also been clinically classified into cross-sectional image–positive and –negative PA (4, 5). Cross-sectional image–positive PA includes APA and aldosterone-producing adrenocortical carcinoma, whereas image-negative PA includes microAPA, IHA, and other non-APA lesions, including unilateral multiple adrenocortical micronodules (UMN) and unilateral adrenocortical hyperplasia (6, 7). Recently, the combined use of cross-sectional imaging and adrenal venous sampling (AVS) has enhanced the localization of small cortical lesions, but lesions smaller than 7 mm in diameter remain difficult to detect (8). In addition, diagnosis of APA-negative, “unilateral” PA has become more frequent in clinical practice as a result of the increased use of AVS (9, 10). At this juncture, approximately 30% of PA cases are considered cross-sectional image negative (8). Two-thirds of patients with PA lesions have been histologically diagnosed as having adrenocortical hyperplasia (1–3, 7), but CYP11B2 expression has not been examined.
Nanba et al. (11) previously classified PA into 2 different groups, i.e., APA and non-APA, in both bilateral and unilateral cases, based upon CYP11B2 immunohistochemistry (IHC) results, but a detailed histopathological analysis of non-APA lesions was not reported.
Somatic mutations of several genes, including KCNJ5, ATP1A1, ATP2B3, and CACNA1D, have been reported to cause aldosterone overproduction in APA (12–14). KCNJ5 somatic mutations have been frequently detected in APA cases, and, in particular, in 60% to 70% of Japanese APA cases (15). In contrast, in a cohort of histologically normal adrenal glands from patients with unknown blood pressure status who underwent renal transplantation, Nishimoto et al. (16) reported that CACNA1D somatic mutations were detected in 26% of aldosterone-producing cell clusters (APCCs), whereas no KCNJ5 mutations were identified. However, histopathological classification and somatic mutation status of aldosterone-driver genes of cross-sectional image–negative cases have not been well characterized.
Therefore, in this study, we retrospectively reviewed the histopathological features, IHC-based localization of CYP11B2 and other steroidogenic enzymes expression, and somatic mutation status of aldosterone-regulating genes from a cohort of cross-sectional image–negative, non-APA, aldosterone-secreting glands from patients with hypertension to develop a more comprehensive classification and elucidate the pathobiology of these adrenocortical disorders.
Materials and Methods
Study cases
Resected adrenals from 25 consecutively available cases (21 cases from Tohoku University Hospital from 2005 to 2015, and 4 cases from Asahikawa Red Cross Hospital from 2014 to 2015) that met the following criteria were included in our retrospective study. All cases had hypertension associated with aldosterone hypersecretion based upon laboratory data (aldosterone and renin levels). All cases demonstrated improvement in their hypertensive status based on lowered blood pressure after surgery and decreased number of antihypertensive medications. Two confirmation tests were used: a captopril loading test (Tohoku University) and a flosemide loading test followed by walking (Asahikawa Red Cross Hospital). All of the cases examined were negative by cross-sectional imaging. On the basis of histologic examination [hematoxylin and eosin (H&E) and CYP11B2 IHC], these cases did not have microAPAs based on the preservation of adrenal zonation patterns and the absence of histologically consistent neoplastic progression such as nuclear atypia, fibrous capsule, and well circumscribed margins.
Laterality of all cases was clinically categorized by the results of AVS. We used the criteria of laterality determination, using a postadrenocorticotropic hormone stimulation cutoff value of 2.6 of the lateralization index (17).
Clinicopathological characteristics of these 25 cases are summarized in Table 1. Six nonpathological adrenal glands (NA) obtained from surgery for renal cell carcinoma and other nonendocrine/hypertension-related entities were used as controls. The research protocol for this study was institutional review board approved at both Tohoku University School of Medicine and Asahikawa Red Cross Hospital, Japan.
Table 1.
Summary of Clinicopathological Characteristics of 25 Cases
| No. | Age | Sex | Laterality | Post B2 Histological Dx | PAC (ng/dL) | PRA | ARR | Captopril Challenge ARRa | Lateralization Index in AVS Before Cosyntropin Loading | Lateralization Index in AVS After Cosyntropin Loading | Serum K | Preoperation Status |
Postoperation Status |
Spironolactone Body | Paradoxical Hyperplasia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | SBP | DBP | No. of Antihypertensive Drugs | SBP | DBP | No. of Antihypertensive Drugs | Nodule | ZG | ||||||||||||
| 1 | 25 | M | Bilateral | DH | DH | 11.4 | 0.8 | 14.3 | 2.1 | 2.77 | 1.21 | 4.4 | 122 | 62 | 7 | 117 | 71 | 1 | - | - | |
| 2 | 27 | F | Bilateral | DH | DH | 44.6 | 2.7 | 16.5 | 5.1 | 1.5 | 1.92 | 4.3 | 112 | 68 | 1 | 120 | 82 | 0 | - | - | |
| 3 | 32 | F | Bilateral | DH | DH | 13.9 | 0.6 | 23.2 | 1.8 | 2.19 | 1.06 | 3.4 | 113 | 67 | 3 | 115 | 87 | 0 | - | - | |
| 4 | 43 | F | Bilateral | DH | DH | 38.2 | 1.8 | 21.2 | 4.3 | 1.51 | 1.35 | 4 | 120 | 82 | 5 | 111 | 73 | 1 | + | + | |
| 5 | 44 | M | Bilateral | DH | DH | 14.8 | 0.4 | 37 | 18.2 | 7.38 | 1.23 | 4.7 | 119 | 74 | 4 | 120 | 69 | 0 | + | + | |
| 6 | 44 | F | Bilateral | DH | DH | 19.9 | 2.3 | 8.7 | 5.2 | 1.33 | 1.03 | 3.4 | 119 | 64 | 3 | 118 | 82 | 1 | - | - | |
| 7 | 44 | F | Bilateral | DH | DH | 19.7 | 0.4 | 49.3 | 10.7 | 1.71 | 1.49 | 4.7 | 111 | 76 | 2 | 127 | 82 | 0 | - | - | |
| 8 | 42 | M | Unilateral | Not resected | DH | 17.4 | 0.7 | 24.9 | 9.1 | 1.41 | 2.71 | 4.8 | 120 | 70 | 8 | 117 | 84 | 0 | - | - | |
| 9 | 49 | M | Unilateral | Not resected | DH | 47 | 0.1 | 470 | 171 | 9.73 | 18.7 | 3.1 | 118 | 88 | 4 | 110 | 74 | 0 | - | + | |
| 10 | 51 | M | Bilateral | Not resected | DH | 15.6 | 0.7 | 22.3 | 6 | 2.22 | 1.67 | 3.5 | 122 | 84 | 3 | 103 | 70 | 0 | + | - | |
| 11 | 61 | M | Bilateral | Not resected | DH | 13 | 0.5 | 26 | 46.5 | 6.49 | 2.47 | 4.3 | 119 | 69 | 2 | 118 | 74 | 0 | + | - | |
| 12 | 47 | F | Bilateral | DH | Not resected | 16.9 | 0.6 | 28.2 | 46.3 | 3.77a | 1.52a | 3.5 | 99 | 66 | 3 | 98 | 81 | 0 | - | - | |
| 13 | 32 | M | Bilateral | MN | MN | 7.2 | 0.3 | 24 | 5.2 | 2.18 | 1.22 | 4.1 | 121 | 86 | 5 | 119 | 78 | 1 | - | - | Weak/weak |
| 14 | 36 | F | Bilateral | MN | MN | 17.4 | 0.5 | 34.8 | 25.7 | 1.54 | 2.03 | 4.8 | 120 | 86 | 2 | 129 | 88 | 1 | - | - | +/+ |
| 15 | 37 | F | Bilateral | MN | MN | 11.3 | 0.4 | 28.3 | 39.5 | 1.26 | 1.33 | 4.4 | 115 | 59 | 4 | 119 | 76 | 1 | + | - | +/+ |
| 16 | 40 | M | Bilateral | MN | MN | 13.8 | 0.1 | 138 | 35.7 | 1.63 | 1.51 | 3.9 | 126 | 81 | 6 | 126 | 86 | 1 | + | - | −/− |
| 17 | 47 | F | Bilateral | MN | MN | 11 | 0.2 | 55 | 48.5 | 2.47 | 1.61 | 3.5 | 126 | 88 | 4 | 100 | 73 | 1 | - | - | +/+ |
| 18 | 30 | M | Bilateral | Not resected | MN | 14.4 | 1.6 | 9 | 4.6 | 4.87 | 1.43 | 3.7 | 143 | 88 | 6 | 139 | 84 | 3 | + | - | Weak |
| 19 | 43 | F | Unilateral | Not resected | MN | 15.5 | 0.8 | 19.4 | (1.3/47.7) 36.7b | 3.04 | 7.95 | 3.3 | 125 | 97 | 1 | 123 | 98 | 0 | - | - | + |
| 20 | 49 | F | Bilateral | Not resected | MN | 18.4 | 0.7 | 26.3 | N/A | 2.67 | 1.71 | 3.3 | 136 | 84 | 1 | 133 | 89 | 0 | - | - | Weak |
| 21 | 61 | F | Unilateral | Not resected | MN | 10.3 | 0.4 | 25.8 | (0.9/52.8) 58.6b | 5.27 | 46.8 | 4.2 | 164 | 103 | 1 | 146 | 83 | 0 | - | - | + |
| 22 | 61 | F | Unilateral | Not resected | MN | 36.2 | 0.3 | 120.7 | (0.3/36.2) 120.7b | 3.41 | 10.5 | 3.9 | 158 | 98 | 1 | 156 | 90 | 0 | - | - | + |
| 23 | 69 | M | Bilateral | Not resected | MN | 9.9 | 0.4 | 24.8 | 12.3 | 2.53 | 1.63 | 3.9 | 123 | 84 | 4 | 144 | 87 | 2 | + | - | + |
| 24 | 69 | F | Unilateral | Not resected | MN | 23.5 | 0.2 | 117.5 | 28.7 | 11.1 | 4.91 | 4.5 | 124 | 66 | 2 | 116 | 63 | 0 | - | - | + |
| 25 | 36 | M | Unilateral | MN | Not resected | 20.2 | 0.4 | 50.5 | 15.2 | 3.19 | 4.6 | 4 | 116 | 70 | 5 | 133 | 95 | 0 | - | - | + |
Twenty-five consecutive cases with available resected adrenals from 2005 to 2015 that met the following criteria were retrospectively selected from 2 institutions: clinical hypertension associated with aldosterone hypersecretion, improvement of hypertensive status according to blood pressure, and the number of antihypertensive drugs before and after surgery. Twenty-one cases were from Tohoku University Hospital (from 2005 to 2015), and 4 cases from Asahikawa Red Cross Hospital (from 2014 to 2015). Biological PA confirmation was determined by the captopril loading test (Tohoku University), and the flosemide loading test followed by walking (Asahikawa Red Cross Hospital). All cases were negative according to cross-sectional imaging. No cases had microAPAs (as assessed by histology and CYP11B2 IHC). All patients underwent unilateral or bilateral adrenal resection for persistent hypertension resistant to multiple antihypertensive medications, cardiovascular complications, juvenile hypertension, or some adrenal gland surface irregularities according to cross-sectional imaging. Lateralization of all cases was clinically categorized by AVS, using the cutoff value of 2.6 of lateralization index after adrenocorticotropic hormone loading (17). Twenty-five cases were classified as ZG hyperplasia, similar to that seen adjacent to APAs. As in APA cases, ZGs are morphologically hyperplastic but negative for CYP11B2, suggesting the inability to produce aldosterone. In addition, ZG paradoxical hyperplasia was generally less pronounced in MN than APA. Adjacent ZGs in some MN cases demonstrated even atrophic changes. Spironolactone bodies were detected both in nodular and nonnodular cortical cells in DH, but only in cortical cells of micronodules in MN, and these cells harboring spironolactone bodies were positive for CYP11B2.
Abbreviations: ARR, aldosterone renin ratio; DBP, diatolic blood pressure; Dx, diagnosis; F, female; M, male; N/A, not available; PAC, plasma aldosterone concentration; PRA, plasma renin activity; SBP, systolic blood pressure.
Canulation failure.
PRA/PAC (ARR) value 2 hours after flosemide loading followed by walk.
Immunohistochemical analysis
All surgical adrenal specimens were submitted for histopathological examination by fixation in 10% buffered formalin and embedded in paraffin. An average of 6 H&E-stained tissue sections per case (depending on adrenal size), prepared from formalin-fixed, paraffin-embedded (FFPE) blocks, were carefully reviewed. After review of all tissue sections, CYP11B2 IHC was performed (18). Other steroidogenic enzymes were also immunostained in representative sections following the H&E and CYP11B2 assessment. The thickness of the tissue sections was set at 3 μm in all cases. IHC protocols used in this study are summarized in Supplemental Table 1 (11.8KB, docx) . The immunoreactivity of steroidogenic enzymes was semiquantified using H-score analysis [= Σ (number of cells in whole target area × score 1+, 2+, 3+) / 500 cells] (19, 20). When there was interobserver variability between Y.Y. and Y.N. for the relative intensity of the positive cells, the slides were reevaluated using double-headed light microscopy by these 2 observers, and the relative immune intensity of the cases was subsequently determined by this light microscopic evaluation.
We also analyzed the CYP11B2-positive areas as a percentage of the total adrenal cortex tissue within these sections using digital analysis software (HALO Area Quantification, version 1.0; Leica, Buffalo Grove, IL) as follows: CYP11B2-positive area (%) = CYP11B2 positive / entire adrenocortical area (μm2). All immunostained tissue sections were digitally scanned and captured using Image Scope AT2 (Leica) for analysis.
DNA extraction
As described previously, because unilateral resected non-APA PA cases have been frequently encountered, we selected 7 unilateral cases for analysis of somatic mutation status of aldosterone-driver genes, on the basis of the results of AVS. We prepared serial tissue sections of these cases as follows for DNA isolation from CYP11B2-positive areas: H&E (3 μm), CYP11B2 (3 μm), 8 unstained sections at 10-μm thickness for DNA extraction, CYP11B2 (3 μm), H&E (3 μm). We macrodissected CYP11B2-positive and -negative areas from unstained sections (using the adjacent CYP11B2 IHC slides as a guide) under a dissecting microscope using a scalpel. Genomic DNA was extracted using the AllPrepDNA/RNA FFPE Kit (QIAGEN, Germantown, MD), as described (16).
Next-generation sequencing
Genomic DNA isolated from both CYP11B2-positive (n = 35; micronodule, n = 32; ZG, n = 3) and CYP11B2-negative (n = 7) areas was subjected to targeted next-generation sequencing (NGS) to identify somatic mutations in aldosterone-driver genes using an updated version of our previous approach (16). Specifically, sequencing of CYP11B2-positive areas was performed using the Ion AmpliSeq Library kit 2.0 (Thermo Fisher Scientific, Waltham, MA) with barcode incorporation and 2 custom Ion Ampliseq multiplex polymerase chain reaction panels (APAv1 and APAv2; Thermo Fisher Scientific) targeting essentially the same genes but with different amplicons to distinguish true mutations from sequencing artifacts. These panels targeted genes previously reported as recurrently somatically mutated in APA (full coding sequence of KCNJ5, CACNA1D, ATP1A1, and ATP2B3 on both panels). Of particular importance, the individual amplicons covering these genes are different between the 2 panels. CYP11B2-negative regions from each sample were processed as described previously, except that they were only sequenced on the APAv1 panel. Templates were prepared using the Ion PI Hi-Q OT2 200 Kit sequencer (Life Technologies, Carlsbad, CA), according to the manufacturer's instructions. NGS was performed using the Ion PI Hi-Q Sequencing 200Kit and Ion PI Chip v3 on the Ion Torrent Proton sequencer (Life Technologies), according to the manufacturer's instructions.
Data analysis was performed essentially as described previously (16), but with additional stringency and incorporation of results from both panels. For CYP11B2-positive specimens, variants culled by default proton low-stringency somatic variant filtering were further filtered to identify potential driving somatic mutations by removing synonymous or noncoding variants, those with flow-corrected read depth <300 in both panels, flow-corrected variant allele–containing reads <40 in both panels, variant allele frequencies (flow-corrected variant allele–containing reads / flow-corrected read depth) <0.05 in both panels, flow-corrected variant allele calling forward to reverse read ratio <0.2 or >5 in both panels, or indels within homopolymer runs ≥4. Variants occurring exclusively in reads containing other variants (single nucleotide variants or indels) or those occurring in the last mapped base of a read were excluded in this study. Variants passing default proton low stringency somatic variant filtering in the matched normal sample or any other normal sample were considered germline/artifacts and also excluded. Last, all of the variants that passed these filtering criteria in CYP11B2-positive samples were visually confirmed in Integrative Genomics Viewer (IGV; Broad Institute, Cambridge, MA; https://www.broadinstitute.org/igv/), along with the paired normal sample to confirm lack of substantial read support for the culled variant.
Statistical analysis
The comparison of immunoreactivity (H-score) of each steroidogenic enzyme examined in this study, the percentage of CYP11B2-positive area, and clinicopathological factors among these cases were analyzed by Mann-Whitney U test. P values < 0.05 were considered significant.
Results
Cross-sectional image–negative hypertensive adrenals associated with aldosterone hypersecretion were first tentatively classified into the following 2 histological subtypes: multiple adrenocortical micronodules (MN) and diffuse hyperplasia (DH) of ZG, on the basis of the intra-adrenal patterns of CYP11B2 immunoreactivity as described later (Fig. 1). Each histological subtype also had a corresponding unilateral and bilateral counterpart, i.e., unilateral MN (UMN) and bilateral MN, unilateral DH, and bilateral DH (BDH).
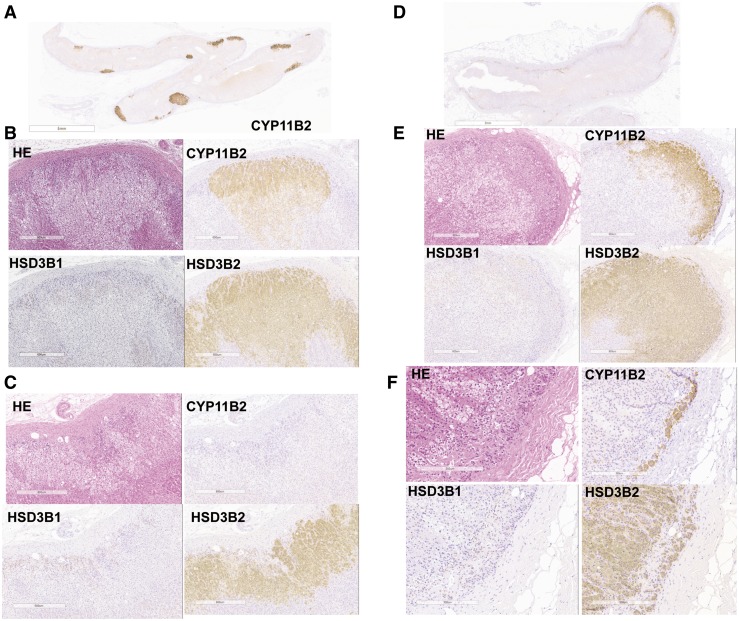
Figure 1.
Representative histological illustrations of 2 histological subtypes detected in adrenals from image-negative hypertension associated with aldosterone hypersecretion. (A) MN, low-power field. (B) Micronodules in MN, high-power field. (C) Adjacent adrenal cortex in MN, high-power field. Multiple CYP11B2-positive adrenocortical micronodules were detected, whereas CYP11B2-positive cells were rarely detected in adjacent ZG. HSD3B1 immunoreactivity was weakly detected in cortical micronodules and moderately positive in adjacent ZG, representing “paradoxical hyperplasia.” HSD3B2 immunoreactivity was markedly detected in adrenocortical nodules but weakly positive or absent in adjacent ZG. CYP11B2 immunoreactivity of adrenocortical nodules in MN was marked in the subcapsular area and weak in the outer ZF, gradually diminishing from the capsule, suggesting that zonation of adrenal cortex is still preserved in these cortical nodules. (D) DH (nodular pattern), low-power field. (E) Micronodules in DH, high-power field. (F) Adjacent adrenal cortex in DH, high-power field. CYP11B2-positive cells were diffusely detected in hyperplastic ZG along with multiple CYP11B2-positive cortical micronodules. HSD3B1 immunoreactivity was very weak or absent both in cortical micronodules and adjacent ZG, whereas HSD3B2 was present in both cortical micronodules and adjacent ZG. HE, hematoxylin and eosin.
MN
MN was defined as the presence of CYP11B2-positive cortical micronodules located in the CYP11B2-negative ZG [Fig. 1(A–C)]. Immunolocalization patterns of steroidogenic enzyme in these nodules were generally negative for CYP11B1 and CYP17A1 and positive for HSD3Bs (predominantly HSD3B2 and HSD3B1 were weak). The adjacent ZGs were associated with “paradoxical hyperplasia” (21), that is, they were histologically hyperplastic but negative for CYP11B2 and HSD3Bs (HSD3B1 weakly positive). The ZGs of adjacent adrenals in MN cases demonstrated various degrees of paradoxical hyperplasia, as was reported previously in APA cases; they were morphologically hyperplastic but negative for CYP11B2 and HSD3B2. However, this histological change in ZGs was generally less pronounced in MN than APA. Adjacent ZGs in some MN cases demonstrated even atrophic changes. Spironolactone bodies were histologically identified only in the CYP11B2-positive cortical micronodules in the cases treated with spironolactone before surgery.
DH
DH was defined as histologically diffuse hyperplastic CYP11B2-positive ZG that harbored CYP11B2-positive adrenocortical micronodules [Fig. 1(D–F)]. The cortical micronodules were not necessarily readily recognizable in all of the sections. DH was further subclassified into the following 2 histologically distinctive subtypes on the basis of the presence or absence of micronodules: DH, nodular pattern, and DH, nonnodular pattern. Spironolactone bodies were recognized both in the CYP11B2-positive hyperplastic ZGs and cortical micronodules. HSD3B2 immunoreactivity was marked in hyperplastic ZG.
Laterality of the lesions
Among the 25 cases examined in this study, 7 and 18 were clinically diagnosed as unilateral and bilateral, respectively, according to the results of AVS, based on the criteria of the cutoff value as lateralization index of 2.6 (17). The unilateral cases were further subclassified into 5 UMN and 2 unilateral DH, and the bilateral cases into 8 bilateral MN (MN/MN) and 10 BDH (DH/DH) (Table 1). All of the resected bilateral cases demonstrated similar pathological features in both right and left adrenals.
Steroidogenic enzyme IHC
CYP11B2
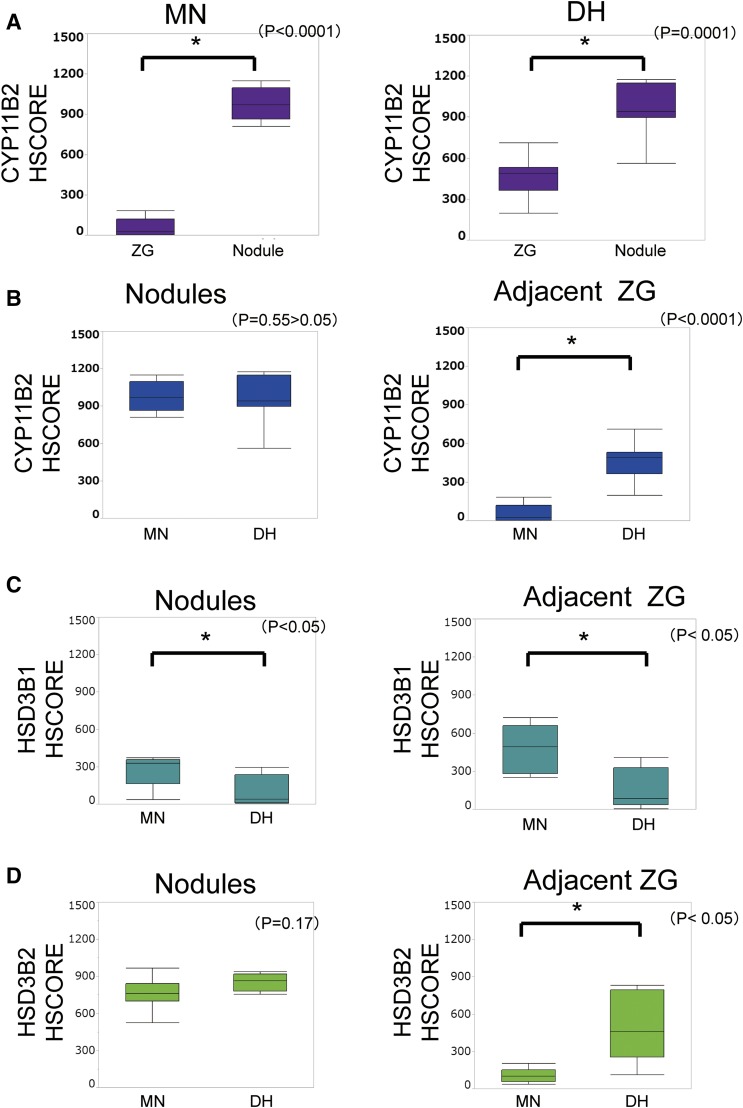
In both MN and DH, CYP11B2 immunoreactivity (H-score) was significantly higher in adrenocortical nodules than in adjacent ZGs [Fig. 2(A); MN, P < 0.0001; DH, P = 0.0001; Mann-Whitney U test], indicating the potential for aldosterone biosynthesis predominantly in adrenocortical nodules in MN and DH. ZGs of the adjacent adrenal cortex of MN were morphologically hyperplastic but predominantly negative for CYP11B2, consistent with the concept of paradoxical hyperplasia [Fig. 2(B)]. Therefore, aldosterone biosynthesis was considered focal or localized in the ZG of MN.
Figure 2.
Immunoreactivity of steroidogenic enzymes in MN and DH. (A) Comparison of CYP11B2 immunoreactivity between cortical nodules and adjacent ZGs. CYP11B2 immunoreactivity was significantly more abundant in cortical nodules than in adjacent ZGs (MN, *P < 0.0001; DH, *P = 0.0001). (B) Comparison of CYP11B2 immunoreactivity between MN and DH. CYP11B2 immunoreactivity was significantly more abundant in nonnodular part of the ZGs in DH than in MN (*P < 0.0001). (C) Comparison of HSD3B1 immunoreactivity between cortical nodules and adjacent ZGs. HSD3B1 immunoreactivity was significantly more abundant both in cortical nodules and adjacent ZGs in MN than in those in DH (nodules, *P = 0.04; adjacent ZG, *P = 0.0128). (D) Comparison of HSD3B2 immunoreactivity between cortical nodules and adjacent ZGs. HSD3B2 immunoreactivity was significantly more abundant in adjacent ZGs in DH than in those in MN (*P = 0.0192). However, there were no significant differences between DH and MN with respect to HSD3B2 immunoreactivity in cortical nodules (P = 0.17).
HSD3Bs
HSD3B1 immunoreactivity was significantly higher in both adrenocortical micronodules and adjacent ZGs in MN than in DH [Fig. 2(C); nodules, P = 0.04; adjacent ZG, P = 0.01; Mann-Whitney U test]. HSD3B2 immunoreactivity was significantly higher in adjacent ZGs in DH than in MN [Fig. 2(D); P = 0.02; Mann-Whitney U test]. However, no significant differences in HSD3B2 immunoreactivity were detected between micronodules of DH and MN (P = 0.17; Mann-Whitney U test).
Quantitative analysis of CYP11B2-positive area in UMN vs NA
The percentages of the CYP11B2-positive areas were compared between UMN and NA using digital image analysis [Fig. 3(A)]. The percentage of the CYP11B2-positive area in UMN was significantly higher than that in NA [Fig. 3(B); P = 0.0061; Mann-Whitney U test]. Although limited by the retrospective nature of the study and the small number of cases examined, a cutoff for the CYP11B2-positive area of ∼1% of the adrenal cortex area could differentiate UMN from NA (100%).
Figure 3.
Results of digital image analysis of CYP11B2-positive area (unilateral MN vs NA). (A) Percentage of CYP11B2-positive areas against the adrenal cortex. (B) Comparison of CYP11B2-positive areas between UMN and NA. CYP11B2-positive areas in UMN were significantly higher than those in NA. More than approximately 1% as the cutoff value corresponds to “hyperplastic change” pathologically.
Somatic gene mutation status associated with aldosterone overproduction
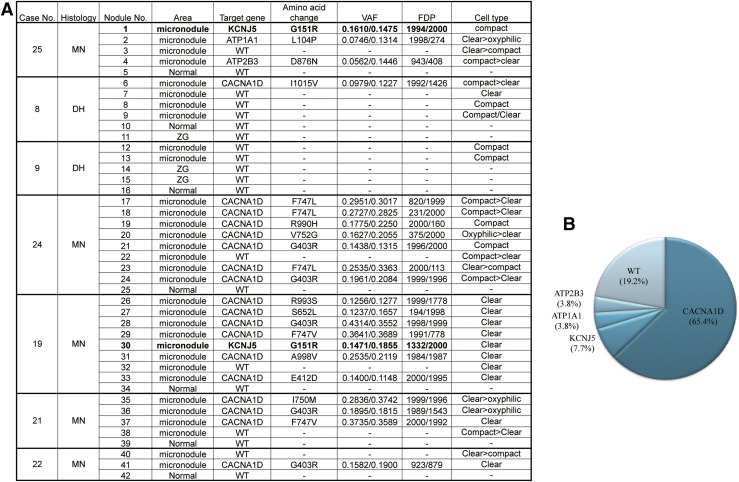
We further explored the characteristics of the CYP11B2 cortical micronodules detected in these histological subtypes by evaluating the frequency and spectrum of somatic mutations in aldosterone-regulating genes in the 7 unilateral cases described previously. DNA was isolated from 26 CYP11B2-positive cortical micronodules from these 5 MN cases, and from 6 nodules and 3 nonnodular part of CYP11B2-positive ZGs from the 2 DH cases. Somatic mutations of aldosterone-driver genes were detected significantly more frequently in CYP11B2-positive adrenocortical micronodules in MN [n = 21 of 26 (81%)] vs DH [n = 1 of 6 (17%); P = 0.0063; 2-sided Fisher’s exact test]. Among 26 adrenocortical micronodules, mutations in CACNA1D (65%), KCNJ5 (8%), ATP1A1 (4%), and ATP2B3 (4%) were detected (Fig. 4). Of particular interest, KCNJ5 somatic mutations were identified in 2 of 5 (40%) MN cases (case no. 19 and no. 25, 1 micronodule each; Fig. 4). Representative images of the cortical micronodule with KCNJ5 mutation are presented in Supplemental Fig. 1 (30MB, tif) . One nodule harboring mutant KCNJ5 (from case no.19) was composed of clear cells [zona fasciculata (ZF)–like cells], whereas the other nodule with mutation (from case no. 25) was composed of compact cells (ZG-like cells). Only 1 of 6 (17%) sequenced DH micronodules had any somatic mutations (in CACNA1D), whereas 17 of 26 (65%) sequenced MN micronodules had CACNA1D mutations [MN, n = 17 of 26 (65%) vs DH, n = 1 of 6 (17%); P = 0.06; 2-sided Fisher’s exact test]. A summary of the somatic mutations in these cases is illustrated in Fig. 4. No somatic mutations were detected in CYP11B2-negative normal adrenal cortex, which was used as the negative control in each case.
Figure 4.
Aldosterone-driver gene somatic mutation profiles in MN and DH. (A) Seven unilateral cases (MN, 5 cases; DH, 2 cases) were analyzed with NGS. In total, 42 CYP11B2-positive areas were isolated using macrodissection (with corresponding CYP11B2-negative adrenal cortex for control). Variant allele frequency (VAF) and flow-corrected read depth (FDP) are shown from both panels used to sequence the CYP11B2-positive micronodules. (B) Aldosterone-driver somatic gene mutation profiles in MN. Mutations were detected in 81% (n = 21 of 26) of micronodules recognized in MN. Among these, the mutations of KCNJ5 (n = 2 of 26; 7.7%), ATP1A1 (n = 1 of 26; 3.8%), ATP2B3 (n = 1 of 26; 3.8%), and CACNA1D (n = 17 of 26; 65.4%) were detected in the cortical micronodules of MN, respectively. In the cases examined in this study, 19.2% (n = 5 of 26) of micronodules were wild type (WT).
Discussion
This study comprehensively subclassifies cross-sectional image–negative hyperaldosteronism on the basis of detailed histopathological features, CYP11B2 IHC, and somatic mutation status.
MN vs DH
Cross-sectional image–negative hyperaldosteronism could be subclassified into 2 histologically distinctive subtypes: MN and DH. Both MN and DH were considered to be distinct disorders on the basis of CYP11B2 IHC, i.e., aldosterone biosynthesis had occurred diffusely or focally in the ZG of adrenal cortex. In MN cases, HSD3B1 was detected in both cortical micronodules and adjacent ZGs, but HSD3B2 was relatively abundant only in the micronodules and absent in adjacent ZG. Therefore, the adjacent ZGs in MN demonstrated the “paradoxical hyperplasia” (21) that was detected in adjacent ZGs in APA (7), and these findings were also consistent with the nature of autonomous aldosterone production in MN. In addition, these steroidogenic enzyme profiles in the cortical micronodules were consistent with the previous report that first proposed the concept of UMN (7). However, "UMN" as previously described could possibly include regenerative adrenocortical nodules composed of ZF-like clear cells that are negative for CYP11B2 and positive for CYP17A1 and CYP11B1 (9, 10). Therefore, in this study, we defined MN as CYP11B2-positive multiple cortical micronodules.
The number of unilateral resected MN cases has been recently increasing as a result of AVS development, as described previously. These cases are usually considered as having surgical indications because the patients are thought to present with a relatively early clinical stage of PA.
DH is a pure histological entity, considered to more frequently manifest in bilateral lesions and therefore termed BDH. In our present study, the great majority of DH cases were bilateral (83%; n = 10 of 12). In DH cases examined, more than 50% of nonnodular parts of the ZG were CYP11B2-positive areas. The term IHA has been used as a rather nonspecific umbrella term for a long period of time in adrenocortical pathology to describe the bilateral PA lesions associated with ZG hyperplasia, with or without adrenocortical nodules, regardless of underlying etiologies (6, 22).
Doi et al. (23) reported that HSD3B1 as well as HSD3B2 was immunolocalized in the ZG, and HSD3B2 was also predominantly detected in the ZGs of both NA and IHA cases. However, results of our present study indicate that HSD3B1 expression is relatively low in both micronodules and the adjacent ZG in DH cases, whereas HSD3B2 immunoreactivity is abundant in both micronodules and adjacent hyperplastic ZG.
Through an optimized NGS approach that is compatible with minute amounts of FFPE tissue, somatic mutations in aldosterone-driver genes were significantly more frequent in CYP11B2-positive cortical nodules in MN (81%) vs DH (17%). Of particular interest, KCNJ5 somatic mutations were detected in 2 of 5 MN cases (2 of 26 micronodules) but not in either of the 2 DH cases (0 of 6 micronodules) profiled herein. In addition, no somatic mutation was detected in the nonnodular part of CYP11B2-positive ZG in DH cases. Our results are in contrast with the prevailing thought that the capacity to produce aldosterone in non-APA lesions, especially in unilateral cases, is relatively lower than that in APA (24). However, contralateral sides of adrenal glands in bilateral disease cases are required for further clarification.
Cell morphology of CYP11B2-positive adrenocortical micronodules
Adrenocortical micronodules were composed of various types of cortical cells, mainly clear cells (ZF-like, lipid-rich cells) and compact cells (ZG-like, spherical small cells). However, there were some other types, for instance, oxyphilic cells, which had eosinophilic or relatively eosinophilic abundant cytoplasm, occasionally containing lipid droplets. However, in our present study, there were no significant correlations between cell morphology and aldosterone-driver gene somatic mutation status [Fig. 4(A)]. The great majority of these adrenocortical micronodules were composed of mixed cell types, which indicated that zonation of micronodules was at least partially preserved.
MN vs microAPA
Histopathological differential diagnosis between MN and microAPA is extremely difficult. Results of previously reported studies demonstrated that the term microAPA has not necessarily been rigorously defined, but in general, tumors of less than 7 mm in the greatest dimension on the basis of imaging methods are currently considered to represent microAPA (8, 25, 26). MicroAPAs often do not have classical APA-associated histological features, such as a fibrous capsule, intratumoral heterogeneity of clear and compact cortical cells, and/or the occasionally detected tumor cell atypia with enlarged nuclei (8). However, results of our present study demonstrated that CYP11B2 immunoreactivity of adrenocortical micronodules in MN was marked in the adrenal subcapsular area, and these cells extended inward from the capsule [Fig. 1(A) and 1(B)]. This finding also indicated that adrenal cortex zonation was at least partially preserved in these adrenals in non-APA hyperaldosteronism. Therefore, histologically discernible nuclear atypia, patterns of CYP11B2 immunoreactivity, and preservation of adrenal cortex zonation could be of value in histopathological differentiation of microAPAs from MN.
MN vs multiple APCCs
Nishimoto et al. (16) defined APCC as CYP11B2-positive cell clusters within the ZG area normally involved in aldosterone biosynthesis in NA. In our present study, we defined MN as a distinct histopathological entity in the cases associated with autonomous aldosterone hypersecretion, because the prevalence of aldosterone-driver gene somatic mutation was much higher in MN than APCC [MN, n = 21 of 26 (81%) vs APCC, n = 8 of 23 (35%); P = 0.0015; 2-sided Fisher’s exact test] (16), and the CYP11B2-positive area was larger in MN than in APCC. These findings may be related to the aldosterone hypersecretion detected in these patients.
Importantly, however, MN in this cohort of hypertensive cases resembled normal adrenals with multiple CYP11B2-expressing APCCs in nonhypertensive cases (16). Our findings therefore suggest that APCCs in nonhypertensive adrenals could progress to the MN subtype of PA, a concept that is supported by the similar spectrum of somatic mutations in aldosterone-driver gene somatic mutations. However, histopathological differentiation between adrenocortical nodules in MN and APCCs detected in NA is still considered extremely difficult. Therefore, in our present study, we quantified histologic “hyperplastic change” by analyzing the ratio of CYP11B2 area in the whole adrenal cortex using digital image analysis. Results of this particular quantitative histological approach revealed that “pathologic” CYP11B2 status occurred when 1% of the adrenal cortex became positive for CYP11B2 (Fig. 4). Therefore, the cases in which CYP11B2-positive cortical nodules occupy more than 1% of the adrenal glands are considered to represent MN “histologically.” However, this definition would have to be prospectively validated and confirmed using a larger number of cases.
KCNJ5 somatic mutations were detected in the micronodules of MN cases (2 of 26). The patterns of adrenocortical zonation with more marked CYP11B2 immunoreactivity in outer areas, as in normal adrenal glands, were still preserved in the cortical micronodules harboring KCNJ5 mutations. However, no APCC had KCNJ5 mutations in a relatively large autopsy study of adrenals from a Japanese population (27), in contrast with a relatively high rate of KCNJ5 mutations (70%) in Japanese patients with APA (15). Nonetheless, whether these cortical micronodules harboring KCNJ5 mutations detected in MN have the potential to progress to APA or microAPA is unknown, and further investigations are required for clarification.
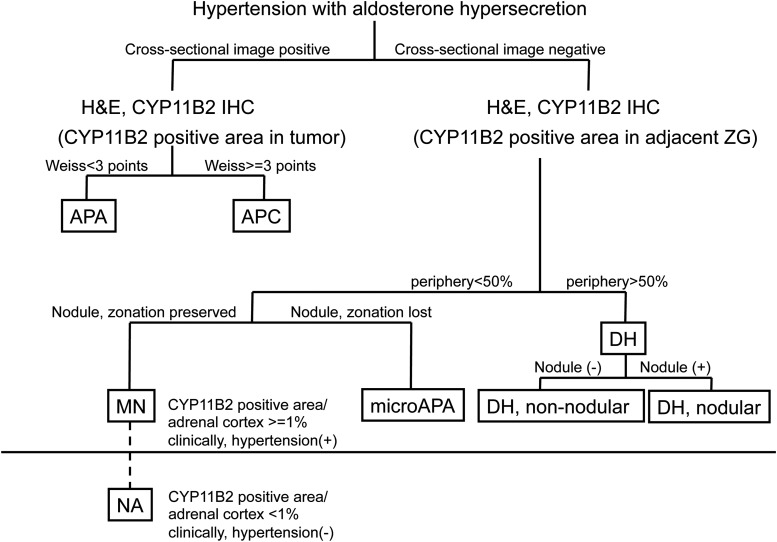
Conclusion
CYP11B2 immunolocalization is pivotal in defining cellular etiology and histological subtypes in cross-sectional image–negative hypertension associated with hyperaldosteronism that included PA. Here we classified the histological subtypes of non-APA adrenocortical lesions with aldosterone hypersecretion into 2 distinctive entities: DH (diffuse hyperplasia pattern) and MN (focal nodular pattern) (Fig. 5). Results of this study indicate that DH and MN are 2 different entities in terms of aldosterone biosynthesis. CACNA1D mutation is most frequently detected in CYP11B2-positive micronodules of MN. We consider MN as a histological subtype of PA, which we hypothesize may develop from APCC accumulation, although this requires additional investigation. Furthermore, unilateral cases of MN should be thought to represent a relatively early clinical stage of PA, and possibly considered as indicated for surgery. However, additional investigation and clinical long-term postoperative prognosis will be required to determine whether some of these cortical nodules harboring KCNJ5 mutations could represent the precursor lesions of APA.
Figure 5.
Diagram of classification of cross-sectional image–negative lesion in a case of hypertension associated with aldosterone hypersecretion. Cross-sectional image–negative hypertension associated with hyperaldosteronism that included PA was subclassified into 2 histologically distinctive subtypes on the basis of whether CYP11B2-positive cells occupied more than 50% of the ZG: MN (periphery <50%) and DH (periphery >50%). Both MN and DH were considered distinct disorders on the basis of CYP11B2 IHC, i.e., aldosterone biosynthesis occurs diffusely or focally in the ZG of the adrenal cortex. Subsequently, the preservation of zonation in CYP11B2 adrenocortical nodules, or lack of zonation preservation, is the distinctive differential diagnostic feature of micronodules and microAPA. Both MN and DH have unilateral and bilateral counterparts, respectively. APC, aldosterone-producing carcinoma.
Acknowledgments
Acknowledgments
This work was supported by JSPS KAKENHI (grant no. JP15H04711), the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. DK106618 to W.E.R. and S.A.T.) and the National Heart, Lung and Blood Institute (grant no. HL27255 to C.E.G.-S.). S.A.T. is supported by the A. Alfred Taubman Medical Research Institute and the Prostate Cancer Foundation. Y.Y. is supported by a scholarship from the Takeda Science Foundation.
Disclosure Summary: S.A.T. has received travel support from Thermo Fisher Scientific and is a consultant and equity holder in Strata Oncology. The remaining authors have nothing to disclose.
Footnotes
- APA
- aldosterone-producing adenoma
- APCC
- aldosterone-producing cell cluster
- AVS
- adrenal venous sampling
- BDH
- bilateral diffuse hyperplasia
- DH
- diffuse hyperplasia
- FFPE
- formalin fixed, paraffin embedded
- H&E
- hematoxylin and eosin
- IHA
- idiopathic hyperaldosteronism
- IHC
- immunohistochemistry
- MN
- multiple adrenocortical micronodules
- NA
- nonpathological adrenal glands
- NGS
- next-generation sequencing
- PA
- primary aldosteronism
- UMN
- unilateral multiple adrenocortical micronodules
- ZF
- zona fasciculata
- ZG
- zona glomerulosa.
References
- 1.Funder JW. Medicine. The genetics of primary aldosteronism. Science. 2011;331(6018):685–686. [DOI] [PubMed] [Google Scholar]
- 2.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F; PAPY Study Investigators . A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. [DOI] [PubMed] [Google Scholar]
- 3.Williams JS, Williams GH, Raji A, Jeunemaitre X, Brown NJ, Hopkins PN, Conlin PR. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006;20(2):129–136. [DOI] [PubMed] [Google Scholar]
- 4.Mulatero P, Dluhy RG, Giacchetti G, Boscaro M, Veglio F, Stewart PM. Diagnosis of primary aldosteronism: from screening to subtype differentiation. Trends Endocrinol Metab. 2005;16(3):114–119. [DOI] [PubMed] [Google Scholar]
- 5.Ishidoya S, Kaiho Y, Ito A, Morimoto R, Satoh F, Ito S, Ishibashi T, Nakamura Y, Sasano H, Arai Y. Single-center outcome of laparoscopic unilateral adrenalectomy for patients with primary aldosteronism: lateralizing disease using results of adrenal venous sampling. Urology. 2011;78(1):68–73. [DOI] [PubMed] [Google Scholar]
- 6.Ganguly A. Primary aldosteronism. N Engl J Med. 1998;339(25):1828–1834. [DOI] [PubMed] [Google Scholar]
- 7.Omura M, Sasano H, Fujiwara T, Yamaguchi K, Nishikawa T. Unique cases of unilateral hyperaldosteronemia due to multiple adrenocortical micronodules, which can only be detected by selective adrenal venous sampling. Metabolism. 2002;51(3):350–355. [DOI] [PubMed] [Google Scholar]
- 8.Omura M, Sasano H, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res. 2006;29(11):883–889. [DOI] [PubMed] [Google Scholar]
- 9.Katayama Y, Takata N, Tamura T, Yamamoto A, Hirata F, Yasuda H, Matsukuma S, Daido Y, Sasano H. A case of primary aldosteronism due to unilateral adrenal hyperplasia. Hypertens Res. 2005;28(4):379–384. [DOI] [PubMed] [Google Scholar]
- 10.Hirono Y, Doi M, Yoshimoto T, Kanno K, Himeno Y, Taki K, Sasano H, Hirata Y. A case with primary aldosteronism due to unilateral multiple adrenocortical micronodules. Endocr J. 2005;52(4):435–439. [DOI] [PubMed] [Google Scholar]
- 11.Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, Okumura A, Kawa G, Tanabe A, Naruse M. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013;98(4):1567–1574. [DOI] [PubMed] [Google Scholar]
- 12.Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440–444, e1–e2. [DOI] [PubMed] [Google Scholar]
- 14.Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6(6):19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Okada S, Rokutanda N, Takata D, Koibuchi Y, Horiguchi J, Oyama T, Takeyoshi I, Mori M. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab. 2012;97(4):1311–1319. [DOI] [PubMed] [Google Scholar]
- 16.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K, Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112(33):E4591–E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh F, Abe T, Tanemoto M, Nakamura M, Abe M, Uruno A, Morimoto R, Sato A, Takase K, Ishidoya S, Arai Y, Suzuki T, Sasano H, Ishibashi T, Ito S. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007;30(11):1083–1095. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS Jr. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–5425. [PubMed] [Google Scholar]
- 20.Konosu-Fukaya S, Nakamura Y, Satoh F, Felizola SJ, Maekawa T, Ono Y, Morimoto R, Ise K, Takeda K, Katsu K, Fujishima F, Kasajima A, Watanabe M, Arai Y, Gomez-Sanchez EP, Gomez-Sanchez CE, Doi M, Okamura H, Sasano H. 3β-Hydroxysteroid dehydrogenase isoforms in human aldosterone-producing adenoma. Mol Cell Endocrinol. 2015;408:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasano H, Okamoto M, Sasano N. Immunohistochemical study of cytochrome P-45011/beta-hydroxylase in human adrenal cortex with mineralo- and glucocorticoid excess. Virchows Archiv A Pathol Anat Histopathol. 1988;413(4):313–318. [DOI] [PubMed] [Google Scholar]
- 22.Mattsson C, Young WF Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006;2(4):198–208, quiz, 1, 230. [DOI] [PubMed] [Google Scholar]
- 23.Doi M, Satoh F, Maekawa T, Nakamura Y, Fustin JM, Tainaka M, Hotta Y, Takahashi Y, Morimoto R, Takase K, Ito S, Sasano H, Okamura H. Isoform-specific monoclonal antibodies against 3β-hydroxysteroid dehydrogenase/isomerase family provide markers for subclassification of human primary aldosteronism. J Clin Endocrinol Metab. 2014;99(2):257–262. [DOI] [PubMed] [Google Scholar]
- 24.Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y, Ito S. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono Y, Nakamura Y, Maekawa T, Felizola SJ, Morimoto R, Iwakura Y, Kudo M, Seiji K, Takase K, Arai Y, Gomez-Sanchez CE, Ito S, Sasano H, Satoh F. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension. 2014;64(2):438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karashima S, Takeda Y, Cheng Y, Yoneda T, Demura M, Kometani M, Ohe M, Mori S, Yagi K, Yamagishi M. Clinical characteristics of primary hyperaldosteronism due to adrenal microadenoma. Steroids. 2011;76(12):1363–1366. [DOI] [PubMed] [Google Scholar]
- 27.Omata K, Hovelson DH, Liu C-J, Satoh F, Sasano H, Rainey WH, Tomins SA. Aldosterone producing cell clusters accumulate with age in normal adrenal glands. Proceedings of the Endocrine Society; April 1–4, 2016; Boston, MA. Presentation OR13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]