Abstract
Context:
Fibroblast growth factor (FGF)23 is a critical determinant of phosphate homeostasis. The role of FGF23, however, in regulating physiologic changes in serum phosphate and renal phosphate handling across childhood is not well described. In addition, animal models have suggested a role for FGF23 in regulating renal calcium excretion.
Objective:
To assess changes in FGF23 concentrations across childhood in relation to changes in mineral ions and hormones of mineral ion homeostasis.
Design:
This was a cross-sectional study.
Setting:
The study was conducted at a Clinical Research Center at a tertiary care hospital.
Patients or Other Participants:
Ninety healthy girls ages 9 to 18 years were recruited from the surrounding community.
Main Outcome Measures:
The associations of intact and C-terminal FGF23 concentrations with measures of mineral ion homeostasis were determined by univariable and multivariable linear regression.
Results:
Serum phosphate and renal phosphate excretion varied with age, as expected (R = −0.49, P < 0.001 and R = −0.48, P < 0.001, respectively). Neither intact nor C-terminal FGF23 varied with age, and FGF23 was not correlated with serum or urinary phosphate. Intact FGF23 was positively correlated with serum calcium (R = 0.39, P < 0.001) and negatively correlated with urinary calcium/creatinine ratio (R = −0.27, P = 0.011).
Conclusions:
The changes in serum and urinary phosphate handling across childhood do not appear to be determined by alterations in FGF23 concentrations. These data may point to a role for FGF23 in calcium regulation in human physiology.
The authors examine predictors of FGF23 in a cohort of healthy girls ages 9 to 18 years and find it to be correlated with decreased urinary calcium but not with measures of phosphate homeostasis.
Fibroblast growth factor (FGF)23, a secretory product of osteocytes, plays an integral role in phosphate homeostasis (1). FGF23 signaling via FGF receptors and its coreceptor Klotho leads to downregulation of the two sodium-phosphate cotransporters in the proximal renal tubules, namely NPT2a and NPT2c, thus promoting urinary phosphate excretion (2–4). In addition, FGF23 lowers serum concentrations of 1,25-dihydroxyvitamin D [1,25(OH)2D] by both inhibiting 1-α-hydroxylase (CYP27B1) and stimulating the catabolic enzyme 24-hydroxylase (CYP24A1) (2). FGF23 circulates in two forms, the intact, active form, and an inactive C-terminal fragment generated from regulated proteolytic cleavage (5).
Several disease states stem from altered regulation of FGF23 production; elevated FGF23 concentrations lead to hypophosphatemic states, including X-linked hypophosphatemia, autosomal dominant and autosomal recessive forms of hypophosphatemia, and tumor-induced osteomalacia (6–8). Conversely, low concentrations of or resistance to FGF23 leads to hyperphosphatemic tumoral calcinosis (9, 10). However, the role of FGF23 in normal physiology is less well established.
Late childhood and adolescence provide a unique window into phosphate homeostasis as serum phosphate concentrations are physiologically higher in younger children and decrease steadily with increasing age through late adolescence (11, 12). The reason for this pattern is unclear but may relate to the important roles of phosphate in growth plate development, bone matrix mineralization, and osteoblast maturation, all critical processes in childhood bone growth and modeling (13). The factors driving this physiologic pattern remain poorly understood.
The objective of this study therefore was to investigate FGF23 concentrations, both intact and total (intact and C-terminal), across late childhood and adolescence in healthy girls to better understand their function and regulation in normal physiology. The study was limited to girls for analytic efficiency as the timing of the physiologic decrease in serum phosphate varies between girls and boys, similar and possibly related to differences in the timing of the onset of puberty (11, 12). Our hypothesis was that FGF23 concentrations would be lower in younger children and would rise across late childhood and adolescence coincident with the decrease in serum phosphate.
Materials and Methods
Subjects
This was a cross-sectional investigation of clinical and biochemical predictors of intact and C-terminal FGF23 circulating concentrations in children. Healthy girls ages 9 to 18 years were recruited from the community via advertisements and mass mailings. Subjects were limited to girls due to hypothesized effects of puberty on circulating FGF23 and the differential timing and hormonal milieu of puberty in boys and girls. Subjects with a history of clinically significant cardiac, hepatic, gastrointestinal, renal, oncologic, or endocrine disease were excluded as well as subjects taking any bone-active medications (including vitamin D >1000 international units/day and hormonal birth control), subjects with a body mass index ≥99th percentile for age, and subjects with a fracture in the preceding 3 months. A screening visit was conducted, and subjects found to have abnormal thyrotropin (>7.0 or <0.1 micro-international units/mL), diabetes mellitus (HgbA1c ≥6.5% or fasting plasma glucose ≥126 mg/dL), or anemia (Hgb <11 g/dL) were additionally excluded. Of 109 subjects screened, 101 met eligibility criteria and 90 completed the study visit. Subjects self identified race and ethnicity. This study was approved by the Partners Human Research Committee. Informed consent was obtained from subjects who were 18 years old and from a parent or guardian for subjects who were minors. Informed assent was obtained from minors. This trial was registered as NCT01180946 at ClinicalTrials.gov.
Clinical and biochemical investigations
A standardized physical examination including Tanner staging and anthropometric measurements was performed. Vitamin D and calcium intake were determined by dietary recall questionnaire. All blood samples were drawn in the morning in the fasting state, and urinary assays were performed on fasting second-voided samples. The renal tubular threshold for phosphate per glomerular filtration rate (TP/GFR) was calculated, as described (14, 15). Serum intact FGF23 was measured by enzyme-linked immunosorbent assay (ELISA) (Kainos, Tokyo, Japan) with a sensitivity of 3 pg/mL and intra- and interassay coefficient of variations (CVs) of ≤3% and ≤4%, respectively. Plasma C-terminal FGF23 was measured by ELISA (Immutopics, San Clemente, CA) with a sensitivity of 1.5 relative units (RU)/mL and intra- and interassay CVs of ≤3% and ≤5%, respectively. The 25-hydroxyvitamin D (25OHD) was measured by liquid chromatography–tandem mass spectrometry with a lower limit of detection of 6 ng/mL and an interassay CV of 6% to 9%. The 1,25-dihydroxyvitamin D (1,25(OH)2D) was measured by column chromatography, followed by radioimmunoassay (LabCorp, Burlington, NC). Parathyroid hormone (PTH) was measured by chemiluminescent immunoassay (Beckman Coulter, Brea, CA) with a sensitivity of 1 pg/mL and intra- and interassay CVs of 3% and 6%, respectively. Propeptide of type I procollagen (P1NP) was measured by radioimmunoassay (Orion Diagnostica, Espoo, Finland) with a sensitivity of 2 mcg/L and intra- and interassay CVs of 6% to 10%. C-terminal telopeptide of type I collagen (CTX) was measured by ELISA with a sensitivity of 0.02 ng/mL and intra- and interassay CVs of 3% and 11%, respectively (Immunodiagnostic Systems, Boldon, UK). Insulin-like growth factor-1 (IGF-1) was measured by ELISA (Immunodiagnostic Systems) with a sensitivity of 3.1 mcg/L and intra- and interassay CVs of 7%. Urine calcium was measured by colorimetric assay and urine creatinine by kinetic assay. Corrected calcium (mg/dL) was calculated as [total calcium (mg/dL) + 0.8 × (4.4 − serum albumin [g/L])].
Imaging
DXA imaging of the whole body less head and of the lumbar spine was obtained (Hologic QDR Discovery A, Bedford, MA). The z scores were generated using Hologic Apex 3.3 software and were adjusted for height z score, as described (16).
Statistical analyses
We used Stata 12.1 (StataCorp LP, College Station, TX) for all analyses. The Shapiro–Wilk test for normality was performed for all continuous variables. Clinical and laboratory data are reported as mean ± standard deviation, except as noted. We used linear regression to assess the associations of clinical and biochemical variables. We used multivariable linear regression to control these associations for age as well as to generate a multivariable prediction model for intact FGF23 concentrations, including all variables found to be significantly associated in univariate correlations, as well as variables thought to be biologically significant. Correlations with a P value of <0.05 are reported as significant. This was an exploratory data analysis, and thus no formal power calculations were performed. A post hoc analysis demonstrated that, with a sample size of 90 and a null hypothesis of no correlation, we had 80% power to detect a correlation coefficient R of at least 0.3 with a P value of <0.05.
Results
Subject characteristics
Table 1 describes the 90 girls in this study, with a median age of 14.0 years. Median bone age was slightly higher at 15.0 years, consistent with secular changes in bone age advancement (17). The racial and ethnic distribution reflected that of the Boston area. Height and weight were similar to population reference values, as reflected in the respective z scores. We measured circulating concentrations of calcium, albumin-corrected calcium, phosphate, and other key factors in mineral metabolism as well as measures of urinary excretion of calcium (urine calcium/creatinine ratio), phosphate (TP/GFR), and sodium (urine sodium/creatinine ratio). As expected, several of these varied with age: in particular, serum calcium, phosphate, 1,25(OH)2D, TP/GFR, and urine sodium/creatinine decreased with age, whereas serum creatinine increased with age, but remained within the age-specific normal range. IGF-1 had an inverted U-shaped relationship with age with a peak at approximately age 14 to 15 years. Bone turnover markers P1NP and CTX were elevated in younger girls with a peak at 11 to 12 years, and then declined progressively. Thirty-two (35%) subjects had a serum 25OHD <20 ng/mL, consistent with deficiency (18). Of these, none had a PTH concentration outside of the normal range, although one subject with a 25OHD of 20 ng/mL had an elevated PTH of 80 pg/mL. Intact FGF23 was normally distributed with a mean of 42.1 pg/mL, whereas C-terminal FGF23, which includes both intact and the C-terminal fragment, was mildly right skewed with a mean of 102.4 RU/mL and a median of 89.3 RU/mL.
Table 1.
Clinical Characteristics of Subjects (n = 90) and Correlation of Biochemical Analytes With Age
|
Correlation With Age (Years) |
||||
|---|---|---|---|---|
|
Variable |
β | R | P | |
| Age (years) | 14.0 (12.2, 16.2) | |||
| Bone age (years) | 15.0 (12.0, 17.0) | |||
| Race | ||||
| White | 69 (76%) | |||
| Black | 5 (6%) | |||
| Asian | 2 (2%) | |||
| Multiple | 10 (11%) | |||
| None of the above | 4 (4%) | |||
| Hispanic ethnicity | 9 (10%) | |||
| Height (cm) | 160.0 (150.2, 165) | |||
| Height z score | 0.1 (−0.6, 1.0) | |||
| Weight (kg) | 53.4 (43.0, 61.0) | |||
| Weight z score | 0.4 (−0.2, 1.0) | |||
| BMI (kg/m2) | 20.2 (18.6, 23.4) | |||
| BMI z score | 0.3 (−0.3, 1.0) | |||
| Breast Tanner stage | ||||
| 1 (prepubertal) | 12 (13%) | |||
| 2–4 (pubertal) | 35 (39%) | |||
| 5 (postpubertal) | 43 (48%) | |||
| Calcium intake (diet and supplements) (mg) | 857 (607, 1311) | |||
| Vitamin D intake (diet and supplements) (IU) | 132 (73, 261) | |||
| Calcium (mg/dL) | 9.6 ± 0.3 | −0.05 | −0.37 | <0.001 |
| Albumin (g/L) | 4.5 ± 0.2 | 0.01 | 0.12 | 0.262 |
| Corrected calcium (mg/dL) | 9.5 ± 0.4 | −0.05 | −0.40 | <0.001 |
| Phosphate (mg/dL) | 4.3 ± 0.5 | −0.10 | −0.49 | <0.001 |
| Creatinine (mg/dL) | 0.6 ± 0.1 | 0.03 | 0.61 | <0.001 |
| 25OHD (ng/mL) | 21.8 ± 6.7 | 0.48 | 0.19 | 0.074 |
| 1,25(OH)2D (pg/mL) | 50.7 ± 15.4 | −2.99 | −0.51 | <0.001 |
| PTH (pg/mL) | 35.0 ± 11.9 | 0.05 | 0.01 | 0.916 |
| Urine calcium/creatinine (mg/mg) | 0.07 ± 0.05 | 0.00 | −0.16 | 0.142 |
| TP/GFR (mg/dL) | 3.9 ± 0.6 | −0.12 | −0.53 | <0.001 |
| Urine sodium/creatinine (mEq/mg) | 0.12 ± 0.08 | −0.02 | −0.50 | <0.001 |
| IGF-1 (ng/mL) | 194.7 ± 65.7 | n/aa | ||
| P1NP (μg/L) | 333.6 ± 270.6 | n/aa | ||
| CTX (ng/mL) | 1.38 ± 0.73 | n/aa | ||
| Intact FGF23 (pg/mL) | 42.1 ± 11.3 | −0.36 | −0.09 | 0.426 |
| C-terminal FGF23 (RU/mL) | 102.4 ± 47.8 | 0.67 | 0.04 | 0.739 |
Data expressed as median (25th percentile, 75th percentile), number (percent), or mean ± standard deviation, as appropriate. Subjects who self-identified as multiracial were white and black (3), black and Native American (3), and Asian and white (4). β, R, and P values calculated by univariate linear regression. Abbreviations: BMI, body mass index; IU, international units.
Correlation is nonlinear, as described in the text.
Correlations of measures of phosphate homeostasis with FGF23
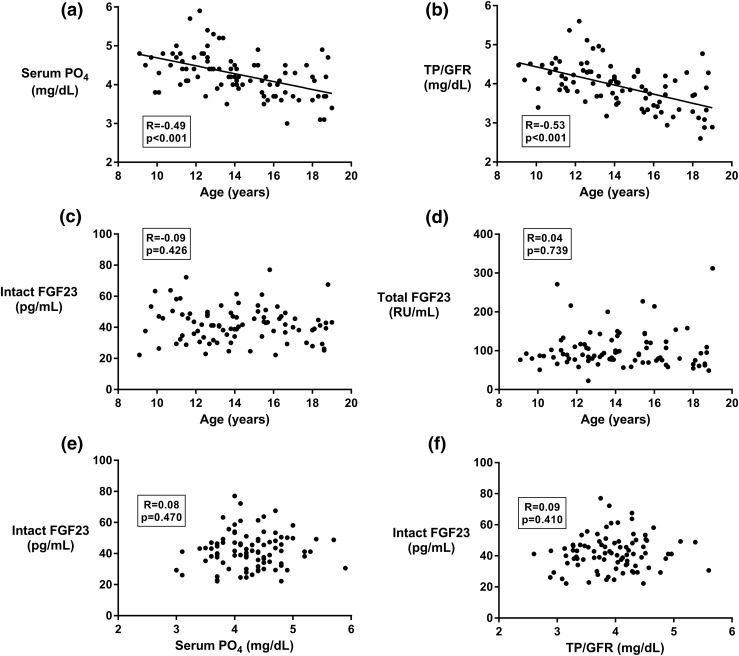
Figure 1(a) and 1(b) shows scatterplots of serum phosphate concentration and of TP/GFR with age. Consistent with previous reports, we observed a significant decrease of both phosphate and TP/GFR with increasing age. Contrary to our hypothesis, neither intact nor C-terminal FGF23 varied with age, as shown in Fig. 1(c) and 1(d). Intact FGF23 did not correlate with serum phosphate nor with TP/GFR, as shown in Fig. 1(e) and 1(f). Similarly, C-terminal FGF23 did not correlate with either serum phosphate (R = 0.01, P = 0.898) or TP/GFR (R = 0.02, P = 0.865).
Figure 1.
Scatterplots showing correlations of (a) serum phosphate with age, (b) TP/GFR with age, (c) intact FGF23 with age, (d) C-terminal FGF23 with age, (e) intact FGF23 with serum phosphate, and (f) intact FGF23 with TP/GFR. PO4, phosphate.
Associations of FGF23 with other measures of mineral ion homeostasis
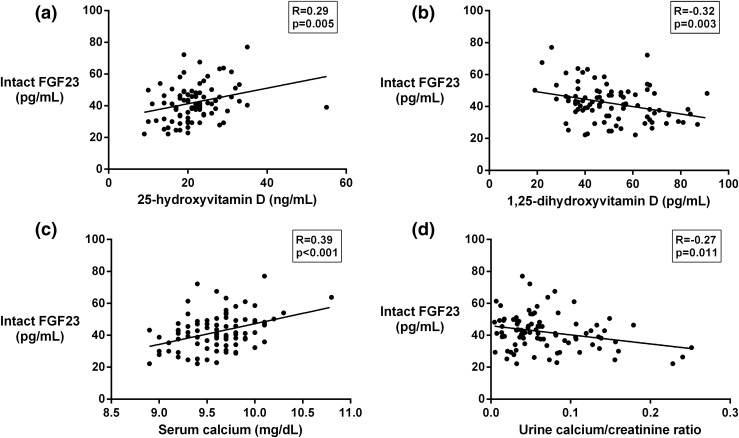
As shown in Fig. 2(a) and 2(b), intact FGF23 was positively correlated with 25OHD and negatively correlated with 1,25(OH)2D. Unexpectedly, intact FGF23 was also positively correlated with serum calcium and negatively correlated with the fasting urinary calcium/creatinine ratio, as seen in Fig. 2(c) and 2(d). We did not detect any correlation between intact FGF23 and calcium intake (R = 0.16, P = 0.123). Of note, we did not observe significant correlations of calcium intake with either serum calcium (R = 0.12, P = 0.250) or fasting urinary calcium/creatinine (R = 0.10, P = 0.376).
Figure 2.
Scatterplots showing correlations of (a) intact FGF23 with 25-hydroxyvitamin D, (b) intact FGF23 with 1,25-dihydroxy-vitamin D, (c) intact FGF23 with serum calcium, and (d) intact FGF23 with the urinary calcium/creatinine ratio.
One subject was incidentally noted to have a mildly elevated serum calcium of 10.8 mg/dL with a corresponding albumin-corrected calcium of 10.3 mg/dL; after exclusion of this outlier, the correlation of intact FGF23 and serum calcium remained significant (R = 0.34, P = 0.001). These analyses were repeated with albumin-corrected calcium in place of total calcium, and the observed correlations were very similar (association with intact FGF23: R = 0.38, P < 0.001, after exclusion of outlier: R = 0.35, P < 0.001).
Intact FGF23 was not associated with PTH (R = −0.08, P = 0.460). C-terminal FGF23 was not associated with 25OHD, 1,25(OH)2D, serum or urine calcium, and PTH. Intact FGF23 was not associated with IGF-1 in the complete cohort (R = 0.14, P = 0.184). In a post hoc analysis, intact FGF23 was associated with IGF-1 in the pubertal and postpubertal girls (R = 0.27, P = 0.016, n = 78), but not in the prepubertal girls (R = −0.21, P = 0.517, n = 12). There were no correlations of intact or C-terminal FGF23 with P1NP or CTX in the full cohort nor after stratifying by pubertal stage.
Associations with DXA measures of bone mineral content and density
Because FGF23 has been positively correlated with bone turnover markers in a cohort of younger children (19) and has been demonstrated to impair bone mineralization in vitro (20, 21), we investigated whether circulating concentrations were associated with measures of bone mass and density. Neither intact nor C-terminal FGF23 correlated with total body less head bone mineral content height-adjusted z score (R = 0.06, P = 0.560 and R = 0.01, P = 0.923, respectively) or with spine bone mineral density height-adjusted z score (R = 0.00, P = 0.977 and R = 0.01, P = 0.914, respectively).
Adjusted correlations of intact FGF23
Given that several metabolites vary physiologically with age, as described previously, we investigated whether FGF23 was associated with these metabolites after adjusting for age. As shown in Table 2, the correlations of intact FGF23 with 25OHD, 1,25(OH)2D, serum calcium, albumin-corrected serum calcium, and urine calcium/creatinine ratio persisted after adjustment. In addition, after adjustment for age, we found a positive correlation of FGF23 with serum creatinine.
Table 2.
Unadjusted and Age-Adjusted Associations of Intact FGF23
|
Unadjusted |
Adjusted for Age |
|||||
|---|---|---|---|---|---|---|
|
Analyte |
β | R | P | β | Partial R | P |
| Calcium (mg/dL) | 12.9 | 0.39 | <0.001 | 13.7 | 0.38 | <0.001 |
| Corrected calcium (mg/dL) | 11.9 | 0.38 | <0.001 | 12.9 | 0.38 | <0.001 |
| Phosphate (mg/dL) | 1.6 | 0.08 | 0.470 | 0.96 | 0.04 | 0.704 |
| Creatinine (mg/dL) | 12.7 | 0.13 | 0.223 | 28.1 | 0.23 | 0.031 |
| 25OHD (ng/mL) | 0.49 | 0.29 | 0.005 | 0.54 | 0.31 | 0.003 |
| 1,25(OH)2D (pg/mL) | −0.23 | −0.32 | 0.003 | −0.36 | −0.42 | <0.001 |
| PTH (pg/mL) | −0.07 | −0.08 | 0.460 | −0.07 | −0.08 | 0.466 |
| Urine calcium/creatinine | −56.7 | −0.27 | 0.011 | −59.9 | −0.28 | 0.008 |
| TP/GFR (mg/dL) | 1.74 | 0.09 | 0.410 | 1.69 | 0.07 | 0.501 |
We next created a multivariable prediction model for intact FGF23 entering those factors found to be correlated on univariate analyses [serum calcium, urine calcium/creatinine ratio, 25OHD, and 1,25(OH)2D] as well as other variables thought to be biologically important, including age, serum phosphate, TP/GFR, and serum creatinine. Independent predictors of intact FGF23 in this model included serum calcium (β = 11.4, P = 0.001), urine calcium/creatinine ratio (β = −50.3, P = 0.012), and 1,25(OH)2D (β = −0.27, P = 0.001). There was a trend toward 25OHD as an additional independent predictor (β = 0.30, P = 0.062). R2 for the overall model was 0.38.
Predictors of urinary calcium excretion
Given the significant negative correlation of intact FGF23 with fasting urinary calcium, we investigated the correlation of other potential determinants of urinary calcium. As shown in Table 3, we found a significant correlation with urinary sodium and a significant inverse correlation with serum PTH. We did not find correlations with serum calcium (total or albumin corrected), 1,25(OH)2D, or calcium intake. We next created a multivariable prediction model for fasting urine calcium/creatinine, entering those factors found to be correlated on univariate analyses (intact FGF23, PTH, and urine sodium). Each of these factors remained independent predictors in this model.
Table 3.
Associations of Fasting Urine Calcium/Creatinine Ratio
|
Univariate Associations | ||||
|---|---|---|---|---|
| Analyte | β | Standardized β | R | P |
| Serum calcium (mg/dL) | 6.28 × 10−3 | 0.039 | 0.04 | 0.719 |
| Serum albumin-corrected calcium (mg/dL) | 1.04 × 10−3 | 0.007 | 0.00 | 0.949 |
| 1,25(OH)2D (pg/mL) | 0.33 × 10−3 | 0.096 | 0.10 | 0.378 |
| PTH (pg/mL) | −1.42 × 10−3 | −0.295 | −0.30 | 0.006 |
| Calcium intake (diet and supplements) (mg) | 0.10 × 10−3 | 0.096 | 0.10 | 0.376 |
| Urine sodium/creatinine (mEq/mg) | 0.264 | 0.394 | 0.39 | <0.001 |
| Intact FGF23 (pg/mL) | −1.30 × 10−3 | −0.271 | −0.27 | 0.011 |
| Multivariable Model (Adjusted R2 for the Model: 0.25) | ||||
|---|---|---|---|---|
| Analyte | β | Standardized β | Partial R | P |
| PTH (pg/mL) | −1.25 × 10−3 | −0.253 | −0.28 | 0.010 |
| Urine sodium/creatinine (mEq/mg) | 0.219 | 0.327 | 0.35 | 0.001 |
| Intact FGF23 (pg/mL) | −1.33 × 10−3 | −0.276 | −0.30 | 0.005 |
For the multivariable model, we entered all covariates found to be statistically significant on univariate analysis.
Discussion
Contrary to our initial hypothesis, in this cross-sectional study of 9- to 18-year-old girls, we did not observe any age-related changes in intact FGF23 nor in C-terminal FGF23 despite observing the expected decline in serum phosphate. In addition, we did not observe any correlation of either form of FGF23 with serum phosphate concentrations or urinary phosphate handling. Notably, TP/GFR, a measure of the renal tubular threshold for phosphate reabsorption, decreased with age, consistent with the previously described physiology that changes in serum phosphate are consequent to alterations in renal phosphate handling. As neither FGF23 nor PTH, the two major hormones promoting renal phosphate excretion, demonstrated age-related changes in concentration, these data suggest that these phosphatonins, rather than being the primary determinants of serum phosphate and renal phosphate excretion, serve to defend a phosphate set point determined by other factors.
The lack of correlation of intact FGF23 with serum phosphate in our cohort is consistent with the findings of a large cohort study of older men (22). In contrast, in a large cohort of premenopausal women, the authors found a significant, although weak, correlation of intact FGF23 with serum phosphate (r = 0.12 to 0.16) (23). Similar weak but significant correlations have also been seen in pediatric populations (19, 24). In particular, Gkentzi et al. (24), in a study of 159 children aged 2 to 18 years, found that both intact and C-terminal FGF23 were positively associated with both serum phosphate (r = 0.18 and r = 0.24, respectively) and tubular maximum reabsorption of phosphate/GFR (r = 0.29 and r = 0.31, respectively), although they did not observe an association of either form of FGF23 with age. Of note, both intact and C-terminal FGF23 concentrations were substantially higher, and 25OHD concentrations were substantially lower in the children in our cohort, possibly related to dietary and other environmental differences between the United States and Greece. In addition, our cohort was limited to preadolescent and adolescent girls, whereas Gkentzi et al. (24) studied both boys and girls with a wider age range. These factors may contribute to the discrepancies in observed correlations between phosphate and FGF23.
Consistent with its known effects to decrease the synthesis and increase the catabolism of 1,25(OH)2D, we observed a negative correlation of intact FGF23 with 1,25(OH)2D in this cohort, although no correlation with C-terminal FGF23 (2, 25). Similar correlations have been reported previously in adults (23, 26). In contrast, Gkentzi et al. (24) did not observe any correlation of these hormones in children. We also observed a positive correlation of serum 25OHD with FGF23; previous reports have shown mixed results, including positive, negative, and absent correlations (13, 19, 24, 27, 28). These discrepancies may reflect the complex coordination of vitamin D and mineral ion metabolism and the interdependent effects of dietary calcium, dietary phosphate, and serum vitamin D metabolites. In particular, given that 1,25(OH)2D is known to stimulate FGF23 production (29, 30), an increase in 25OHD may provide increased substrate for local production of 1,25(OH)2D with autocrine and/or paracrine effects on FGF23 production; this effect may differ in populations with differing distributions of circulating 25OHD concentrations. Our results are consistent with a previous report from our group demonstrating that high-dose vitamin D treatment of subjects with low circulating 25OHD leads to increases in circulating FGF23 (31).
We observed an initially unexpected negative relationship of intact FGF23 with urinary calcium excretion. Emerging data in rodent models however demonstrate that FGF23, in addition to its effects on renal phosphate handling, also plays a critical role in renal handling of other ions, including calcium and sodium (32). In particular, deletion of FGF23 in a mouse model leads to an ∼75% decrease in the fully glycosylated transient receptor potential vanilloid-5 (TRPV5) calcium channel at the apical membrane of distal tubule cells, with an associated ∼75% increase in urine calcium excretion (33). Conversely, injection of recombinant FGF23 to wild-type mice leads to upregulation of TRPV5 and a profound reduction of urinary calcium excretion (33). Clinical studies have also hinted at a relationship of FGF23 with calcium homeostasis in human physiology. Consistent with our observations, a negative correlation of FGF23 concentrations and both fractional excretion of calcium and 24-hour urinary calcium excretion was recently reported in a large cohort of adults with normal renal function, although only C-terminal FGF23 was assayed in that study (34). In a Gambian cohort of children with dietary calcium deficiency-induced rickets, FGF23 concentrations were quite elevated and decreased with dietary calcium treatment over 1 year, although remained higher than those of control children (35). Interestingly, in this cohort, serum calcium was within the reference range and not different from control subjects despite dietary calcium intake of only ∼200 mg/d, perhaps due to the effect of FGF23 to enhance renal calcium reabsorption and maintain normocalcemia. As expected, we found serum PTH and urine sodium excretion to be additional significant predictors of urinary calcium excretion. Notably, in our multivariable model, each of these determinants remained significant with fairly minimal changes in the estimated effect size, suggesting that they operate by independent mechanisms. The independence of PTH and FGF23 in particular is consistent with the model proposed by Andrukhova and colleagues (36, 37) in which FGF23 leads to increased TRPV5 at the apical membrane of distal tubule cells, which is subsequently phosphorylated and activated under the influence of PTH. The absence of observed association of urine calcium with total calcium intake is most likely due to the small size of this cohort, given that the previously reported correlations under normal environmental conditions are of very small magnitude, particularly in pediatric subjects (38, 39).
The positive association of FGF23 with serum calcium was also unexpected. This relationship may be secondary to increased renal reabsorption leading to higher serum calcium. However, data from other studies suggest that circulating calcium may have a direct effect on FGF23 production. In cultured osteoblasts, calcium stimulates FGF23 mRNA synthesis, an effect inhibited by calcium channel blockers (40). In several rodent models of hypocalcemia, including severe dietary calcium deficiency, hypoparathyroidism, 1,25(OH)2D deficiency, and absence of the vitamin D receptor, FGF23 concentrations are low (40–43). In addition, normalization of serum calcium by increased dietary calcium in these models leads to increases in FGF23 (40–43). Conversely, patients with elevated serum calcium in the setting of primary hyperparathyroidism were shown in one study to have elevated FGF23 concentrations, even after controlling for PTH (44). In addition, in patients with renal disease, FGF23 is positively associated with serum calcium and with use of calcium-based phosphate binders (45, 46). However, in the aforementioned cohort of adult patients with normal renal function, there was no association of serum calcium with FGF23 (34). The effect of serum calcium to regulate FGF23 is most likely a chronic rather than an acute effect, as Wesseling-Perry et al. (47) demonstrated that acute changes in serum calcium by infusions of calcium gluconate (to raise serum calcium) and of sodium citrate (to lower serum calcium) had no effect on circulating FGF23 over 2 hours, whereas PTH concentrations changed rapidly.
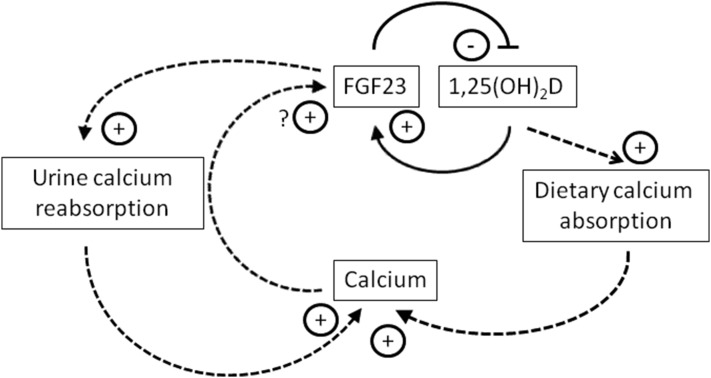
A direct effect of serum calcium to stimulate FGF23 is surprising in light of the increasingly well-established role of FGF23 to enhance urinary calcium reabsorption and would seem to generate an unusual positive-feedback loop. These effects, however, may be better understood when considered in light of the related known negative-feedback loop between 1,25(OH)2D and FGF23, in which 1,25(OH)2D stimulates the production of FGF23, which then decreases circulating 1,25(OH)2D (1) (Fig. 3). Serum calcium may then participate in an indirect negative feedback loop, in which stimulation of FGF23 by calcium leads to downregulation of 1,25(OH)2D and thus decreased gut absorption of calcium, ultimately working to decrease serum calcium concentrations. Conversely, in settings of frank hypocalcemia, lower FGF23 concentrations may be permissive for increased 1,25(OH)2D to promote gastrointestinal absorption of calcium. This may help to explain the observation that in patients treated for hypoparathyroidism, whereas FGF23 concentrations are elevated, they are not sufficiently elevated to normalize serum phosphate (i.e., inappropriately low) (48). The effect of FGF23 to promote urinary calcium reabsorption may function as a counterbalance to the effects of FGF23 on 1,25(OH)2D; for example, if FGF23 is high and 1,25(OH)2D is thus suppressed, increased renal reabsorption of calcium may counter the decreased gastrointestinal absorption in a drive to maintain eucalcemia. The balance among the effects of FGF23 on calcium most likely depends on multiple interdependent factors, including dietary calcium and phosphate content and circulating vitamin D metabolites.
Figure 3.
Proposed model of FGF23 interactions with calcium and modulators of calcium homeostasis. FGF23 and 1,25(OH)2D interact in a previously described feedback loop in which 1,25(OH)2D stimulates FGF23, and FGF23 reduces 1,25(OH)2D levels by inhibiting CYP27B1 and stimulating CYP24A1 activity (solid lines). Data from this cohort suggest that FGF23 and serum calcium may also interact in an indirect feedback loop, in which increased serum calcium stimulates FGF23, thereby inhibiting 1,25(OH)2D synthesis, decreasing dietary calcium absorption, and thus decreasing serum calcium (dashed lines). In addition, the effect of FGF23 to enhance urinary calcium reabsorption, thereby decreasing urinary calcium excretion and increasing serum calcium, counters the effect of FGF23 to diminish 1,25(OH)2D-mediated dietary calcium absorption, and thus works to maintain serum calcium stability.
Our study had several strengths, including a well-phenotyped cohort of healthy pediatric subjects with a physiologically wide range of serum phosphate concentrations. In addition, we measured both intact and C-terminal FGF23, allowing us to more thoroughly investigate the relationships of FGF23 with bone mineral ions and regulatory hormonal networks. There were also some limitations. Our study is cross-sectional; a longitudinal intraindividual investigation of serum phosphate and FGF23 might have brought out a more subtle relationship. We did not have measures of dietary phosphate intake in part due to known imprecision regarding phosphate content in nutritional databases (49). Our study was limited to girls given the differences in serum phosphate between boys and girls and should thus be repeated in boys to investigate whether alterations in the sex steroid milieu affect FGF23 regulation (50). Given the relatively small sample size, we did not perform subgroup analyses in subjects of different ethnic backgrounds. In addition, we did not measure soluble Klotho, which may play a role in FGF23 regulation as well as a direct role in distal tubule TRPV5 regulation (51). Furthermore, a 24-hour urine collection rather than a fasting spot urine calcium/creatinine ratio would have provided a more complete assessment of urinary calcium handling during both fasting and postprandial states (52).
In summary, in our cohort of healthy girls, the age-related decline in TP/GFR was associated with the decline in serum phosphate concentrations, but we observed no age-associated change in either intact or C-terminal FGF23. These data suggest that FGF23 is not a major determinant of the phosphate set point, but rather serves to maintain serum phosphate at the set point established by other factors. In contrast, intact FGF23 was associated with serum calcium and negatively associated with urinary calcium excretion. These data confirm and extend data from rodent models and from cohorts of both healthy adults and children and adults with disorders of mineral metabolism. Further longitudinal studies in varied physiologic states are needed to more clearly elucidate the complex and interdependent regulatory networks maintaining calcium and phosphate homeostasis.
Acknowledgments
We thank the research subjects and families for participation, and the research nurses and bionutritionists at the Massachusetts General Hospital General Clinical Research Center for expert support.
Acknowledgments
This work was supported by National Institutes of Health Grants T32DK007028 (to D.M.M.), F32HD071759 (to D.M.M.), K23DK073356 (to S.M.B.), P01DK11794 (subproject IV; to H.J.), K23DK105350 (to D.M.M.), and UL1RR025758; Harvard Clinical and Translational Science Center; and National Center for Research Resources. This work was also supported by a Massachusetts General Hospital Physician-Scientist Development Award (to S.M.B.), a Harvard Medical School Office for Diversity Inclusion and Community Partnership Award (to S.M.B.), and a Claflin Distinguished Scholar Award (to S.M.B.).
Clinical trial registry: ClinicalTrials.gov no. NCT01180946 (registered 11 August 2010).
Disclosure Summary: H.J. is a named inventor on patents for methods to measure FGF23. All remaining authors have nothing to disclose.
Footnotes
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25OHD
- 25-hydroxyvitamin D
- CTX
- C-terminal telopeptide of type I collagen
- CV
- coefficient of variation
- ELISA
- enzyme-linked immunosorbent assay
- FGF
- fibroblast growth factor
- IGF-5
- insulin-like growth factor-1
- P1NP
- propeptide of type I procollagen
- PTH
- parathyroid hormone
- RU
- relative unit
- TP/GFR
- tubular threshold for phosphate per glomerular filtration rate
- TRPV5
- transient receptor potential vanilloid-5.
References
- 1.Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118(12):3820–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. [DOI] [PubMed] [Google Scholar]
- 3.Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Jüppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145(7):3087–3094. [DOI] [PubMed] [Google Scholar]
- 4.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143(8):3179–3182. [DOI] [PubMed] [Google Scholar]
- 6.ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. [DOI] [PubMed] [Google Scholar]
- 7.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98(11):6500–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. [DOI] [PubMed] [Google Scholar]
- 9.Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, White KE. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146(9):3883–3891. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7(4):318–319. [PubMed] [Google Scholar]
- 11.Loh TP, Metz MP. Trends and physiology of common serum biochemistries in children aged 0-18 years. Pathology. 2015;47(5):452–461. [DOI] [PubMed] [Google Scholar]
- 12.Zierk J, Arzideh F, Rechenauer T, Haeckel R, Rascher W, Metzler M, Rauh M. Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem. 2015;61(7):964–973. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, Feng JQ. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2011;26(5):1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodehl J, Gellissen K, Weber HP. Postnatal development of tubular phosphate reabsorption. Clin Nephrol. 1982;17(4):163–171. [PubMed] [Google Scholar]
- 15.Alon U, Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr Nephrol. 1994;8(2):250–251. [DOI] [PubMed] [Google Scholar]
- 16.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calfee RP, Sutter M, Steffen JA, Goldfarb CA. Skeletal and chronological ages in American adolescents: current findings in skeletal maturation. J Child Orthop. 2010;4(5):467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, Kundt G, Haffner D. Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Ann Clin Biochem. 2012;49(Pt 6):546–553. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23(6):939–948. [DOI] [PubMed] [Google Scholar]
- 21.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89(2):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsell R, Mirza MA, Mallmin H, Karlsson M, Mellström D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O, Larsson TE. Relation between fibroblast growth factor-23, body weight and bone mineral density in elderly men. Osteoporos Int. 2009;20(7):1167–1173. [DOI] [PubMed] [Google Scholar]
- 23.Imel EA, Liu Z, McQueen AK, Acton D, Acton A, Padgett LR, Peacock M, Econs MJ. Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone. 2016;86:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gkentzi D, Efthymiadou A, Kritikou D, Chrysis D. Fibroblast growth factor 23 and Klotho serum levels in healthy children. Bone. 2014;66:8–14. [DOI] [PubMed] [Google Scholar]
- 25.Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278(11):9843–9849. [DOI] [PubMed] [Google Scholar]
- 26.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187–1196. [DOI] [PubMed] [Google Scholar]
- 27.Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellström D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O, Larsson TE. Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol. 2008;158(1):125–129. [DOI] [PubMed] [Google Scholar]
- 28.Lane NE, Parimi N, Corr M, Yao W, Cauley JA, Nielson CM, Ix JH, Kado D, Orwoll E; Osteoporotic Fractures in Men (MrOS) Study Group . Association of serum fibroblast growth factor 23 (FGF23) and incident fractures in older men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. 2013;28(11):2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins MT, Lindsay JR, Jain A, Kelly MH, Cutler CM, Weinstein LS, Liu J, Fedarko NS, Winer KK. Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res. 2005;20(11):1944–1950. [DOI] [PubMed] [Google Scholar]
- 30.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280(4):2543–2549. [DOI] [PubMed] [Google Scholar]
- 31.Burnett-Bowie SA, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol. 2012;7(4):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erben RG, Andrukhova O. FGF23 regulation of renal tubular solute transport. Curr Opin Nephrol Hypertens. 2015;24(5):450–456. [DOI] [PubMed] [Google Scholar]
- 33.Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B, Erben RG. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2014;33(3):229–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhayat NA, Ackermann D, Pruijm M, Ponte B, Ehret G, Guessous I, Leichtle AB, Paccaud F, Mohaupt M, Fiedler GM, Devuyst O, Pechère-Bertschi A, Burnier M, Martin PY, Bochud M, Vogt B, Fuster DG. Fibroblast growth factor 23 and markers of mineral metabolism in individuals with preserved renal function. Kidney Int. 2016;90(3):648–657. [DOI] [PubMed] [Google Scholar]
- 35.Prentice A, Ceesay M, Nigdikar S, Allen SJ, Pettifor JM. FGF23 is elevated in Gambian children with rickets. Bone. 2008;42(4):788–797. [DOI] [PubMed] [Google Scholar]
- 36.Andrukhova O, Streicher C, Zeitz U, Erben RG. Fgf23 and parathyroid hormone signaling interact in kidney and bone. Mol Cell Endocrinol. 2016;436:224–239. [DOI] [PubMed] [Google Scholar]
- 37.de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol. 2009;20(8):1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matkovic V, Ilich JZ, Andon MB, Hsieh LC, Tzagournis MA, Lagger BJ, Goel PK. Urinary calcium, sodium, and bone mass of young females. Am J Clin Nutr. 1995;62(2):417–425. [DOI] [PubMed] [Google Scholar]
- 39.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4(12):1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David V, Dai B, Martin A, Huang J, Han X, Quarles LD. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154(12):4469–4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289(5):F1088–F1095. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Ortiz ME, Lopez I, Muñoz-Castañeda JR, Martinez-Moreno JM, Ramírez AP, Pineda C, Canalejo A, Jaeger P, Aguilera-Tejero E, Rodriguez M, Felsenfeld A, Almaden Y. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23(7):1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn SJ, Thomsen AR, Pang JL, Kantham L, Bräuner-Osborne H, Pollak M, Goltzman D, Brown EM. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol Metab. 2013;304(3):E310–E320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154(1):93–99. [DOI] [PubMed] [Google Scholar]
- 45.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y. FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int. 2004;65(5):1943–1946. [DOI] [PubMed] [Google Scholar]
- 46.Cancela AL, Oliveira RB, Graciolli FG, dos Reis LM, Barreto F, Barreto DV, Cuppari L, Jorgetti V, Carvalho AB, Canziani ME, Moysés RM. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract. 2011;117(1):c74–c82. [DOI] [PubMed] [Google Scholar]
- 47.Wesseling-Perry K, Wang H, Elashoff R, Gales B, Jüppner H, Salusky IB. Lack of FGF23 response to acute changes in serum calcium and PTH in humans. J Clin Endocrinol Metab. 2014;99(10):E1951–E1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89(9):4489–4492. [DOI] [PubMed] [Google Scholar]
- 49.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16(3):186–188. [DOI] [PubMed] [Google Scholar]
- 50.Cannata-Andía JB, Carrillo-López N, Naves-Díaz M. Estrogens and bone disease in chronic kidney disease: role of FGF23. Curr Opin Nephrol Hypertens. 2010;19(4):354–358. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36(2):174–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28(2):120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]