Abstract
Context:
Although bone is a common site for tumor metastases, the burden of bone events [bone metastases and skeletal-related events (SREs)] in patients with thyroid cancer is not well known.
Objective:
To measure the prevalence of bone events and their impact on mortality in patients with thyroid cancer.
Patients, Design, and Setting:
We identified patients diagnosed with thyroid cancer between 1991 and 2011 from the linked Surveillance Epidemiology and End Results–Medicare dataset. Multivariable logistic regression was used to identify the risk factors for bone metastases and SREs. We used Cox proportional hazards regressions to assess the impact of these events on mortality, after adjusting for patient and tumor characteristics.
Results:
Of the 30,063 patients with thyroid cancer, 1173 (3.9%) developed bone metastases and 1661 patients (5.5%) developed an SRE. Compared with papillary thyroid cancer, the likelihood of developing bone metastases or an SRE was higher in follicular thyroid cancer [odds ratio (OR), 2.25; 95% confidence interval (CI), 1.85 to 2.74 and OR, 1.40; 95% CI, 1.15 to 1.68, respectively] and medullary thyroid cancer (OR, 2.16; 95% CI, 1.60 to 2.86 and OR, 1.62; 95% CI, 1.23 to 2.11, respectively). The occurrence of a bone event was associated with greater risk of overall and disease-specific mortality [hazard ratio (HR), 2.14; 95% CI, 1.94 to 2.36 and HR, 1.59; 95% CI, 1.48 to 1.71, respectively]. Bone events were a poor prognostic indicator even when compared with patients with other distant metastases (P < 0.001 and P < 0.001 for overall and disease-specific mortality, respectively).
Conclusions:
Bone events in patients with thyroid cancer are a poor prognostic indicator. Patients with follicular and medullary thyroid cancers are at especially high risk for skeletal complications.
Bone events in patients with thyroid cancer are a poor prognostic indicator. Patients with follicular and medullary thyroid cancers are at especially high risk for skeletal complications.
In the last decade the incidence of thyroid cancer has steadily risen by 4.5% each year (1, 2). It is estimated that by 2019, thyroid cancer will surpass colorectal cancer to become the third most common cancer in women (1, 2). In the United States, ~95% of patients with thyroid cancer were diagnosed with localized or regional disease, but ~5% presented with distant disease (2). Despite the excellent prognosis of patients with localized/regional disease (5-year survival rate, 99%), survival dropped remarkably in the presence of distant metastases (2, 3).
Bone is the second most common site for thyroid cancer metastases (4). Bone provides a congenial environment for tumor cell proliferation and usually portends a poor prognosis (5, 6). Bone metastases also result in high morbidity because of the occurrence of skeletal-related events (SREs). SREs include pathologic fractures, spinal cord compression, need for bone irradiation, or bone surgery. Although comprehensive studies on bone metastases and SREs in other cancers exist, very little is known about bone metastases and SREs in thyroid cancer (7–11).
Because comprehensive data on the occurrence and implications of bone metastases and SREs are lacking, we performed a large population-based study of all patients with thyroid cancer diagnosed between 1991 and 2011 using the Surveillance Epidemiology and End Results (SEER)–Medicare database. In addition to identifying factors that increase the risk of bone events, we examined their impact on overall and disease-specific mortality. We hypothesized that the presence of bone events would be an independent predictor of poor outcome.
Methods
Data source and patient population
The SEER database is a program of the National Cancer Institute and provides data on clinical, demographic, and cause of death information for patients diagnosed with cancer. SEER data cover ~28% of the US population (2). As of 2011, Medicare covers 93% of the US population age >65 years and provides data on covered health services, including diagnosis and treatment. The SEER-Medicare dataset linked these two large population-based sources of data in 1991, therefore providing detailed information about Medicare beneficiaries with cancer.
Data from patients diagnosed with thyroid cancer between January 1, 1991, and December 31, 2011, were extracted from the SEER-Medicare database. All patients with thyroid cancer were included in the study. Data were censored at death or at last follow-up. The study was exempt from institutional review board approval because it used publicly available data that could not be tracked to human subjects.
Measures
Using the International Classification of Diseases (ICD-9) and Current Procedure Terminology (CPT-4) codes, we identified 35,734 patients with thyroid cancer diagnosed between 1991 and 2011. Patients with other cancers known to commonly involve bone, such as breast, prostate, lymphoma, and multiple myeloma (n = 5671) were excluded from the analyses. After excluding these other cancers, our cohort comprised of 30,063 patients. Patients were stratified into three age groups: ≤50, 51 to 65, and >65 years. Patient’s race was categorized by the SEER database as white, black, Hispanic, Asian, North American Native, or other. Asian, Hispanics, North American Native, and other were grouped together as other. Measures of socioeconomic status were categorized as follows: household income (<$30,000; $30,000 to $34,999; $35,000 to $45,999, and >$46,000) and percent of population >25 with a high school degree (<14%, 14% to 19.9%, 20% to 28.9%, and >29%). Tumor histology was limited to ICD-9 using codes for papillary (including variants), follicular (including variants), Hürthle cell, medullary, and anaplastic thyroid cancer. Tumor size was categorized as <1, 1 to 1.9, 2.0 to 4.0, and >4 cm. Patients were stratified according to their SEER stage at diagnosis: localized disease confined to the primary site, regional disease with spread to regional lymph nodes, and distant disease with evidence of metastatic spread. Missing data on race (n = 218, 0.7%), household income (n = 2721, 9.1%), high school degree (n = 2721, 9.1%) tumor size (n = 3517, 11.7%), and unknown/other stage (n = 770, 2.5%) were excluded from the analyses. The total number of patients after exclusion of missing data was 26,350.
A bone event was defined as the occurrence of either bone metastases or SREs. Using ICD-9 and CPT-4 codes, we identified patients with bone metastases and SREs. SREs were defined as pathologic fractures, spinal cord compression, need for bone radiation, and bone surgery. Claims involving SREs can be identified from these two sets of codes. For details on the ICD-9 and CPT-4 codes used, refer to the Supplemental Data (36KB, docx) .
Statistical analysis
Descriptive statistics were used to describe the characteristics of the cohort, including frequency and occurrence of bone metastases and SREs. Multivariable logistic regression models were fit to identify factors associated with the occurrence of a bone event (we used separate models for bone metastases and SRE). We used Cox proportional hazard regression to analyze the association between the occurrence of bone events and death. Hazard ratios (HRs) and 95% confidence intervals (CIs) from the Cox model are reported. Mortality rates were stratified by localized/regional disease without bone-related events, distant disease without bone-related events, and distant disease with bone-related events and were considered significant if P < 0.05. Mortality rates among the different subgroups were compared using the log-rank test. Survival was calculated as the time from diagnosis of thyroid cancer to the date of death or until last follow-up, whichever occurred first. Kaplan-Meier survival plots were constructed for disease-specific and overall mortality. All data were analyzed using SAS version 9.3 (SAS Institute).
Results
The median follow-up time for the cohort was 74 months. As shown in Table 1, the average patient age was 64 years; papillary thyroid cancer was the most common type of thyroid cancer (82.1%), and most patients had localized disease (61.8%). The study population was largely women (71.4%) and white (79.2%). There were 1173 (3.9%) patients who developed bone metastases and 1661 patients (5.5%) that developed an SRE. Bone events (either bone metastases and/or SREs) were identified in 2457 patients (8.2%).
Table 1.
Sociodemographics and Tumor Characteristics of Patients With Thyroid Cancer Diagnosed Between 1991 and 2011
| Characteristic | N (%) |
|---|---|
| Age, y | |
| ≤50 | 3961 (13.2) |
| 51–65 | 12,379 (41.2) |
| >65 | 13,723 (45.6) |
| Sex | |
| Female | 21,474 (71.4) |
| Male | 8589 (28.6) |
| Race | |
| White | 23,640 (79.2) |
| Black | 2284 (7.7) |
| Other | 3921 (13.1) |
| Household income | |
| ≥$46,000 | 6257 (22.9) |
| $35,000–$45,999 | 6862 (25.1) |
| $30,000–$34,999 | 4198 (15.3) |
| <$30,000 | 10,025 (36.7) |
| Percent of no high school degree | |
| <14% | 8224 (30.1) |
| 14%–19.9% | 5121 (18.7) |
| 20%–28.9% | 6191 (22.6) |
| ≥29% | 7806 (28.6) |
| Histology | |
| Papillary | 24,674 (82.1) |
| Follicular | 2467 (8.2) |
| Hürthle cell | 1344 (4.5) |
| Medullary | 870 (2.9) |
| Anaplastic | 708 (2.3) |
| SEER stage | |
| Localized | 18,096 (61.8) |
| Regional | 9361 (32.0) |
| Distant | 1836 (6.2) |
| Tumor size, cm | |
| ≤1 | 9993 (37.6) |
| 1.1–2 | 6482 (24.4) |
| 2.1–4 | 6391 (24.1) |
| >4 | 3680 (13.9) |
| Events | |
| Bone metastases | 1173 (3.9) |
| SRE | |
| Pathologic fracture | 1192 (4.0) |
| Spinal cord compression | 281 (0.9) |
| Surgery or radiation to bone | 298 (1.0) |
| Bone metastases and/or SREa | 2457 (8.2) |
Total number of SREs was 1771, but the number of patients was 1661.
Of the 2457 patients that developed a bone event, 218 (8.9%) had evidence of bone events at the time of diagnosis. Of the 1173 patients who developed bone metastases, 372 (32%) also developed an SRE, with 268 (23%) having a pathologic fracture.
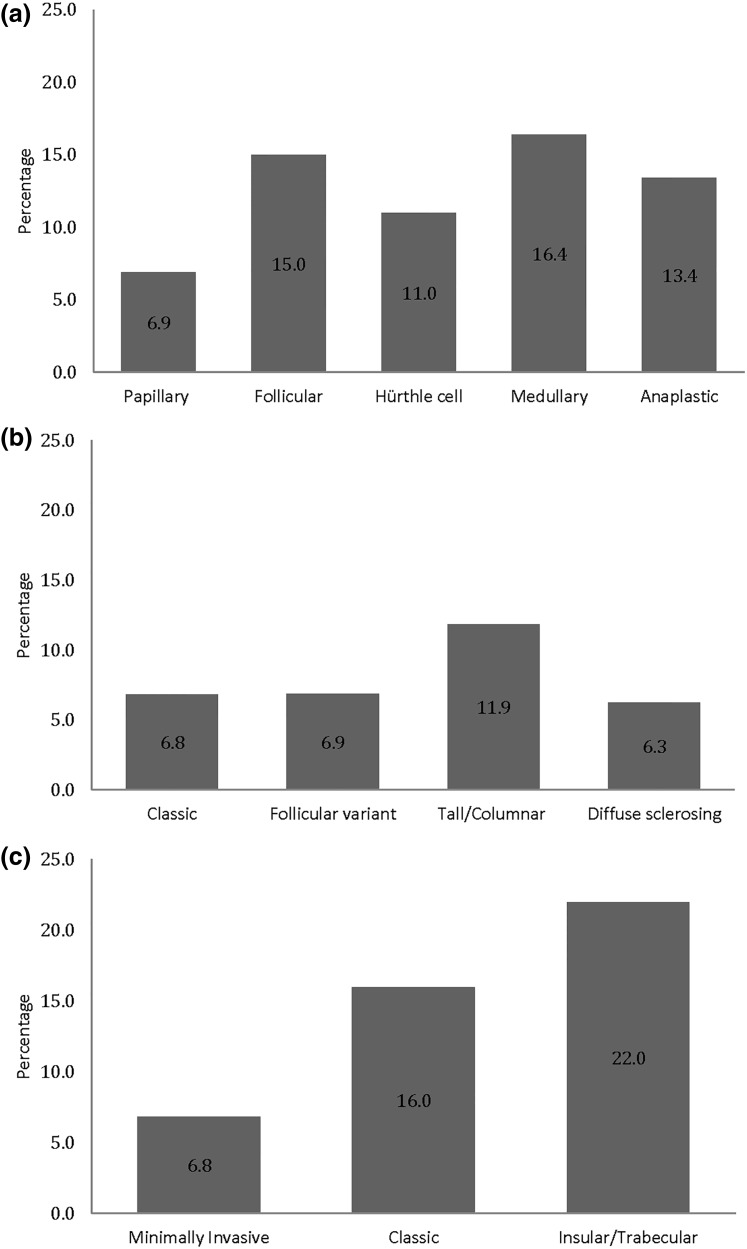
Compared with papillary thyroid cancer, the occurrence of bone events was higher in patients with follicular (15.0%) and medullary thyroid cancer (16.4%) (Fig. 1). Among the variants of papillary thyroid cancer, 11.9% of tall/columnar cell variant had bone events versus 6.9% of follicular variant, 6.8% of classic, and 6.3% of diffuse sclerosing. Similarly, 22% of insular/trabecular variant developed bone events compared with 6.8% of minimally invasive and 16% of classic variants of follicular thyroid cancer.
Figure 1.
(a) Percentage of bone metastases and/or SREs in thyroid cancer. (b) Percentage of bone metastases and/or SREs in variants of papillary thyroid cancer. (c) Percentage of bone metastases and/or SREs in variants of follicular thyroid cancer.
In multivariable analysis shown in Table 2, patients >65 years of age were more likely to develop bone metastases and SREs than those ≤50 years of age [odds ratio (OR), 1.87; 95% CI, 1.45 to 2.45 and OR, 2.40; 95% CI, 1.96 to 2.97, respectively]. Patients with follicular and medullary thyroid cancers were more likely to develop bone metastases (OR, 2.25; 95% CI, 1.85 to 2.74 and OR, 2.16; 95% CI, 1.60 to 2.86, respectively) and SREs (OR, 1.40; 95% CI, 1.15 to 1.68 and OR, 1.62; 95% CI, 1.23 to 2.11, respectively). In addition, as shown in Table 2 based on SEER staging, regional and distant disease were associated with greater odds of developing bone metastases (OR, 2.21; 95% CI, 1.86 to 2.62 and OR, 12.66; 95% CI, 10.35 to 15.49, respectively) and SREs (OR, 1.22; 95% CI, 1.07 to 1.39 and OR, 2.96; 95% CI, 2.43 to 3.61, respectively). Furthermore, the difference between the rate of occurrence of bone events between follicular and medullary thyroid cancer was not significant (P = 0.83).
Table 2.
Multivariable Analysis of Factors Associated With Bone Metastases and SREs in Patients With Thyroid Cancer
| Characteristic | Bone Metastases [OR (95% CI)] | SREs [OR (95% CI)] |
|---|---|---|
| Histology | ||
| Papillary | 1.0 (Ref) | 1.0 (Ref) |
| Follicular | 2.25(1.85–2.74) | 1.40 (1.15–1.68) |
| Hürthle cell | 1.77 (1.34–2.32) | 1.20 (0.93–1.54) |
| Medullary | 2.16 (1.60–2.86) | 1.62 (1.23–2.11) |
| Anaplastic | 0.40 (0.26–0.60) | 0.89 (0.63–1.25) |
| Age, y | ||
| ≤50 | 1.0 (Ref) | 1.0 (Ref) |
| 51–65 | 1.44 (1.11–1.90) | 1.20 (0.97–1.51) |
| >65 | 1.87 (1.45–2.45) | 2.40 (1.96–2.97) |
| Sex | ||
| Female | 1.0 (Ref) | 1.0 (Ref) |
| Male | 1.37 (1.18–1.58) | 0.70 (0.61–0.79) |
| Race | ||
| White | 1.0 (Ref) | 1.0 (Ref) |
| Black | 0.78 (0.57–1.05) | 0.60 (0.46–0.77) |
| Other | 1.11 (0.91–1.34) | 0.87 (0.73–1.02) |
| SEER stage | ||
| Localized | 1.0 (Ref) | 1.0 (Ref) |
| Regional | 2.21 (1.86–2.62) | 1.22 (1.07–1.39) |
| Distant | 12.66 (10.35–15.49) | 2.96 (2.43–3.61) |
| Tumor size, cm | ||
| ≤1 | 1.0 (Ref) | 1.0 (Ref) |
| 1.1–2 | 1.05 (0.84–1.32) | 0.92 (0.79–1.07) |
| 2.1–4 | 1.38 (1.12–1.71) | 0.99 (0.85–1.16) |
| >4 | 1.54 (1.22–1.95) | 0.98 (0.81–1.18) |
Abbreviation: Ref, reference.
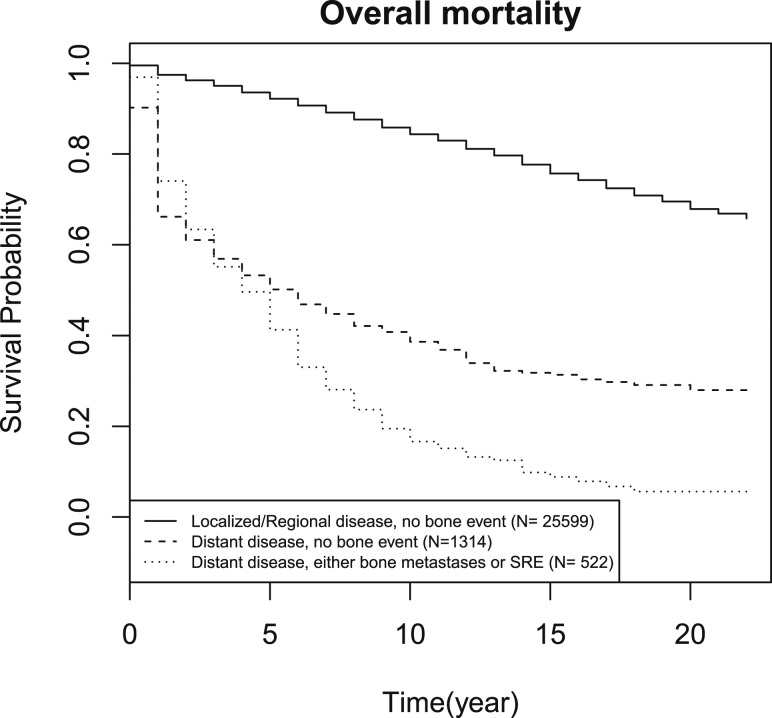
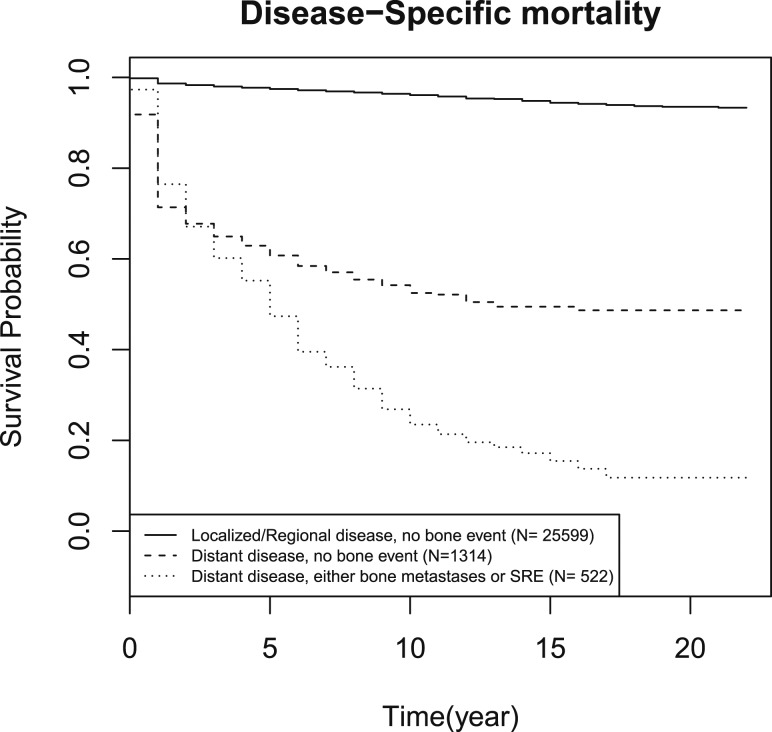
Results from the Cox proportional hazards regression (Table 3) demonstrate that older age and male sex are associated with higher mortality. After controlling for histology, age, sex, race, and SEER stage, the presence of a bone event independently correlated with higher risk of overall (HR, 2.14; 95% CI, 1.94 to 2.36) and disease-specific mortality (HR, 1.59; 95% CI, 1.48 to 1.71) (Table 3). Even in patients with distant disease, the occurrence of bone events was associated with higher overall and disease-specific mortality than those who did not develop a bone event (P < 0.001 and P < 0.001, respectively) (Figs. 2 and 3). In the presence of bone events, overall and disease-specific mortality was higher in papillary (P < 0.001 and P < 0.001, respectively) and medullary thyroid cancer (P < 0.001 and P < 0.001, respectively). In contrast, bone events were not predictive of either overall or disease-specific mortality in follicular (P = 0.84 and P = 0.31, respectively) and anaplastic thyroid cancer (P = 0.07 and P = 0.18, respectively). Data are shown in the Supplemental Data (36KB, docx) .
Table 3.
Relationship Between Bone Metastases and/or SRE and Patient Mortality
| Characteristic | Overall Mortality [HR (95% CI)] | Disease-Specific Mortality [HR (95% CI)] |
|---|---|---|
| Histology | ||
| Papillary | 1.0 (Ref) | 1.0 (Ref) |
| Follicular | 1.22 (1.12–1.33) | 1.35 (1.18–1.55) |
| Hürthle cell | 1.26 (1.12–1.41) | 1.36 (1.13–1.65) |
| Medullary | 1.53 (1.35–1.74) | 2.47 (2.07–2.94) |
| Anaplastic | 7.15 (6.45–7.94) | 10.55 (9.28–11.99) |
| Age, y | ||
| ≤50 | 1.0 (Ref) | 1.0 (Ref) |
| 51–65 | 1.57 (1.39–1.77) | 2.41 (1.85–3.15) |
| >65 | 6.03 (5.37–6.76) | 8.58 (6.65–11.07) |
| Sex | ||
| Female | 1.0 (Ref) | 1.0 (Ref) |
| Male | 1.36 (1.29–1.44) | 1.18 (1.08–1.29) |
| Race | ||
| White | 1.0 (Ref) | 1.0 (Ref) |
| Black | 1.38 (1.25–1.53) | 1.04 (0.87–1.25) |
| Other | 1.03 (0.96–1.12) | 1.07 (0.95–1.20) |
| SEER stage | ||
| Localized | 1.0 (Ref) | 1.0 (Ref) |
| Regional | 1.70 (1.60–1.82) | 5.84 (5.07–6.74) |
| Distant | 4.88 (4.48–5.31) | 20.52 (17.53–24.02) |
| Bone metastases and/or SREs | ||
| No | 1.0 (Ref) | 1.0 (Ref) |
| Yes | 1.59 (1.48–1.71) | 2.14 (1.94–2.36) |
Abbreviation: Ref, reference.
Figure 2.
Kaplan-Meier curves of overall mortality for patients with localized or regional disease and no bone events, distant disease with no bone events, and distant disease with bone events.
Figure 3.
Kaplan-Meier curves of disease-specific mortality for patients with localized or regional disease and no bone events, distant disease with no bone events, and distant disease with bone events.
Excluding patients with missing income and education data did not have a substantial effect on the results.
Discussion
In our large, population-based study of bone events in patients with thyroid cancer, we found that the frequency of bone events is higher in follicular and medullary thyroid cancer. Older patients and those with regional/distant disease are at greater risk for bone events. Of clinical importance, bone events were an independent poor prognostic indicator, even when compared with other patients with distant metastases but without bone events.
Although thyroid cancer is predisposed to bone metastases, the existent data are limited to older, retrospective, single institution studies with small cohorts mainly focused on differentiated thyroid cancer (4). Additionally, our study included all types of thyroid cancer variants. We observed that bone metastases occurred in 3.9% (1173) of the patients, and this is similar to previous reports from other studies (range, 3.8% to 12.7%) (12–20). Prior studies done largely on differentiated thyroid cancers reported that the rates of bone metastases were higher in follicular thyroid cancer (13, 15, 18, 20, 21). In our comprehensive cohort, we saw a high occurrence of bone events in both follicular and medullary thyroid cancer. Until recently, data on bone events in medullary thyroid cancer were limited (22). Xu et al. (23) evaluated the risk of bone metastases and SREs in 188 patients with medullary thyroid cancer from a single institution. In their retrospective chart review, 19% of patients were confirmed to have bone metastases; of these, 48% developed one or more SRE (23). In our population-based cohort, we observed a 16.4% occurrence of bone metastases or SREs with medullary thyroid cancer. Moreover, in our study, the difference in the rate of occurrence was not significant between medullary and follicular thyroid cancer. Prior studies were unable to perform between-group comparisons because they were limited by their sample size (16, 24, 25). Contrary to the findings by Farooki et al. (10) in 2012, wherein they reported 78% occurrence of SREs in differentiated thyroid cancers, we found 32% of patients with bone metastases developed an SRE. The difference in the rates is most likely explained by a higher percentage of patients with follicular thyroid cancer in their study (34%) versus our study (8%) and modalities used for detection of bone metastases (10). In our study, pathologic fractures were the most frequently noted SREs (23%), which is lower than that reported by Farooki et al. (10) We observed that 8.9% of the patients had evidence of bone events at the time of diagnosis of thyroid cancer, which is lower than reports from other studies (13, 15, 18, 20). The observed differences are likely caused by the differences in procedure/modalities used to detect bone events in these studies. Because of our large population-based sample, we were able to evaluate the variants of differentiated thyroid cancer. We found that aggressive variants of differentiated thyroid cancer, such as tall/columnar variant of papillary thyroid cancer and insular/trabecular variant of follicular thyroid cancer, have a higher percentage of bone metastases or SREs. Prior to our study, data on SREs in variants of differentiated thyroid cancer were limited.
Known prognostic factors, such as age, time of detection of bone metastases, and radioactive iodine avidity, have previously been shown to affect overall survival (3, 26, 27). Even though bone metastases occur less frequently than pulmonary metastases, the prognosis for those with bone metastases is generally believed to be worse (21, 28). In the presence of bone metastases, 10-year survival drops to 13% to 53% (15, 19–21, 29). Similarly, we found that bone events were a strong predictor of overall and disease-specific mortality. This was strikingly observed in patients with distant disease, where overall and disease-specific mortality were higher in the presence of bone events. Contrary to previous data we found the presence of a bone event is associated with higher overall and disease-specific mortality in papillary and medullary thyroid cancer but not in follicular thyroid cancer (23, 25).
The strengths of this study are the longitudinal population-based setting, the large sample size, and inclusion of all types of thyroid cancer (including variants of papillary and follicular thyroid cancer). Moreover, we evaluated patients with all stages of thyroid cancer and not only those with distant disease, therefore providing an overall estimate of the risk of skeletal complications. Health care claims are an integral source of information on bone metastases and SREs. The linking of SEER and Medicare provides information across different sources. Similar to studies evaluating SREs in other cancers, we found that the identification of an SRE depends on utilization of diagnosis and procedure codes found on health care claims (30–32). These claims have not been validated to identify bone metastases and SREs. There may be services that are not captured on claims, and similarly there may be services not covered by Medicare and therefore not found in the database. Therefore, although it is likely we have missed true cases and the occurrence (cumulative rates) of bone metastases and SREs is underreported, it is also possible that false-positive cases were included in our analysis. Our results are generalizable to the population, although Medicare includes mostly older patients and therefore applicability of these findings in the younger patients may be reduced. In addition, we were unable to evaluate for the number and location of bone metastases and treatment with bisphosphonates, which may potentially have an impact on bone events. Despite these limitations, the findings in our study parallel the results from the other single institution studies.
Bone metastases and SREs are a serious complication in thyroid cancer. Patients with follicular and medullary thyroid cancer have a greater likelihood of developing bone events. The occurrence of a bone event is an independent poor prognostic indicator. Our data are reflective of the population at large, and our findings emphasize the need to appropriately evaluate bone events, especially in high-risk groups. Improved disease-specific survival remains central to cancer research; however, symptom relief and protection against skeletal complications should not be overlooked. Prospective studies evaluating the benefits of denosumab and bisphosphonates in thyroid cancer are needed. It is imperative to focus on the pathogenesis, prevention, and treatment of bone events in these uniquely high-risk patients.
Acknowledgments
The authors thank Brittany Gay for assisting with production of tables and figures and for reviewing the manuscript.
Acknowledgments
M.H. was supported by grant number R01CA201198 from the National Cancer Institute and R01HS024512 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Agency for Healthcare Research and Quality.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
Abbreviations:
- CI
- confidence interval
- CPT-4
- Current Procedure Terminology
- HR
- hazard ratio
- ICD-9
- International Classification of Diseases
- OR
- odds ratio
- SEER
- Surveillance Epidemiology and End Results
- SRE
- skeletal-related event.
References
- 1.Aschebrook-Kilfoy B, Schechter RB, Shih YC, Kaplan EL, Chiu BC, Angelos P, Grogan RH. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1252–1259. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER. Surveillance, Epidemiology, and End Results Program. Available at: www.seer.cancer.gov. Accessed May 2016.
- 3.Banerjee M, Muenz DG, Chang JT, Papaleontiou M, Haymart MR. Tree-based model for thyroid cancer prognostication. J Clin Endocrinol Metab. 2014;99(10):3737–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muresan MM, Olivier P, Leclère J, Sirveaux F, Brunaud L, Klein M, Zarnegar R, Weryha G. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15(1):37–49. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 6.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. [DOI] [PubMed] [Google Scholar]
- 7.Broder MS, Gutierrez B, Cherepanov D, Linhares Y. Burden of skeletal-related events in prostate cancer: unmet need in pain improvement. Support Care Cancer. 2015;23(1):237–247. [DOI] [PubMed] [Google Scholar]
- 8.Clemons M, Gelmon KA, Pritchard KI, Paterson AH. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: the state of the art. Curr Oncol. 2012;19(5):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman RE. Skeletal complications of malignancy. Cancer. 1997; 80(8 Suppl)1588–1594. [DOI] [PubMed] [Google Scholar]
- 10.Farooki A, Leung V, Tala H, Tuttle RM. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2012;97(7):2433–2439. [DOI] [PubMed] [Google Scholar]
- 11.Poon M, Zeng L, Zhang L, Lam H, Emmenegger U, Wong E, Bedard G, Lao N, Chow R, Chow E. Incidence of skeletal-related events over time from solid tumour bone metastases reported in randomised trials using bone-modifying agents. Clin Oncol (R Coll Radiol). 2013;25(7):435–444. [DOI] [PubMed] [Google Scholar]
- 12.Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, Enkaoua E, Turpin G, Chiras J, Saillant G, Hejblum G. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86(4):1568–1573. [DOI] [PubMed] [Google Scholar]
- 13.Fanchiang JK, Lin JD, Huang MJ, Shih HN. Papillary and follicular thyroid carcinomas with bone metastases: a series of 39 cases during a period of 18 years. Changge-ng Yi-xue Zazhi. 1998;21(4):377–382. [PubMed] [Google Scholar]
- 14.Lin JD, Huang MJ, Juang JH, Chao TC, Huang BY, Chen KW, Chen JY, Li KL, Chen JF, Ho YS. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9(12):1227–1235. [DOI] [PubMed] [Google Scholar]
- 15.Marcocci C, Pacini F, Elisei R, Schipani E, Ceccarelli C, Miccoli P, Arganini M, Pinchera A. Clinical and biologic behavior of bone metastases from differentiated thyroid carcinoma. Surgery. 1989;106(6):960–966. [PubMed] [Google Scholar]
- 16.McCormack KR. Bone metastases from thyroid carcinoma. Cancer. 1966;19(2):181–184. [DOI] [PubMed] [Google Scholar]
- 17.Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, Robbins RJ. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10(3):261–268. [DOI] [PubMed] [Google Scholar]
- 18.Proye CA, Dromer DH, Carnaille BM, Gontier AJ, Goropoulos A, Carpentier P, Lefebvre J, Decoulx M, Wemeau JL, Fossati P, Sulman C. Is it still worthwhile to treat bone metastases from differentiated thyroid carcinoma with radioactive iodine? World J Surg. 1992;16(4):640–645, discussion 645–646. [DOI] [PubMed] [Google Scholar]
- 19.Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F, Ricard M, Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37(4):598–605. [PubMed] [Google Scholar]
- 20.Wu K, Hou SM, Huang TS, Yang RS. Thyroid carcinoma with bone metastases: a prognostic factor study. Clin Med Oncol. 2008;2:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. [DOI] [PubMed] [Google Scholar]
- 22.Giraudet AL, Vanel D, Leboulleux S, Aupérin A, Dromain C, Chami L, Ny Tovo N, Lumbroso J, Lassau N, Bonniaud G, Hartl D, Travagli JP, Baudin E, Schlumberger M. Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J Clin Endocrinol Metab. 2007;92(11):4185–4190. [DOI] [PubMed] [Google Scholar]
- 23.Xu JY, Murphy WA Jr, Milton DR, Jimenez C, Rao SN, Habra MA, Waguespack SG, Dadu R, Gagel RF, Ying AK, Cabanillas ME, Weitzman SP, Busaidy NL, Sellin RV, Grubbs E, Sherman SI, Hu MI. Bone metastases and skeletal-related events in medullary thyroid carcinoma. J Clin Endocrinol Metab. 2016;101(12):4871–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heitz P, Moser H, Staub JJ. Thyroid cancer: a study of 573 thyroid tumors and 161 autopsy cases observed over a thirty-year period. Cancer. 1976;37(5):2329–2337. [DOI] [PubMed] [Google Scholar]
- 25.Tickoo SK, Pittas AG, Adler M, Fazzari M, Larson SM, Robbins RJ, Rosai J. Bone metastases from thyroid carcinoma: a histopathologic study with clinical correlates. Arch Pathol Lab Med. 2000;124(10):1440–1447. [DOI] [PubMed] [Google Scholar]
- 26.Choi YM, Kim WG, Kwon H, Jeon MJ, Lee JJ, Ryu JS, Hong EG, Kim TY, Shong YK, Kim WB. Early prognostic factors at the time of diagnosis of bone metastasis in patients with bone metastases of differentiated thyroid carcinoma. Eur J Endocrinol. 2016;175(3):165–172. [DOI] [PubMed] [Google Scholar]
- 27.Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67(3):501–508. [DOI] [PubMed] [Google Scholar]
- 28.Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf). 2005;63(1):87–93. [DOI] [PubMed] [Google Scholar]
- 29.Beierwaltes WH, Nishiyama RH, Thompson NW, Copp JE, Kubo A. Survival time and “cure” in papillary and follicular thyroid carcinoma with distant metastases: statistics following University of Michigan therapy. J Nucl Med. 1982;23(7):561–568. [PubMed] [Google Scholar]
- 30.Aly A, Onukwugha E, Woods C, Mullins CD, Kwok Y, Qian Y, Arellano J, Balakumaran A, Hussain A. Measurement of skeletal related events in SEER-Medicare: a comparison of claims-based methods. BMC Med Res Methodol. 2015;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onukwugha E, Yong C, Mullins CD, Seal B, McNally D, Hussain A. Skeletal-related events and mortality among older men with advanced prostate cancer. J Geriatr Oncol. 2014;5(3):281–289. [DOI] [PubMed] [Google Scholar]
- 32.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, Blackburn J, Arora T, Kilgore ML. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999-2006. Prostate Cancer Prostatic Dis. 2011;14(2):177–183. [DOI] [PubMed] [Google Scholar]