Abstract
The epidemiology of vector-borne zoonoses depends on the movement of both hosts and vectors, which can differ greatly in intensity across spatial scales. Because of their life history traits and small size, vector dispersal may be frequent, but limited in distance. However, little information is available on vector movement patterns at local spatial scales, and particularly for ticks, transmitting the greatest diversity of recognized infectious agents. To test the degree to which ticks can disperse and disseminate pathogens at local scales, we investigated the temporal dynamics and population structure of the soft tick Ornithodoros maritimus within a colony of its seabird host, the Yellow-legged gull Larus michahellis. Ticks were repeatedly sampled at a series of nests during the host breeding season. In half of the nests, ticks were collected (removal sampling), in the other half, ticks were counted and returned to the nest. A subsample of ticks was screened for known bacteria, viruses and parasites using a high throughput real-time PCR system to examine their distribution within the colony. The results indicate a temporal dynamic in the presence of tick life stages over the season, with the simultaneous appearance of juvenile ticks and hatched chicks, but no among-nest spatial structure in tick abundance. Removal sampling significantly reduced tick numbers, but only from the fourth visit onward. Seven bacterial isolates, one parasite species and one viral isolate were detected but no spatial structure in their presence within the colony was found. These results suggest weak isolation among nests and that tick dispersal is likely frequent enough to quickly recolonize locally-emptied patches and disseminate pathogens across the colony. Vector-mediated movements at local scales may therefore play a key role in pathogen emergence and needs to be considered in conjunction with host movements for predicting pathogen circulation and for establishing effective control strategies.
Keywords: Argasidae, Within-nest dynamics, Dispersal, Epidemiology
Graphical abstract
Highlights
-
•
A temporal dynamic in the abundance of tick stages was found over the season.
-
•
Destructive sampling reduced tick abundance near the end of the sampling period.
-
•
No spatial structure in the ticks or infectious agents was detected.
-
•
Relatively frequent tick movements among nests were suggested.
1. Introduction
Vector-borne zoonoses are maintained, by definition, in complex transmission cycles that can include different vector and reservoir host species (Sonenshine and Mather, 1994, Brisson et al., 2008, Keesing et al., 2006). Locally, vector spatial dynamics mediate pathogen population structure among hosts (Gómez-Díaz et al., 2010), the outcome of host-parasite co-evolutionary interactions (Gandon and Michalakis, 2002) and ultimately the dynamics of disease emergence (Ostfeld et al., 2005). Understanding the spatial and temporal dynamics of vectors is thus of major importance to prevent or control outbreaks, especially as global change favors the redistribution of biodiversity (Tylianakis et al., 2008, Ogden, 2013). However, very little information is currently available on local scale movements of vectors. Indeed, few studies have focused on vector dispersal patterns even in major disease-causing systems (but see Abrahan et al., 2011 for Triatominae vectors of Chagas disease and Service (1997) for a review on mosquito dispersal). Ticks are among the most important vectors of diseases worldwide and are able to transmit bacteria, viruses and eukaryotic parasites (Jongejan and Uilenberg, 2004) to a wide variety of vertebrate hosts including birds, reptiles and mammals (Sonenshine and Roe, 2014). As for other vectors, work on local scale movements of ticks has been limited. McCoy et al. (2003a) found that cliff topography and nest site organization are of key importance for predicting local gene flow of the seabird tick Ixodes uriae. Likewise, soft ticks of the Ornithodoros turicata species showed low inter-burrow movement and specific depth preferences within tortoise burrows (Adeyeye and Butler, 1989). These studies highlight the variety of different biotic and abiotic factors that may come into play when trying to understand local scale vector structure.
Ornithodoros maritimus Vermeil and Marguet (1967) is a member of the soft tick (Argasidae) complex O. capensis sensu lato containing eight described species exploiting colonial seabirds in tropical and sub-tropical areas of the globe (Perez-Eid, 2007). Ornithodoros maritimus has been recorded in association with a wide range of seabird host species including cormorants, gulls, alcids and terns in Great Britain, Ireland, France, Italy, Spain and Tunisia (Hoogstraal et al., 1976). It is also known to vector numerous viruses such as Meaban and West Nile viruses (Dietrich et al., 2011, Arnal et al., 2014), and Soldado virus which can induce high mortality rates in bird populations and pruritus in humans (Converse et al., 1975, Feare, 1976). More recently, these ticks have also been found to harbor endosymbiotic bacteria that, in addition to playing a potentially important role in tick biology, may favor the emergence of medically important pathogens, such as the Q fever agent Coxiella burnetii (Duron et al., 2015). Ornithodoros maritimus is largely nidicolous, feeding on the host rapidly at night in the nymphal and adult life stages. This limited contact with the host should result in low tick dispersal among colonies, and should have a cascading effect on pathogen spread (Kada et al., in press).
Here, we investigate the dynamics of the different tick life stages within host nests over the course of a seabird breeding season and test experimentally for spatial structuring among nests at the scale of the breeding colony. To examine the degree to which ticks move among nests, we destructively sampled ticks in half of our study nests to determine whether we could alter within-nest population dynamics by removing ticks. We then used a high throughput real-time PCR system to test for the presence of the most common tick-borne infectious agents including bacteria, parasites and viruses potentially harbored by the ticks and examined their spatial structure within the colony. If ticks are spatially restricted in their dispersal capacity, we expected that removal sampling would lead to the extinction of the local nest population. We also expected that pathogen occurrence in ticks would show strong spatial autocorrelation, with a strong positive correlation among ticks in the same nest and a reduction in this correlation as the distance among nests increased.
2. Material and methods
2.1. Biological system
Like other soft ticks, the life cycle of O. maritimus is polyphasic, composed of three active stages: a single larval stage, several nymphal instars and a sexual adult stage. As larvae, the ticks attach and feed for several hours. In nymphal and adult stages, the ticks feed to repletion in a few minutes during periods when the host is resting – typically at night. Females oviposit after each blood meal, depositing a few hundred eggs each time (Vial, 2009). While not feeding, the ticks of all life stages are found in the nest or surrounding area (nidicolous) where they benefit from stable temperature and humidity conditions (Vial, 2009). Neither O. maritimus, nor its close relatives, have been well-studied under field conditions. However, laboratory data from O. capensis fed on pigeons suggest that a complete cycle may take about 70 days, from engorged adult females to new adult individuals, including 2 or 3 nymphal instars.
O. maritimus regularly exploits the Yellow-legged gull Larus michahellis in breeding colonies across the Mediterranean Sea. These gulls breed in dense colonies (pairs usually nest several meters apart), laying eggs in nests built with a sparse mound of vegetation on the ground or on cliff ledges (Olsen, 2004). These birds are territorial, with relatively limited movements during the breeding season, mainly from feeding areas to the nest area. Outside the breeding season, the species remains gregarious, congregating around ports, harbours and dumps (Olsen, 2004).
2.2. Tick sampling and study location
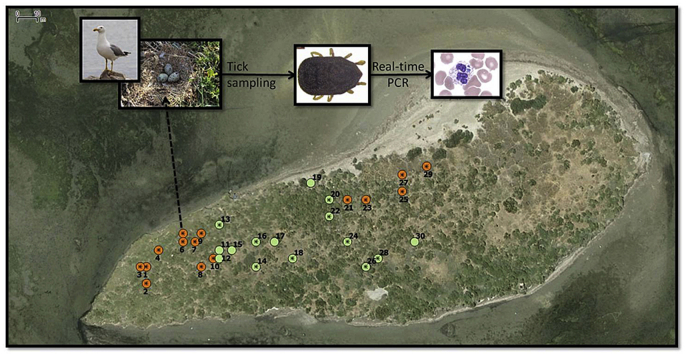
Ticks were collected in the Camargue area of southern France from March to May 2015 on Carteau, a flat island in the Gulf of Fos of 210 m long and 65 m wide, entirely occupied by a breeding colony of Yellow-legged gulls L. michahellis. In 2015, 385 breeding pairs occupied the island over the reproductive season (February to June). We selected, marked and recorded the gps coordinates of 30 nests across the island (Fig. 1). At each visit, a nest was searched for 3 min by two people; one person examined the upper nest materials in a white tray and, the other searched directly inside each nest and all ticks found were removed. For 15 of the 30 focal nests, ticks were returned to the nest once all life stages were identified and counted (non-engorged females, engorged females, males, non-engorged nymphs, engorged nymphs and larvae); we refer to these nests as “counted nests”. In the other 15 nests, all ticks were collected and kept alive in the laboratory; we refer to these as “collected nests” (Fig. 1). The sampling protocol was repeated once per week over six weeks on the same nests. Only during the last visit were all ticks collected from all nests. After collection, ticks were identified and used for DNA/RNA extractions. At each visit, the number of gull eggs, hatching eggs or chicks in each nest was noted. In 2015, gull eggs started hatching during the fourth visit (April 16th).
Fig. 1.
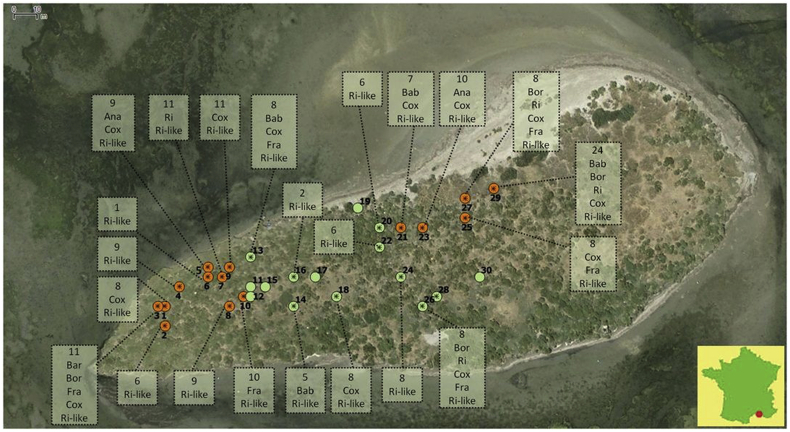
Map showing the position of the 30 tracked nests on Carteau Island, in the Camargue region of France (represented by the red point on the bottom right map). Orange points represent the 15 nests in which ticks were counted and released. The green points are those nests where all ticks were counted and collected. Stars within the points represent the nests in which ticks were used for the screening of infectious agents. Boxes indicate the number of ticks screened and the detected infectious agents: Ana: Anaplasma spp.; Bab: Babesia spp.; Bar: Bartonella spp.; Bor: Borrelia spp.; Cox: Coxiella-like symbiont; Fra: Francisella-like symbiont; Ri: Rickettsia helvetica; Ri-like: Rickettsia-like symbiont. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3. RNA and genomic DNA extraction
Extraction protocols followed those outlined in Moutailler et al. (2016) and Michelet et al. (2014). 201 non engorged adult ticks (162 females and 39 males) collected at the second, fourth, fifth and sixth visits were selected from 24 study nests to test for the presence of infectious agents (∼7ticks/nest). All ticks were washed for 5 min in an ethanol bath, 10 min in two successive water baths and placed individually in sterile tubes and crushed in 300 μL of Dulbecco's modified eagle medium (DMEM) with 10% fetal calf serum using the Precellys®24 Dual homogenizer (Bertin, France). The supernatant was divided into 3 fractions: 100 μL for the DNA extraction, 100 μL for the RNA extraction and the rest was used as back-up and conserved at −80 °C. Genomic tick DNA was then extracted using the Wizard genomic DNA purification kit (Promega, France) according to the manufacturer's instructions. RNA was extracted using the Nucleospin RNA II kit (Macherey Nagel, Germany) according to the manufacturer's instructions. Purified DNA was eluted into 50 μL elution buffer and stored at −20 °C, whereas RNA was eluted into 50 μL of RNase-free water and conserved at −80 °C. Tick DNA and RNA quality was assessed via the amplification of the ITS2 and COI respectively (Michelet et al., 2014, Moutailler et al., 2016).
2.4. High throughput real-time PCR system
Ticks were analyzed for the most common tick-borne infectious agents using the BioMark real-time PCR system (Fluigdim, USA) for high-throughput microfluidic real-time PCR amplification. The DNA primer chip developed by Michelet et al. (2014) includes primers for detecting 28 bacterial species, 12 parasite species and 25 viruses (SM Table A1).
All RNAs were reverse transcribed into cDNAs using random primers and oligos (dT). The remaining methods followed those of Michelet et al. (2014) and Moutailler et al., 2016.
DNA and cDNA pre-amplifications were performed using the TaqMan PreAmp Master Mix (Applied Biosystems, France) according to the manufacturer's instructions. Primers for bacteria or viruses were pooled by combining equal volumes of each primer to have 200 nM of each. The pre-amplification was performed in a final volume of 5 μL containing 2.5 μL TaqMan PreAmp Master Mix (2×), 1.2 μL pooled primer mix (0.2×) and 1.3 μL DNA. Thermal cycling conditions were as follows: one cycle at 95 °C for 10min, 14 cycles at 95 °C for 15s and 4 min at 60 °C (Michelet et al., 2014).
The qPCR reactions were then performed using 6-carboxyfluorescein (FAM) and black hole quencher (BHQ1)-labeled TaqMan probes (Michelet et al., 2014) with TaqMan Gene expression Master Mix, in accordance to the manufacturer's instructions (Applied Biosystem, France). PCR cycling comprised 5 min at 95 °C, 45 cycles at 95 °C for 10s, 15 s at 60 °C and 10 s at 40 °C.
Data were acquired on the BioMark Real-Time PCR system and analyzed using the Fluigdim Real-Time PCR Analysis software to obtain crossing point (CP) values. The assays were performed in duplicate using two negative water controls per chip and Escherichia coli strain EDL933 was added in each run to control for internal inhibition (Michelet et al., 2014).
2.5. Validation of detected infectious agents
Conventional and nested PCRs using different primers than those of the BioMark® system were used to confirm the presence of the detected infectious agents in the samples (Table 1). Amplicons were sequenced by Eurofins MWG Operon (Germany) and assembled using the BioEdit software (Ibis Biosciences, Carlsbad). An online BLAST (National Center for Biotechnology Information) was used to identify the sequenced organisms.
Table 1.
List of pathogens detected using the high-throughput microfluidic real-time PCR amplification. The target gene refers to the gene used for the initial detection of the infectious agent and the confirmation method, the gene and procedure used for verifying the correction identification of the organism. NP: Not performed.
| Pathogens | Species | Target Gene (HT) | Positive ticks - Prevalence % (n = 201) | Confirmation method (gene targeted) | Reference confirmation technique | Confirmation | Sequence reference |
|---|---|---|---|---|---|---|---|
| Bacterium | Anaplasma spp. | msp2 | 3–1.5 | PCR (16S) | Hornok et al., 2008 | Anaplasma spp. | Sequence obtained without known species match |
| Bacterium | Bartonella henselae | pap31 | 1–0.5 | PCR (gltA) | Norman et al., 1995 | none | No sequence obtained |
| Bacteria | Borrelia spp. | 23s | 3–1.5 | Nested PCR (IGS and p66) | Bunikis et al., 2004 | none | No sequence obtained |
| Bacterium | Coxiella spp. | icd | 25–12.4 | NP | – | Coxiella-like symbiont | – |
| Bacterium | Francisella spp | tul4 | 3–1.5 | NP | – | Francisella like symbiont | – |
| Bacterium | Rickettsia helvetica | ITS | 6–3 | NP | – | R. helvetica | – |
| Bacteria | Rickettsia endosymbiont | gltA | 164–81.6 | PCR (gltA) | Regnery et al., 1991 | R. lusitaniae | gb|JQ771933.1 (Milhano et al., 2014) |
| Parasite | Babesia spp. | hsp70 | 6–3 | PCR (18S) | Bonnet et al., 2007 | Babesia spp. | Sequence obtained without known species match |
| Virus | West Nile (WNV) | polyprotein | 6–3 | RT-PCR (NS5) | Scaramozzino et al., 2001 | none | No sequence obtained |
2.6. Statistical analyses
To evaluate the impact of destructive sampling on tick dynamics, we fit Poisson generalized linear mixed models to tick numbers over time. The explanatory variables were i) nest treatment, i.e. whether ticks were collected or counted and ii) an exponential time trend. The effect of collecting on tick dynamics was assessed via the interaction between these two variables. A random nest effect was also introduced to the model to take into account unobserved differences among nests. Females, males and nymphs were studied separately. We did not attempt to analyze data on larvae because of detection issues (see discussion). Calculations were performed with the R software (R-core R Core Team, 2015).
To test for spatial structure in tick numbers among nests, we calculated Moran's I index using the correlogram function in the pgirmess package for R software using total tick numbers and female tick numbers only; this was not done for the other life stages due to the low number of ticks. Ten distance classes among nests were defined using data from the first visit to determine the distance of autocorrelation and identify its potential source. Three distance classes were then defined for counted nests at each subsequent visit to test for potential short, medium and large–scale patterns of tick movement. The same approach, using ten distance classes, was used to test for the presence of structure in the distribution of the detected microorganisms.
3. Results
3.1. Within-season tick dynamics
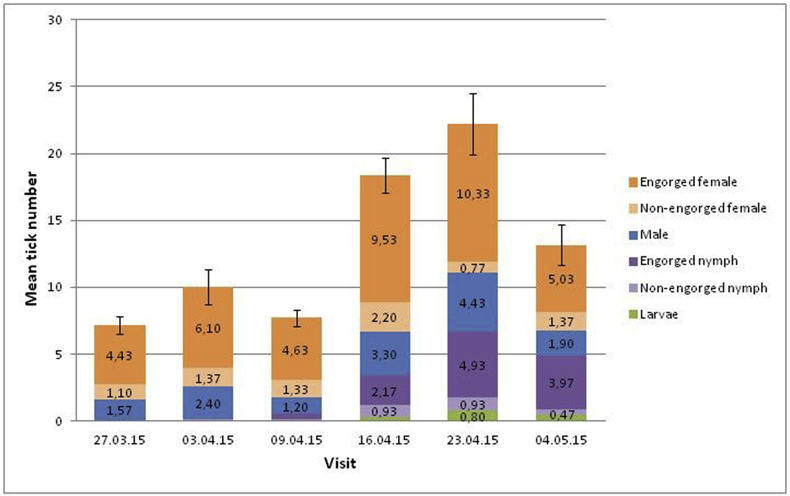
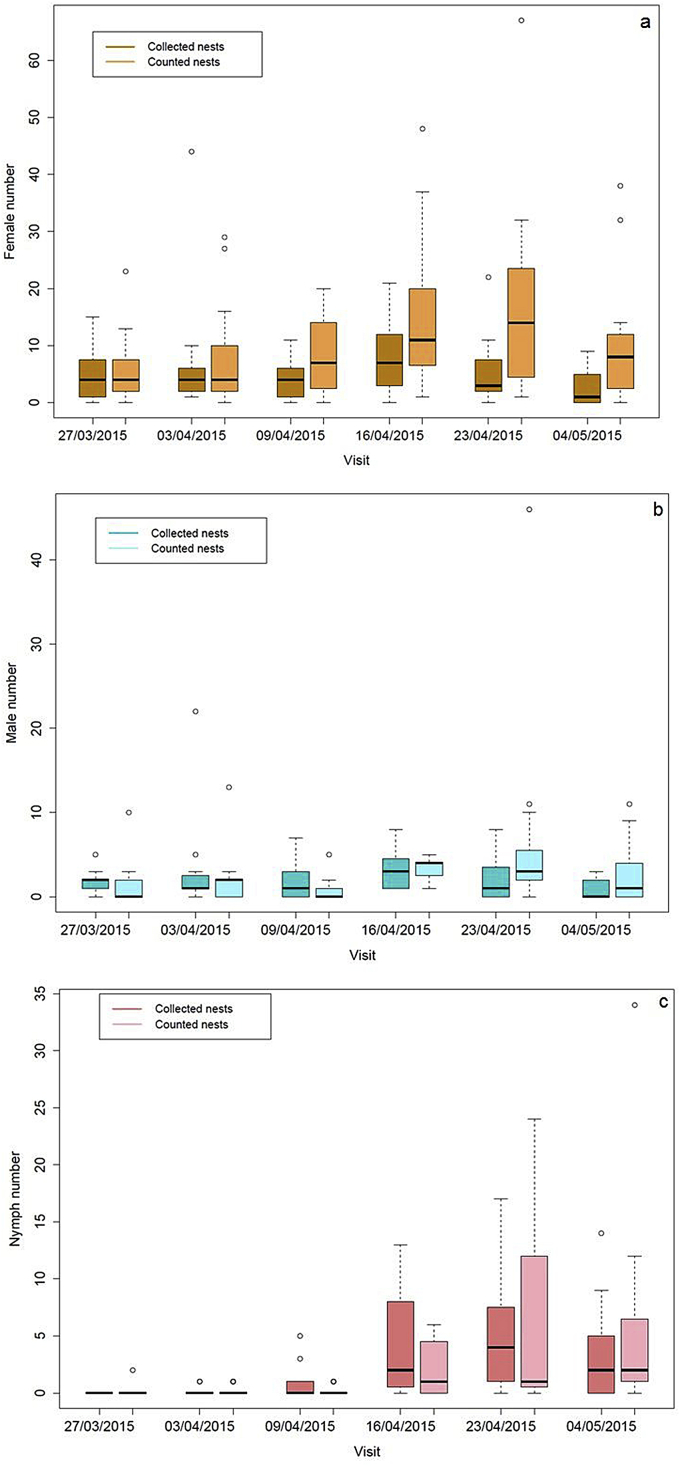
Ticks were found in all nests, but not necessarily at each visit; the prevalence of infested nests per visit varied from 86 to 100%, with all nests infested during visits 4 and 5. The total number of ticks per nest increased over time up to visit 5 and then started to decline (Fig. 2). During the first three visits, we observed predominantly adult ticks (Fig. 3a and b). From the third visit onward, we started to observe engorged and non-engorged nymphs (Fig. 3c) and larvae. The most stable tick life stage over time was engorged females (mean abundance over visits: 6.68 ± 8.89 or see Fig. 2). At the fifth visit, one week after hatching, tick abundance was maximal and all life stages were found.
Fig. 2.
Histogram presenting the mean number of ticks observed in all nests over time. Bars represent mean standard errors of the total number of ticks.
Fig. 3.
Boxplot representations of tick numbers in counted and collected nests over time: a, females only; b, males only; c, nymphs. The box shows the median as a line across the middle and the quartiles (25th and 75th percentiles) at either end. Extremities represent the minimal and maximal values and circles represent outliers.
Table 2 shows the results of the generalized linear models. All slopes were significant, indicating an increase in the population over time, except for the male population in collected nests. For all life stages examined, collecting had a significant impact (i.e., significant interaction), reducing the population size.
Table 2.
Generalized linear model results. As tick number was modeled by an exponential trend, a time slope above 1 indicates an increase in the population size, whereas a time slope less than 1 indicates a decrease compared to expectations. A significant interaction effect denotes a difference in the population size between the two sampling treatments.
| Nest treatment | Time slope | 95% confidence interval | P-value | Interaction p-value |
|---|---|---|---|---|
| Females | ||||
| Counted | 1.15 | 1.11–1.20 | <1.0E-7 | |
| Collected | 0.94 | 0.89–0.99 | 0.023 | <1.0E-7 |
| Males | ||||
| Counted | 1.30 | 1.20–1.40 | <1.0E-7 | |
| Collected | 0.94 | 0.86–1.02 | 0.11 | <1.0E-7 |
| Nymphs | ||||
| Counted | 2.04 | 1.82–2.28 | <1.0E-7 | |
| Collected | 1.67 | 1.51–1.84 | <1.0E-7 | 0.0089 |
3.2. Infectious agents in ticks
Among the 24 nests and 201 non engorged tick individuals analyzed by real-time PCR, 177 (88%) harbored at least one microorganism including 7 bacterial isolates, 1 parasite species and 1 viral isolate (Table 1). After subsequent amplification and sequencing, 159 ticks (79%) were found to harbor only symbiotic bacteria, 4 (2%) were infected by known pathogenic agents, 14 (7%) ticks were co-infected by both symbiotic bacteria and pathogens and 24 (12%) were found uninfected. Three presumably symbiotic bacteria were detected: a Rickettsia-like bacterial isolate was detected in high prevalence across nests (50–100% of sampled ticks) and matched sequences of R. lusitaniae (Milhano et al., 2014) (99% Blast identity), a Coxiella-like bacterial isolate was found in 10 nests with variable prevalence among ticks within nests (9–50%), and a Francisella-like bacterial isolate was detected in 3 nests in low prevalence (10–12.5%). Four bacterial isolates, potentially pathogenic, were detected in low prevalence: 1) an unidentified Anaplasma sp. was found in three nests (within nest prevalence of 2–11%), but did not match known species on GenBank (96% identity to uncultured bacterium), 2) Bartonella henselae was detected in one tick individual in a single nest (within nest prevalence of 9%), but could not be reamplified for confirmation (i.e. no sequence obtained), 3) Borrelia spp. were also detected in three nests (within nest prevalence of 4–12.5%), but again could not be reamplified for confirmation, and 4) R. helvetica was detected and confirmed to occur in five nests (within nest prevalence of 4–25%). Only one parasite species was detected, Babesia sp. and occurred in five nests (within nest prevalence of 8–20%). However, as for the Anaplasma isolate, the reamplified sequences of this Babesia sp. did not match any known parasitic species on GenBank (but showed 93% identity to a plant, Solanum pennellii, accession number: HG975440.1) and it's not known yet what parasitic species was initially amplified. Two co-detections were observed for potential pathogens: Bartonella henselae and R. helvetica in one female tick and Borrelia spp. and R. helvetica in another female tick from a different nest. In addition, co-detections of presumed pathogens also occurred at the nest level: Anaplasma spp., B. henselae and R. helvetica occurred in different ticks in one nest; R. helvetica and Borrelia spp. co-occurred in two nests; Babesia sp., R. helvetica and Borrelia spp. co-occurred in a different nest (see Fig. 1).
Among the 25 tested viruses, only one, the West Nile virus (WNV), was found to occur on Carteau in 6 individual ticks from 5 different nests. Two ticks harboring WNV were also positive for Babesia sp. and for R. helvetica. Nevertheless, we were not able to obtain a sequence of this virus with RT-PCR or nested PCRs and the nature of this particular virus remains to be confirmed.
3.3. Spatial and temporal structuring of ticks and their infectious agents
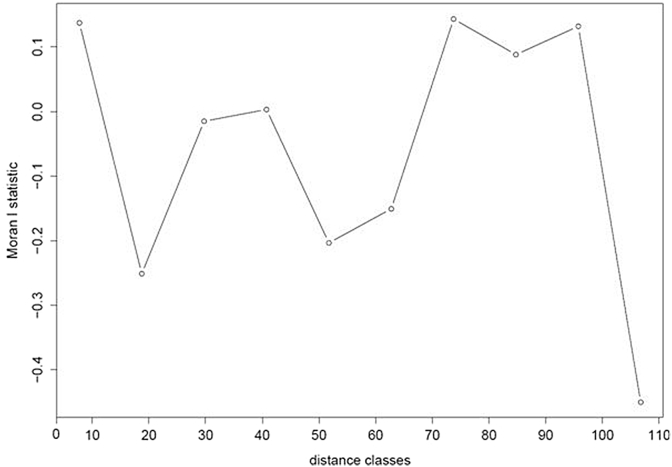
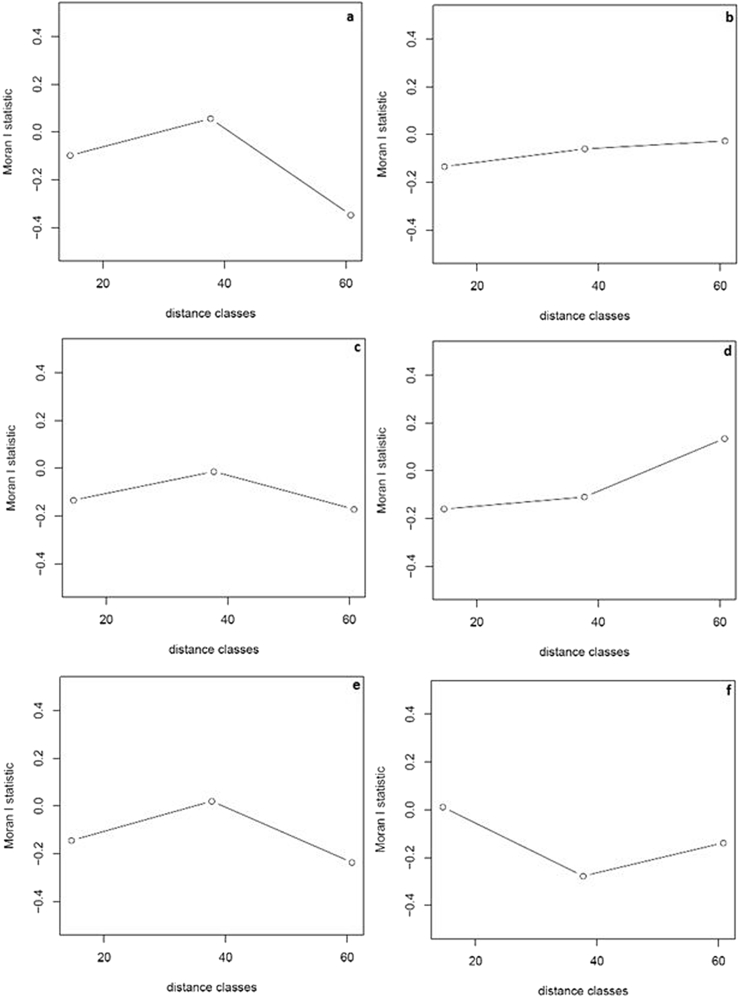
Tests for spatial auto-correlation in tick abundance at the initial visit were non-significant (Fig. 4) and no clear spatial pattern in tick presence could be found for counted nests at subsequent visits (Fig. 5). Likewise, no pattern of spatial structure was found in the occurrence of the infectious agents in the ticks (data not shown).
Fig. 4.
Spatial autocorrelation in total tick number estimated by Moran's I (Sokal and Oden, 1978). Data are from the first visit in the colony and include nests of both treatments. Ten distance classes representing 10 m between marked nests have been defined. No index value was significantly different from zero. The same results were obtained using female count data only (results not shown).
Fig. 5.
Spatial autocorrelation in the total tick number of counted nests, measured as Moran's I, across three distance classes: a, 1st visit; b, 2nd visit; c, 3rd visit; d, 4th visit; e, 5th visit; f, 6th visit. Circles indicate the autocorrelation coefficients. The same results were obtained with female count numbers.
4. Discussion
To understand the degree to which ticks disperse among nests and are responsible for pathogen dissemination at local scales, we investigated the temporal dynamics and structure of a population of the soft tick Ornithodoros maritimus over the course of a host breeding season. We found a temporal dynamic in the abundance of the different tick stages over the season, with the simultaneous appearance of juvenile stages and hatched chicks. Removal sampling reduced tick abundance in the nest from the fourth visit onward, suggesting the presence of some localized populations, but no significant spatial structure was found within the colony for either tick abundance or pathogen presence.
4.1. Within-nest tick dynamics and impact of removal sampling
Little is known about the natural temporal dynamics of host exploitation for O. maritimus. We found that adult ticks were present from the start of the breeding season, with females feeding on brooding adult birds. As engorged females continued to occur and increased in number over time after chick hatching, it is likely that this life stage also feeds on chicks (Figs. 2 and 3a). The proportion of males increased in line with females, potentially to maximize copulation opportunities (Fig. 3b). Nymphs and larvae only started to appear at the third and fourth visits, respectively. However, observations of nymphs and larvae in the nest are extremely difficult due to their small size, particularly when unengorged: 1–5 mm for nymphs and 0.5–1 mm for larvae (Hoogstraal et al., 1976). It is therefore possible that these life stages were active earlier than the fourth visit, but were undetected. Regardless, their numbers still increased significantly at this point in the season with the number of juvenile ticks being maximal at the fifth visit (Fig. 3c). Larvae were also observed feeding on chicks starting at the fourth visit. This delayed peak in activity may reflect an adaptation to host phenology (Magalhães et al., 2007); Due to their longer blood meal (several hours), larvae that feed on stationary chicks may have a higher probability of finding suitable off-host habitat post-bloodmeal than larvae that feed on the more mobile adult birds. There was also a decrease in the total population size of ticks in the nest at the sixth visit. This period coincided with the time when chicks were no longer restricted to the nest site and started to move around the territory of their parents, using different sheltered areas in and around the vegetation. This behavior may favor the return of ticks to overwintering microhabitats where temperature and humidity conditions may be more stable (Hoogstraal, 1985). Adaptations to host phenology have been documented in many parasite-host systems, and notably in phytophagous insects (Filchak et al., 2000, Rouault et al., 2004). However, to distinguish host-associated adaptations from simple constraints associated with climatic factors requires explicit tests. One way to test the hypothesis that life stage appearance and disappearance in ticks has evolved to match host breeding phenology would be to carry out detailed sampling in a series of colonies that vary in breeding dates but share similar climatic conditions.
At the scale of the seabird colony, we tested whether the repeated collection of ticks from nests over six weeks would affect the within-nest dynamics of the tick population. We expected that if ticks were spatially restricted in their dispersal capacity, removal sampling would lead to a strong reduction or extinction of the local nest population. On the whole, tick dynamics were relatively homogenous between collected and counted nests despite removal sampling (Fig. 3). Tick numbers (female, male and nymph) did decline in collected nests relative to counted nests (Table 2), but did not go extinct; the different tick stages were present week after week suggesting that tick dispersal at the colony level does occur and that these nests were locally recolonized.
4.2. Diversity and structure of infectious agents
Microorganisms present in ticks of Carteau Island included both presumed endosymbionts and pathogens. The presence of Coxiella symbionts in O. maritimus was expected based on previous results obtained from this island colony (Duron et al., 2015) and has been recently reported in members of the O. capensis complex from across the globe (Reeves et al., 2006, Duron et al., 2014, Al-Deeb et al., 2016). The Rickettsia-like symbiont we found was previously isolated from a soft tick parasitizing birds in Portugal, O. erraticus (Milhano et al., 2014) and from cell lines of O. capensis collected from coastal Georgia, USA (Mattila et al., 2007). Likewise, the Francisella-like symbiont present in O. maritimus was previously described in both soft (O. moubata (Noda et al., 1997) and Argas persicus (Suitor and Weiss, 1961)) and hard ticks (Niebylski et al., 1997, Sun et al., 2000, Michelet et al., 2013). These different endosymbiotic bacteria are suggested to perform essential functions required for the completion of the tick life cycle, but details on their physiological impacts are scarce for now and we cannot yet explain the high diversity of these organisms within the studied tick population (Duron et al., 2015). Although they likely have no impact on the bird host, recent work has suggested that mutations in endosymbiotic bacteria can directly lead to the emergence of novel pathogenic organisms (Duron et al., 2015).
The prevalence of suspected/known pathogenic agents was much lower within the tick population, as would be expected. Different pathogenic species of Anaplasma are transmitted by hard ticks to mammals (including humans) (Dantas-Torres et al., 2012) and by a soft tick Argas reflexus to pigeons (Jongejan and Uilenberg, 2004). The msp2 target gene is typically used to identify the species of this genus (Rymaszewska, 2010) but the Anaplasma species detected in our study did not match any known species available on public databases and may represent a new species. We also detected one bacterial isolate that matches with Bartonella henselae, the known agent of cat-scratch fever. The first identification of a Bartonella species, related to B. henselae, in a tick was in the soft tick O. sonrai in Senegal but the principal vectors of B. henselae are typically fleas of felids (Chomel et al., 1996). The primers used in our real-time PCR assay were specific to this species, but we were not able to reamplify this bacterium using an alternative gene. This may have been due to low DNA quantity or to slight genetic divergence from B. henselae. These issues require further investigation. Rickettsia helvetica, an infectious agent that can cause meningitis in humans, is well-known to be transmitted by hard ticks (Parola et al., 2005) and notably to wild birds by Ixodes ventalloi in Portugal (Santos-Silva et al., 2006). Our study represents the first confirmed report of this bacterium in association with soft ticks to our knowledge. We also found an unknown species of Babesia in the O. maritimus population. These apicomplexan parasites cause different forms of hemolytic disease, or babesiosis, principally in mammals but also in birds. Most species of Babesia are vectored by hard ticks. Only one soft tick species has so far been recorded to vector these parasites; O. erraticus transmitting Babesia meri to sand rats in Africa (Yabsley and Shock, 2013). The Babesia spp. we found in O. maritimus could not be identified using data available on Genbank. Further work will therefore be required to determine the relationship of this species to other described species of Babesia. The presence of Borrelia spp. and a type of West Nile Virus (a Flavivirus) were also detected in ticks, but could not be confirmed. Both agents are known to be transmitted by different species of soft ticks (Lawrie et al., 2004, Cutler, 2010) and WNV has been previously reported in the Camargue region (Balança et al., 2009, Vittecoq et al., 2013, Pradier et al., 2014). However, a previous study of Yellow-legged gulls on Carteau did not find evidence for the presence of Flavivirus antibodies in eggs, suggesting that WNV and other related viruses may not have circulated in gulls at that time (Arnal et al., 2014). These results therefore also require further confirmation.
Compared to results obtained using the same molecular detection assay and 267 females of the hard tick Ixodes ricinus from nine sites in France (Moutailler et al., 2016), we found a lower diversity of pathogens and at lower prevalence (Table 1) in the soft ticks of Carteau island. This may be a direct consequence of soft tick characteristics; soft ticks feed on the host for only a short time compared to hard ticks and therefore may be more isolated because they have fewer opportunities for host-associated dispersal (Kada et al. in press). Alternatively, soft ticks or host-birds may be more efficient at clearing infections compared to hard ticks and their hosts (Brown and O'Brien, 2011). It should also be noted that the detection of pathogen DNA in the ticks does not mean that these pathogens were still alive and transmissible to a new host. Experimental transmission tests are therefore required now to determine true vector competence. Likewise, data from different colonies across the Mediterranean are needed to evaluate the potential role of O. maritimus in the circulation of these different infectious agents at larger spatial scales.
4.3. Vector movement at the within-colony scale
Removal sampling and the analysis of spatial structure reveal little to no structure for ticks or their infectious agents in the gull colony of Carteau Island, even among nests separated by several meters. Moreover few ticks from a same nest were infected with the same infectious agents, which would have been expected if all ticks in a nest repeatedly fed on the same individual bird. These results suggest frequent within-colony movements likely occur.
Even if topography is known to be an important factor impacting the isolation of hard tick populations and may modify pathogen circulation at small spatial scales (McCoy et al., 2003a), our study suggests that it could be a less important factor in soft ticks. This was also observed in Arnal et al. (2014) where the presence of anti-flavivirus anti-bodies in gull eggs was spatially heterogeneous at the scale of a single colony. Hosts are considered to be the major driver of tick dispersal and may transport them over long distances (e.g., Smith et al., 1996, McCoy et al., 2003b) structuring their populations and their infectious agents. In the case of soft ticks however, feeding times are so short that the role of host movement may be minimal. Moreover, on Carteau Island, we observed that nest sites within a territory are not necessarily reused by hosts from one year to the next. Therefore ticks likely move relatively easily from one nest to another in order to find a suitable host, at least over the area of the territory. Population genetic analyses are now required to determine the degree to which ticks from different nests represent a single panmictic population. As soft ticks differ substantially in life history traits from hard ticks, our results highlight the need to better understand the biology and ecology of nidicolous soft ticks in order to understand their colonization potential and their role in pathogen dissemination. More generally, these results also underline the importance of vector dynamics at local scales, and particularly for predicting and controlling pathogen circulation at larger spatial scales.
Acknowledgements
We thank Olivier Duron, Orianne Tournayre, Florian Binetruy, Jonathan D'Ambrosio and others that helped sample in 2015. Funding for this study was provided by the ANR grant ESPEVEC (ANR blanc ANR-13-BSV7-0018-01) to KDM, and ANSES grant to SM. Logistical support was provided from the Tour du Valat (France). MD was supported by a fellowship from the French Ministry for National Education and Research at University of Montpellier. We also thank the “Tiques et Maladies à tiques” Working Group of the Réseau Ecologie des Interactions Durables (REID) for stimulating discussions and support.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijppaw.2017.05.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abrahan L.B., Gorla D.E., Catalá S.S. Dispersal of Triatoma infestans and other Triatominae species in the arid Chaco of Argentina: flying, walking or passive carriage? The importance of walking females. Memórias do Inst. Oswaldo Cruz. 2011;106:232–239. doi: 10.1590/s0074-02762011000200019. [DOI] [PubMed] [Google Scholar]
- Adeyeye O.A., Butler J.F. Population structure and seasonal intra-burrow movement of Ornithodoros turicata (Acari: argasidae) in gopher tortoise burrows. J. Med. entomology. 1989;26:279–283. doi: 10.1093/jmedent/26.4.279. [DOI] [PubMed] [Google Scholar]
- Al-Deeb M.A., Frangoulidis D., Walter M.C., Kömpf D., Fischer S.F., Petney T., Muzaffar S.B. Coxiella-like endosymbiont in argasid ticks (Ornithodoros muesebecki) from a socotra cormorant colony in umm Al quwain, United Arab Emirates. Ticks Tick-borne Dis. 2016 doi: 10.1016/j.ttbdis.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Arnal A., Gómez-Díaz E., Cerdà-Cuéllar M., Lecollinet S., Pearce-Duvet J., Busquets N., García-Bocanegra I., Pagès N., Vittecoq M., Hammouda A., Samraoui B., Garnier R., Ramos R., Selmi S., González-Solís J., Jourdain E., Boulinier T. Circulation of a Meaban-like virus in Yellow-legged gulls and seabird ticks in the western Mediterranean basin. PLoS One. 2014;9:e89601. doi: 10.1371/journal.pone.0089601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balança G., Gaidet N., Savini G., Vollot B., Foucart A., Reiter P., Boutonnier A., Lelli R., Monicat F. Low West Nile virus circulation in wild birds in an area of recurring outbreaks in southern France. Vector-Borne Zoonotic Dis. 2009;9:737–741. doi: 10.1089/vbz.2008.0147. [DOI] [PubMed] [Google Scholar]
- Bonnet S., Jouglin M., Malandrin L., Becker C., Agoulon A., Hostis M., Chauvin A. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology. 2007;134:197–207. doi: 10.1017/S0031182006001545. [DOI] [PubMed] [Google Scholar]
- Brisson D., Dykhuizen D.E., Ostfeld R.S. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc. R. Soc. Lond. B Biol. Sci. 2008;275:227–235. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., O'Brien V. Ornithological Monographs No. 71, Ornithological Monographs. American Ornithologists’ Union; 2011. Are wild birds important in the transport of arthropod-borne viruses? pp. 1–64. [Google Scholar]
- Bunikis J., Garpmo U., Tsao J., Berglund J., Fish D., Barbour A.G. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology. 2004;150:1741–1755. doi: 10.1099/mic.0.26944-0. [DOI] [PubMed] [Google Scholar]
- Chomel B.B., Kasten R.W., Floyd-Hawkins K., Chi B., Yamamoto K., Roberts-Wilson J., Gurfield A.N., Abbott R.C., Pedersen N.C., Koehler J.E. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converse J.D., Hoogstraal H., Moussa M.I., Feare C.J., Kaiser M.N. Soldado virus (hughes group) from Ornithodoros (alectorobius) capensis (ixodoidea: argasidae) infesting sooty tern colonies in the Seychelles, indian ocean. Am. J. Trop. Med. Hyg. 1975;24:1010–1018. doi: 10.4269/ajtmh.1975.24.1010. [DOI] [PubMed] [Google Scholar]
- Cutler S. j. Relapsing fever – a forgotten disease revealed. J. Appl. Microbiol. 2010;108:1115–1122. doi: 10.1111/j.1365-2672.2009.04598.x. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Chomel B.B., Otranto D. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dietrich M., Gómez-Díaz E., McCoy K.D. Worldwide distribution and diversity of seabird ticks: implications for the ecology and epidemiology of tick-borne pathogens. Vector-Borne Zoonotic Dis. 2011;11:453–470. doi: 10.1089/vbz.2010.0009. [DOI] [PubMed] [Google Scholar]
- Duron O., Jourdain E., McCoy K.D. Diversity and global distribution of the Coxiella intracellular bacterium in seabird ticks. Ticks Tick-borne Dis. 2014;5:557–563. doi: 10.1016/j.ttbdis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Duron O., Noël V., McCoy K.D., Bonazzi M., Sidi-Boumedine K., Morel O., Vavre F., Zenner L., Jourdain E., Durand P., Arnathau C., Renaud F., Trape J.-F., Biguezoton A.S., Cremaschi J., Dietrich M., Léger E., Appelgren A., Dupraz M., Gómez-Díaz E., Diatta G., Dayo G.-K., Adakal H., Zoungrana S., Vial L., Chevillon C. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004892. e1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feare C.J. Desertion and abnormal development in a colony of Sooty terns Sterna fuscata infested by virus-infected ticks. Ibis. 1976;118:112–115. [Google Scholar]
- Filchak K.E., Roethele J.B., Feder J.L. Natural selection and sympatric divergence in the apple maggot Rhagoletis pomonella. Nature. 2000;407:739–742. doi: 10.1038/35037578. [DOI] [PubMed] [Google Scholar]
- Gandon S., Michalakis Y. Local adaptation, evolutionary potential and host–parasite coevolution: interactions between migration, mutation, population size and generation time. J. Evol. Biol. 2002;15:451–462. [Google Scholar]
- Gómez-Díaz E., Doherty P.F., Jr., Duneau D., McCoy K.D. Cryptic vector divergence masks vector-specific patterns of infection: an example from the marine cycle of Lyme borreliosis: divergence and vector-specific infection. Evol. Appl. 2010;3:391–401. doi: 10.1111/j.1752-4571.2010.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H. Argasid and nuttalliellid ticks as parasites and vectors. In: Baker J.R., Muller R., editors. Advances in Parasitology. Academic Press; 1985. pp. 135–238. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H., Clifford C.M., Keirans J.E., Kaiser M.N., Evans D.E. The Ornithodoros (Alectorobius) capensis Group (Acarina: ixodoidea: Argasidae) of the Palearctic and Oriental regions. O. (A.) maritimus: identity, marine bird hosts, virus infections, and distribution in Western Europe and Northwestern Africa. J. Parasitol. 1976;62:799. [PubMed] [Google Scholar]
- Hornok S., Földvári G., Elek V., Naranjo V., Farkas R., de la Fuente J. Molecular identification of Anaplasma marginale and rickettsial endosymbionts in blood-sucking flies (Diptera: Tabanidae, Muscidae) and hard ticks (Acari: ixodidae) Veterinary Parasitol. 2008;154:354–359. doi: 10.1016/j.vetpar.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kada S., McCoy K.D., Boulinier T. Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents. Parasites & Vectors. 2017 doi: 10.1186/s13071-017-2261-y. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F., Holt R.D., Ostfeld R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Lawrie C.H., Uzcátegui N.Y., Gould E.A., Nuttall P.A. Ixodid and argasid tick species and West Nile virus. Emerg. Infect. Dis. 2004;10:653–657. doi: 10.3201/eid1004.030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães S., Forbes M.R., Skoracka A., Osakabe M., Chevillon C., McCoy K.D. Host race formation in the Acari. Exp. Appl. Acarol. 2007;42:225–238. doi: 10.1007/s10493-007-9091-0. [DOI] [PubMed] [Google Scholar]
- Mattila J.T., Burkhardt N.Y., Hutcheson H.J., Munderloh U.G., Kurtti T.J. Isolation of cell lines and a Rickettsial endosymbiont from the soft tick Carios capensis (Acari: argasidae: Ornithodorinae) J. Med. Entomol. 2007;44:1091–1101. doi: 10.1603/0022-2585(2007)44[1091:ioclaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- McCoy K.D., Tirard C., Michalakis Y. Spatial genetic structure of the ectoparasite Ixodes uriae within breeding cliffs of its colonial seabird host. Heredity. 2003;91:422–429. doi: 10.1038/sj.hdy.6800339. [DOI] [PubMed] [Google Scholar]
- McCoy K.D., Boulinier T., Tirard C., Michalakis Y. Host-dependent genetic structure of parasite populations: differential dispersal of seabird tick host races. evolution. 2003;57:288–296. doi: 10.1111/j.0014-3820.2003.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Michelet L., Bonnet S., Madani N., Moutailler S. Discriminating Francisella tularensis and Francisella-like endosymbionts in Dermacentor reticulatus ticks: evaluation of current molecular techniques. Veterinary Microbiol. 2013;163:399–403. doi: 10.1016/j.vetmic.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Boye Pihl T.P., Klitgaard K., BÃ,dker R., Fach P., Moutailler S. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhano N., Palma M., Marcili A., Núncio M.S., de Carvalho I.L., de Sousa R. Rickettsia lusitaniae sp. nov. isolated from the soft tick Ornithodoros erraticus (Acarina: argasidae) Comparative Immunology, Microbiol. Infect. Dis. 2014;37:189–193. doi: 10.1016/j.cimid.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Moutailler S., Moro C.V., Vaumourin E., Michelet L., Tran F.H., Devillers E., Cosson J.-F., Gasqui P., Van V.T., Mavingui P., Vourc’h G., Vayssier-Taussat M. Co-infection of ticks: the rule rather than the exception. PLOS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004539. e0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski M.L., Peacock M.G., Fischer E.R., Porcella S.F., Schwan T.G. Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl. Environ. Microbiol. 1997;63:3933–3940. doi: 10.1128/aem.63.10.3933-3940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H., Munderloh U.G., Kurtti T.J. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A.F., Regnery R., Jameson P., Greene C., Krause D.C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden N. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013;3:46. doi: 10.3389/fcimb.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen K.M. Christopher Helm Publishers Ltd; London: 2004. Gulls of Europe, Asia and North America. [Google Scholar]
- Ostfeld R.S., Glass G.E., Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol. Evol. Special Issue Bump. Book Rev. 2005;20:328–336. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Parola P., Paddock C.D., Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Eid C. Les tiques: identification, biologie, importance médicale et vétérinaire. Lavoisier. 2007 [Google Scholar]
- Pradier S., Sandoz A., Paul M.C., Lefebvre G., Tran A., Maingault J., Lecollinet S., Leblond A. Importance of wetlands management for West Nile virus circulation risk, Camargue, Southern France. Int. J. Environ. Res. Public Health. 2014;11:7740–7754. doi: 10.3390/ijerph110807740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2015. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing.https://www.R-project.org/ Vienna, Austria. [Google Scholar]
- Reeves W.K., Loftis A.D., Sanders F., Spinks M.D., Wills W., Denison A.M., Dasch G.A. Borrelia, Coxiella, and Rickettsia in Carios capensis (Acari: argasidae) from a brown pelican (Pelecanus occidentalis) rookery in South Carolina. USA. Exp. Appl. Acarol. 2006;39:321–329. doi: 10.1007/s10493-006-9012-7. [DOI] [PubMed] [Google Scholar]
- Regnery R.L., Spruill C.L., Plikaytis B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault G., Turgeon J., Candau J.-N., Roques A., Aderkas P. von. Oviposition strategies of conifer seed chalcids in relation to host phenology. Naturwissenschaften. 2004;91:472–480. doi: 10.1007/s00114-004-0554-4. [DOI] [PubMed] [Google Scholar]
- Rymaszewska A. Variability within the msp2 gene in populations of Anaplasma phagocythopilum. Folia Biol. (Praha) 2010;56:269–275. [PubMed] [Google Scholar]
- Santos-Silva M.M., Sousa R., Santos A.S., Melo P., Encarnação V., Bacellar F. Ticks parasitizing wild birds in Portugal: detection of Rickettsia aeschlimannii, R. helvetica and R. massiliae. Exp. Appl. Acarol. 2006;39:331. doi: 10.1007/s10493-006-9008-3. [DOI] [PubMed] [Google Scholar]
- Scaramozzino N., Crance J.-M., Jouan A., DeBriel D.A., Stoll F., Garin D. Comparison of Flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription-PCR assay for detection of Flaviviruses targeted to a conserved region of the NS5 gene sequences. J. Clin. Microbiol. 2001;39:1922–1927. doi: 10.1128/JCM.39.5.1922-1927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service M.W. Mosquito (Diptera: Culicidae) Dispersal—the long and short of it. J. Med. Entomology. 1997;34:579–588. doi: 10.1093/jmedent/34.6.579. [DOI] [PubMed] [Google Scholar]
- Smith R.P., Rand P.W., Lacombe E.H., Morris S.R., Holmes D.W., Caporale D.A. Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease. J. Infect. Dis. 1996;174:221–224. doi: 10.1093/infdis/174.1.221. [DOI] [PubMed] [Google Scholar]
- Sokal R.R., Oden N.L. Spatial autocorrelation in biology: 1. Methodol. Biol. J. Linn. Soc. 1978;10:199–228. [Google Scholar]
- Sonenshine D.E., Mather T.N. Oxford University Press; 1994. Ecological Dynamics of Tick-borne Zoonoses. [Google Scholar]
- Sonenshine D.E., Roe R.M. second ed. vol. 2. Oxford University Press; 2014. (Biology of Ticks). [Google Scholar]
- Suitor E.C., Weiss E. Isolation of a Rickettsia-like microorganism (Wolbachia persica, N. SP.) from Argas persicus (oken) J. Infect. Dis. 1961;108:95–106. [Google Scholar]
- Sun L.V., Scoles G.A., Fish D., O'Neill S.L. Francisella-like endosymbionts of ticks. J. Invertebr. Pathol. 2000;76:301–303. doi: 10.1006/jipa.2000.4983. [DOI] [PubMed] [Google Scholar]
- Tylianakis J.M., Didham R.K., Bascompte J., Wardle D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- Vermeil, Merguet Sur le diagnostic des larves d’Ornithodores du complexe coniceps-capensis (Acarina : Argasidae). Ornithodoros coniceps (Canestrini, 1890) maritimus n. ssp. prévaut dans les îles de Basse-Bretagne. Acarologia. 1967;9:557–565. [Google Scholar]
- Vial L. Biological and ecological characteristics of soft ticks (Ixodida: argasidae) and their impact for predicting tick and associated disease distribution. Parasite. 2009;16:191–202. doi: 10.1051/parasite/2009163191. [DOI] [PubMed] [Google Scholar]
- Vittecoq M., Lecollinet S., Jourdain E., Thomas F., Blanchon T., Arnal A., Lowenski S., Gauthier-Clerc M. Recent circulation of West Nile virus and potentially other closely related Flaviviruses in Southern France. Vector-Borne Zoonotic Dis. 2013;13:610–613. doi: 10.1089/vbz.2012.1166. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Shock B.C. Natural history of zoonotic Babesia: role of wildlife reservoirs. Int. J. Parasitol. Parasites Wildl. 2013;2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.