Abstract
Biodiversity enhances many of nature’s benefits to people, including the regulation of climate and the production of wood in forests, livestock forage in grasslands and fish in aquatic ecosystems. Yet people are now driving the sixth mass extinction event in Earth’s history. Human dependence and influence on biodiversity have mainly been studied separately and at contrasting scales of space and time, but new multiscale knowledge is beginning to link these relationships. Biodiversity loss substantially diminishes several ecosystem services by altering ecosystem functioning and stability, especially at the large temporal and spatial scales that are most relevant for policy and conservation.
Biodiversity loss driven by humans1–3 could substantially diminish the benefits that people derive from nature (ecosystem services)4–6 because the loss of species often alters the pools and fluxes of materials and energy in nature (ecosystem functioning)7–9 (Fig. 1a). In response to requests from governments on the current state of knowledge, the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) is assessing changes in biodiversity and ecosystems, as well as their contributions to people, at both regional and global scales10. Furthermore, halting biodiversity loss is also among the United Nations Sustainable Development Goals (https://sustainabledevelopment.un.org/sdgs), which were established in 2015. It remains difficult, however, to predict the extent to which humandriven changes in biodiversity will alter ecosystem services, especially at the larger spatial and longer temporal scales that are most relevant to policy and conservation. This difficulty stems from a mismatch in the scales of knowledge of the influences and dependence of people on biodiversity.
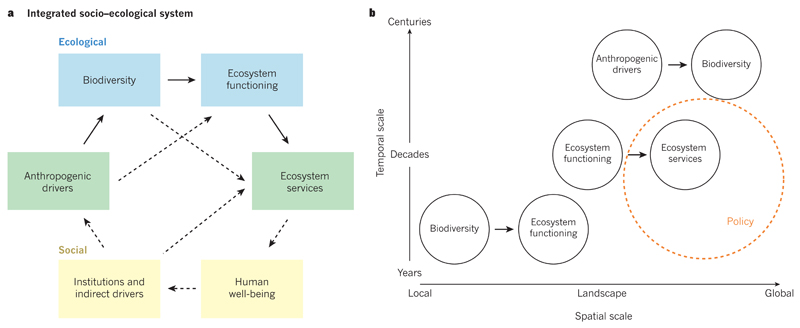
Figure 1. The influence and dependence of people on biodiversity.
a, People influence biodiversity directly by changing land-use, climate and biogeochemical cycles, as well as by introducing species — actions known collectively as anthropogenic drivers. At the global scale, these activities are driving the sixth mass extinction in the history of life on Earth. At the local scale, species losses decrease ecosystem functioning (for example, ecosystem productivity and resource uptake) and stability (the invariability of ecosystem productivity across a period of years). At the intermediate scales such as landscapes or regions, changes in ecosystem functioning can alter the supply of ecosystem services, including the production of wood in forests, livestock forage in grasslands and fish in aquatic ecosystems. It is important to build multiscale knowledge at the intersections of the numerous components of the system. Various system components are positioned in a gradient that spans the social (yellow) to ecological (blue) ends of a socio–ecological continuum. Dashed arrows indicate other important relationships that are beyond the scope of this Review. b, At present, there are mismatches in the spatial and temporal scales at which the relationships between anthropogenic drivers, biodiversity, ecosystem functioning and ecosystem services are best understood. This makes it a challenge to link the cascading effects of human activities on biodiversity, ecosystems and ecosystem services. Furthermore, the scales at which knowledge is available for some of the relationships do not yet align with the scales at which policies and other decisions (orange circle) are often made. Relationships are positioned at the approximate scales at which they are currently best understood.
At the global spatial scale over decades or centuries, the ever-increasing and unprecedented extent and impact of human activities on land and in the oceans is dramatically reducing global biodiversity1–3 (Fig. 1b). There is overwhelming evidence that habitat loss and fragmentation, overexploitation of biological resources, pollution, species invasions and climate change have increased rates of global species extinctions to levels that are much higher than those observed in the fossil record1–3. Human impacts may be immediate, such as when land is cleared for agriculture11, but extinctions often occur decades or centuries after the disturbance, when reductions in populations, restrictions on movement and limitations on the availability of suitable habitats finally take effect12,13. Therefore, the global species extinctions that have been documented in the recent past are only the tip of the iceberg in terms of massive ongoing changes in biodiversity, which include substantial declines in the populations of native species, local extinctions, local gains of new species and spatial homogenization of Earth’s biota13–15.
On the smaller spatial and shorter temporal scales over which species interact with one another, species losses often decrease ecosystem functioning (Fig. 1b), resulting in a reduction in the efficiency with which ecological communities capture biologically essential resources such as soil nutrients and produce biomass8,9. The relationship between biodiversity and ecosystem functioning has been investigated rigorously in the past 25 years through hundreds of biodiversity experiments8,9,16 and dozens of theoretical17,18 and observational studies in a wide range of ecosystems, including grasslands19,20, forests21–23, drylands24 and marine25 systems. The effects of biodiversity on ecosystem function often arise because coexisting species occupy different ecological niches. For instance, species can differ in the ways in which they exploit resources or resist natural enemies, thereby reducing competition and enhancing productivity in diverse communities8,26,27. Results from biodiversity experiments28,29 also support theory that predicts that increased biodiversity enhances the stability of biomass production in ecosystems because it enhances several forms of asynchrony in population dynamics30–33.
At intermediate spatial and temporal scales, including those over which land-use decisions are made, changes in ecosystem functioning alter the supply of ecosystem services (Fig. 1b). Land is often managed to prioritize certain ecosystem services at the expense of others. For example, the production of food or fuel has often been prioritized at the expense of climate regulation and natural beauty. Assessments of a wide range of ecosystem services help to account for a more complete suite of benefits and costs, making it possible to determine whether the bundle of benefits provided by a particular land use outweighs that provided by an alternative land use or habitat. These studies show, for example, that it can sometimes be more valuable in economic terms to manage land to enhance climate regulation and recreation than to expand food production34,35. Many such studies project how, in the next few decades, anthropogenic drivers might diminish or enhance the supply of ecosystem services by altering underlying ecosystem functions at the landscape scale34,35, but without accounting for the effects of drivers on services that are mediated by biodiversity.
In this Review, we examine recent results that expand the scales of knowledge of the relationships between anthropogenic drivers, biodiversity, ecosystem functioning and ecosystem services (Fig. 1a) and begin to link them to one another. We show that the cascading impacts of human activities on biodiversity and ecosystems, as well as their consequences for people, will probably increase at larger spatial and longer temporal scales. Understanding how these relationships shift with scale will help in the assessment of the sustainability of ecosystem services in the face of biodiversity loss. We conclude by suggesting ways to strengthen biodiversity science that supports the development of multiscale environmental policy. Much of the Review focuses on species richness (numbers of species), which is a well-studied, albeit incomplete, surrogate for several other dimensions of biodiversity (Box 1).
Box 1. The dimensions and scales of biodiversity.
Biodiversity is a broad term that represents the variety of life on Earth. There are numerous dimensions of biodiversity, which reflect genetic (for example, genotype), organismal (for example, phenotype), ecological (for example, population, community or ecosystem), taxonomic (for example, species, genus or family) and functional (for example, effect or response traits) attributes at different scales of space (for example, site, country or biome) and time. Diversity can be quantified at multiple nested scales (for example, α-diversity, β-diversity or γ-diversity), using measures of richness (for example, number), evenness (equity of relative abundance), dominance (concentration of abundance) or combinations of such measures (through the Shannon diversity index, the Simpson diversity index or by calculating the probability of interspecific encounter). Although it is prohibitive to consider all possible dimensions and scales of biodiversity, it is crucial to understand the strengths and limitations of each.
Our Review focuses largely on species richness because this measure is commonly used as a surrogate for several dimensions of biodiversity. However, species richness can miss important components of biodiversity that are relevant for ecosystem functioning. For example, phylogenetic diversity or functional traits are sometimes better predictors of ecosystem functioning than species richness100. Ecosystem functioning and services also depend on the interactions that occur between species, including those between predator and prey, herbivore and plant or pollinator and host, as well as the overall number and kinds of species. Furthermore, in most biological communities, whereas only a few species are dominant, many are rare. Species richness does not incorporate measures of abundance that are crucial for many ecosystem functions. Yet species richness may be a useful proxy for unknown differences or interactions between species and could help to account for the fact that the relative abundances of species are not static, varying instead across conditions that change with space and time. Species richness may also be useful for predicting the capacity of a system to respond to potential future conditions, as there is still a high level of uncertainty with regard to the species that will flourish or diminish under new conditions.
Studies are now moving on from efforts to determine which components of biodiversity are the single best predictors of changes in ecosystems, and are instead drawing on the strengths of multiple dimensions of biodiversity and approaches to advance multiscale understanding38.
Scaling anthropogenic impacts
The effects of anthropogenic drivers on biodiversity depend strongly on the spatial and temporal scale being considered. Linking the impacts and dependence of people on biodiversity will require scaling down from long-term global extinction trends to under-explored contemporary trends in local and regional biodiversity (Fig. 1b).
Scaling across space
Although human activities have unarguably driven many global species extinctions in past centuries, the impacts of such activities on biodiversity at sub-global spatial scales are less clear. Rates of global extinctions may be slower than rates of local species loss because a species is not extinct worldwide until it has been lost from every local community. For example, in tropical forests, rates of global species extinctions have been estimated to be three orders of magnitude lower than rates of local population extirpation36. Yet there may be a greater net loss of species at the global scale than at the local scale, if local species losses are offset by local species gains37, which can occur through species introductions or range shifts38. In other words, the loss of global diversity (γ-diversity) can be explained not only by the loss of local diversity (α-diversity), but also by spatial homogenization, which is the loss of β-diversity (the difference in species composition across space). Regardless of whether global extinction rates are slower or faster than the mean rate of net local species loss, which is averaged across all local communities worldwide, there are certainly locations on Earth that have lost a large fraction of species and others that have experienced an increase in the number of species.
Patterns of change in local biodiversity are becoming clearer at many locations worldwide. In areas that have been converted to croplands or pastures, there has been a substantial net loss of local biodiversity11. Specifically, land-use changes have decreased local species richness by around 14% on average worldwide, with losses of up to 76% of species in the worst-affected habitats11. Some of these human-driven losses of local biodiversity have probably emerged over centuries or millennia, given the long history of conversion and use of land by people. In remaining habitats, there have been local species gains at some locations and local species losses elsewhere in the past few decades37,39–41. Some of these gains may have caused a net increase in local species richness, for example, through the introduction of exotic species or colonization by new species that are shifting their ranges in response to climate change. But some apparent gains may simply reflect the recovery of formerly present species following the relaxation of a disturbance42,43. Further studies are needed to determine whether recent species gains are a response to anthropogenic drivers, or whether they reflect community assembly (or recovery), observational errors or other causes. The main drivers of local species loss are better understood. A synthesis of hundreds of experiments and observational studies44 found that local species loss was greater in response to land-use changes (24.8%) and species invasions (23.7%) than to nutrient enrichment (8.2%) or warming (3.6%). Furthermore, species loss was greater for terrestrial biomes (22.4%) than for aquatic biomes (5.9%), and for endothermic animals (33.2%) and primary producers (25.1%), including plants, than for ectothermic animals (10.5%).
It is not yet known whether local species gains at some locations compensate, in terms of ecosystem functioning, for local species losses elsewhere45. Gains of exotic species can have large positive or negative impacts on ecosystem functioning because the traits of such species often differ to those of native species45–47 (Fig. 2). Independently of these shifts in species composition and traits, ecosystem functioning tends to respond more strongly to local species losses than to local species gains (of native or exotic species). This is because the increase in ecosystem functioning per added species becomes smaller as species richness increases8,9,21 (Fig. 2a). Therefore, at any particular level of species richness, the loss of a given number of species will tend to affect ecosystem functioning more than a gain of the same number of species9. Furthermore, at least for plants, the gain of an exotic species might not compensate completely for the loss of a native species, in terms of function. This is because exotic species can exhibit less complementarity48 than native species, which have interacted with each other for a longer period of time, providing a greater opportunity for selection for niche differentiation49. Scaling down knowledge of the effects of anthropogenic drivers on biodiversity to the local scales at which biodiversity and ecosystem functioning relationships are understood will require the development of a much better understanding of the kinds of species that are coming and going, and of the drivers of species gains in local communities.
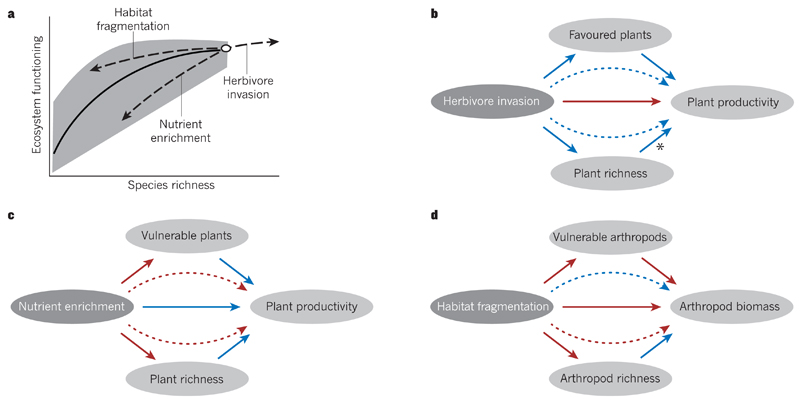
Figure 2. The influence of anthropogenic drivers on ecosystems through effects on the richness and types of species.
a, Most biodiversity experiments consider the dependence of ecosystem functioning on the random loss of species, finding that the decrease in ecosystem functioning per lost species becomes larger as species richness declines (black line). By contrast, non-random changes in ecosystem functioning and species richness (dashed lines) that result from anthropogenic drivers such as herbivore invasion, nutrient enrichment or habitat fragmentation also include shifts in the composition and traits of species that are most vulnerable or favoured, which can reinforce or offset the effects of changes in species richness. The grey region indicates potential variation in ecosystem functioning due to changes in species composition at a particular level of species richness. b–d, Positive (blue arrows) and negative (red arrows) influences of specific anthropogenic drivers of biodiversity change (dark grey ovals) are presented in the style of a structural equation model. Curved dashed arrows indicate indirect effects of specific drivers on ecosystem functioning, and horizontal solid arrows show direct effects on ecosystem functioning that are independent of changes in the composition or richness of species. Non-horizontal solid arrows represent the component relationships of indirect effects. b, Invasion by a herbivorous species has a direct negative effect on plant productivity. However, such invasion can indirectly increase plant productivity by increasing plant species richness, and these positive effects are enhanced when favoured plants contribute substantially to plant productivity46. The asterisk indicates a hypothesized relationship. c, Nutrient enrichment has a direct positive effect on plant productivity. But it can also decrease plant productivity indirectly by decreasing plant species richness, and this negative effect is reinforced when the most vulnerable plants contribute substantially to plant productivity83. d, Habitat fragmentation has a direct negative effect on arthropod biomass. It can also decrease arthropod biomass indirectly by decreasing arthropod species richness, but this negative effect is offset by shifts in species composition when the most vulnerable arthropods make only a small contribution to arthropod biomass53.
Scaling through time
Changes in biodiversity often continue to accumulate decades and even centuries after the initial disturbance. Past and present anthropogenic impacts have led to the accumulation of extinction debts, which means that a large number of species are already committed to extinctions that are yet to occur12,13,50. For instance, habitat fragmentation has created extinction debts that can be expected to unfold in periods of decades or longer, owing to the reduced size and movement of populations12,13,50. Extinction debts have been studied intensively in the past two decades, with several experiments now running for long enough to show that habitat fragmentation gradually reduces species richness in remnant fragments by 13–75% in a decade13. Similarly, the pace of climate change in the past few decades has probably created extinction debts by generating a mismatch between the thermal preferences of many species and the new climate that they are experiencing in their present geographic distribution51. The ability of species to tolerate or avoid changes in climatic conditions is limited, so the failure of some species to adjust their geographic distribution in response to climate change is expected to lead to many local, and eventually global, future extinctions51. Delayed species extinctions were viewed originally as a tragic, deterministic inevitability12 but more recently they have been viewed by some, with greater optimism, as an opportunity to avert an impending extinction crisis through habitat restoration, assisted migration and other conservation actions.
In turn, extinction debts tend to generate biodiversity-dependent debts in ecosystem functioning and ecosystem services with local and global importance43,52,53. For example, habitat loss is likely to lead to carbon emissions not only where forests rich in carbon are converted to cropland, but also from remaining forest fragments in which extinction debts are emerging43. Long before species become extinct worldwide, they first become rare or absent, and are therefore functionally extinct, in many local communities. Consequently, debts of ecosystem functioning and services are likely to occur gradually, rather than emerge only after extinction debts are paid in full. Long-term habitat fragmentation experiments find that ecosystem-functioning debts, in the form of delayed changes in nutrient cycling and changes to plant and consumer biomass, accrue in small and isolated fragments; these functioning debts amounted to a 30% loss of ecosystem functioning after 1 year, which rose to a loss of 80% after a decade13. New research is needed to forecast the magnitudes and rates of extinction, functioning and service debts.
Multiscale effects of biodiversity loss
Ecosystem functioning depends strongly on biodiversity. There is theoretical and empirical evidence to suggest that the effects of species loss on ecosystem functioning often become stronger at larger scales of space and time. Linking the impacts and dependence of people on biodiversity will therefore require a move away from intensively studied local biodiversity effects towards an examination of the under-explored effects that emerge at larger scales (Fig. 1b).
Predicting the effect of biodiversity change on ecosystem functioning at larger spatial scales requires the determination of whether local biodiversity effects are widespread and will therefore accumulate across ecosystems worldwide. Effects of changes in local species richness on ecosystem productivity have been found in naturally assembled grasslands19 and forests21 worldwide. The strength of these local relationships is similar to those found commonly in local-scale biodiversity experiments9. Aggregation of these local effects suggests that the richness of local plant species considerably affects the productivity of forests worldwide21.
Predicting the consequences of biodiversity changes at larger spatial and temporal scales also requires the consideration of positive or negative biodiversity effects that could emerge at larger scales. At the small spatial scales over which species interact, biodiversity effects result from differences between species that lead to selection effects, in which the most productive species dominate the community, or complementarity effects, which include several types of niche partitioning and facilitation, or both26. Theory predicts that the effects of changes in biodiversity on ecosystem functioning and stability could be greater at larger scales than they are, on average, for a particular location and time, owing to the performance-enhancing spatial54 and temporal32 insurance effects of biodiversity (Fig. 3) that can emerge at larger scales.
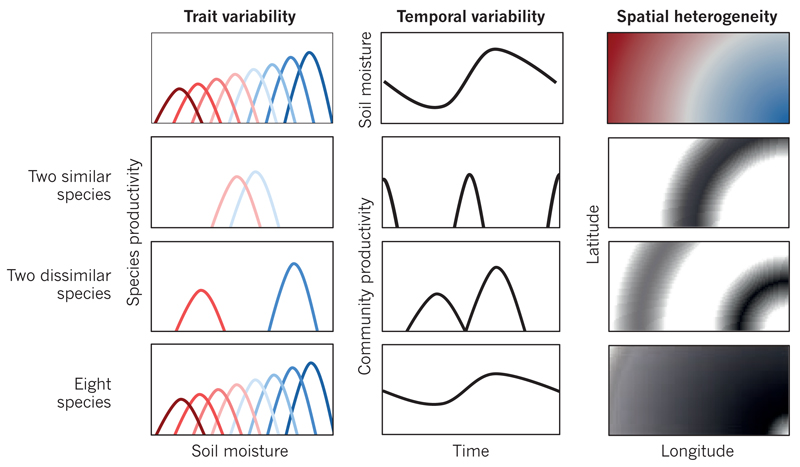
Figure 3. Temporal and spatial insurance effects enhance and stabilize ecosystem productivity.
In a hypothetical example, various plant species (coloured distributions) are most productive (row 1, left) at different levels of the environmental factor soil moisture (dry, red; wet, blue). As soil moisture changes with time (row 1, middle) and space (row 1, right), communities are dominated by the species that is most productive under the particular conditions of each community. In this case, communities that consist of two dissimilar species (row 3) or many species (row 4) are expected to be more productive and to vary less in productivity in time and space than communities with only two similar species (row 2). (Productivity levels in space (rows 2–4, right) are indicated by a gradient from black (high) to white (low).) These performance-enhancing and stabilizing temporal and spatial insurance effects can be thought of as a combination of selection and complementarity effects because they emerge when species have complementary traits and dominate where and when species are most fit.
Spatial insurance effects emerge at larger spatial scales when dispersal enables species to be present and dominate at locations where they are best adapted to the local environment (Fig. 3). At these larger scales, natural ecosystems are heterogeneous and connected by flows of species, energy and resources. This connectivity governs how changes in biodiversity affect ecosystem functioning at various scales55. Theory suggests that spatial insurance effects are maximized at intermediate rates of species dispersal that promote species coexistence, enhance ecosystem functioning and stabilize temporal variability in ecosystem functioning across the landscape54. Habitat fragmentation disrupts connectivity, which leads to species loss and the degradation of ecosystem functions across entire networks of habitat patches13,52,56,57. Empirical19,58,59 and simulation55,60 studies provide evidence that is consistent with the spatial-insurance hypothesis. For example, one study of many grasslands worldwide found that ecosystem productivity is more dependent on species richness across sites than within sites19. Another study found that different sets of species promoted ecosystem functioning at different locations58.
Temporal insurance effects emerge over longer temporal scales. Higher levels of biodiversity tend to reduce the inter-annual variability of ecosystem productivity28,61. This is because species or populations differ in their growth responses to environmental fluctuations31,58 through temporal niche complementarity32, responses to competition30, neutral random demographic variation62 or a combination of all three33. As a result, although no single species can provide ecosystem functioning at all times58, many species31 or populations61 can average out the fluctuations in the environment, providing temporal insurance32 (Fig. 3). Interestingly, temporal insurance effects tend to be stronger as the spatial scale increases because differences in β-diversity desynchronize fluctuations in ecosystem properties at different locations60. Consequently, the properties of ecosystems and services that they provide become less variable and more predictable at larger spatial scales. Anthropogenic drivers could, however, lead to a greater reduction in ecosystem stability at larger spatial scales than at smaller ones if they not only drive local species loss, but also synchronize fluctuations in species by homogenizing biota and abiotic conditions across habitat patches60.
It is less well known that the insurance effects of diversity enhance the temporal mean level of ecosystem productivity32. Therefore, just as spatial insurance effects54 can enhance biodiversity effects at larger spatial scales in heterogeneous landscapes19,58,59, temporal insurance effects32 can enhance biodiversity effects over longer temporal scales in fluctuating environments8,31,58 (Fig. 3). Conversely, if species tend to dominate communities where and when they are least productive, negative biodiversity effects could emerge at larger spatial and temporal scales — a possibility that deserves further consideration. The loss of these temporal insurance effects manifest in several ways, including increases in the temporal variance of ecosystem functioning, decreases in the temporal mean level of ecosystem functioning, and losses of community resistance to perturbations. For example, in grasslands, the loss of local plant diversity substantially reduces the resistance of ecosystem productivity to climate extremes29. New studies are needed to determine how the magnitudes of insurance effects that emerge over space and time compare to those of short-term local biodiversity effects that are evident at certain points in space and time.
As well as the emergence of new biodiversity effects across multiple years, the strength of local, intra-annual biodiversity effects might also shift as anthropogenic drivers gradually alter the niches and competitive hierarchies of species. Recent experimental results suggest that local, intra-annual biodiversity effects in grasslands will have similar or stronger magnitudes under predicted future environmental conditions63–66. For example, increasing the plant species richness of grasslands may lead to greater increases in ecosystem productivity under warmer conditions63 and elevated concentrations of atmospheric carbon dioxide64. And across all of the studies included in a meta-analysis65, increases in grassland plant species richness increased ecosystem productivity by the same amount under conditions of nutrient enrichment or drought as under ambient resource conditions, although individual studies showed a wide range of responses. More work is needed in other types of ecosystems to determine how widely applicable these results are and to understand how anthropogenic drivers affect the many mechanisms by which changes in biodiversity alter ecosystem functioning.
The ecosystem consequences of human-driven changes in biodiversity depend not only on how many species are lost or gained, but also on the kinds of species that increase or decrease in abundance. Some species are more vulnerable to anthropogenic drivers than others67, and some species are more crucial for ecosystem functioning than others68–74. The sheer number of species overall precludes the study of the vulnerability and functional roles of each. Instead, considerable progress has been made by approaches that use functional traits and phylogenetic diversity to predict which kinds of species are most vulnerable or functionally important70–75.
Many kinds of species that are crucial for ecosystem functioning are also vulnerable to anthropogenic drivers of biodiversity loss. For example, large-bodied species tend to be disproportionately vulnerable to extirpation67,76 and are particularly strong controllers of ecosystem functioning and services45,77 such as pollination and dung burial78. Ocean acidification disproportionately threatens calcifying, reef-forming corals that provide an important habitat for vast food webs of marine species that cycle nutrients, provide primary and secondary productivity, support fisheries and provide other values79. Many top predators are both overexploited and strong controllers of nutrient cycling, water quality and other ecosystem services9,77,80–82. Nutrient pollution can shift plant competitive interactions to threaten, for example, native dominant species83 or rare legumes84 and the loss of either can substantially disrupt ecosystem functioning83,85 (Fig. 2c). In all of these cases of non-random changes in biodiversity, the systematic loss of crucial components of biodiversity would affect ecosystems more than would be expected on the basis of the results of most biodiversity experiments and theory (including those we have discussed) that consider random species loss (Fig. 2a).
Further studies are needed to identify important biodiversity components across spatial and temporal scales. Various species of plant will contribute to a particular ecosystem function in different years, at different locations and under different scenarios of anthropogenic change58, and it remains a challenge to predict the kinds of species that will become increasingly dominant or rare in new ecosystems with no historical equivalent in terms of biota and abiotic conditions. Conservation efforts could be short-sighted if they prioritize current crucial components of biodiversity without considering whether the same set of components will remain important in the future.
Ecosystem services depend on biodiversity
Studies are beginning to account for the dependence of ecosystem services on biodiversity. Accounting for these relationships could help to improve forecasts of future supplies of ecosystem services, especially at the larger scales over which global extinctions are advancing (Fig. 1b).
Land managers and land-use policies often prioritize short-term local benefits without accounting fully for the costs to society that will be experienced by people elsewhere and in the future. Assessments of ecosystem services aim to correct these negative externalities by accounting for a fuller suite of benefits and costs, often by considering larger scales. For example, if both the immediate local economic benefits of expanding crop production across the landscape and the long-term global costs of carbon emissions from land conversion are accounted for, it can be more valuable to establish new parklands than to clear further land for agriculture34,35. Similarly, if both the immediate local economic benefits of enhanced crop yields and the long-term widespread costs to health that result from air and water pollution are accounted for, it can be valuable to reduce the level of fertilizer use86.
Most studies of ecosystem services consider intermediate scales of space and time that match the scales at which some decisions are made (for example, land-use decisions in the Willamette River basin in Oregon, United States34, or in the United Kingdom35). However, these scales are often smaller than the scales over which global extinctions are advancing, yet larger than those over which biodiversity effects are best understood (Fig. 1b). Perhaps partly because of this mismatch, most studies of ecosystem services do not account for the direct dependence of ecosystem functioning on biodiversity34,35. Implicitly, they assume that remaining fragments of nature will continue to provide the same flows of benefits to people in the future, regardless of how the biodiversity of such fragments might change over time43. In some cases, involving scales or locations at which biodiversity experiences little change, or ecosystem services depend much more on factors other than biodiversity, this assumption might hold. But in other cases, particularly at large scales, ignoring the dependence of ecosystem services on biodiversity will lead to poor forecasts of future supplies of ecosystem services because not all of the social costs of biodiversity loss will be accounted for.
Several studies have started to determine which ecosystem services depend on biodiversity, either directly87 or indirectly through their underlying ecosystem functions4,5,21,43. For example, there is evidence that maintaining high biodiversity supports the production of crops in agricultural systems, wood in forests, livestock forage in grasslands and fish in aquatic ecosystems5. The maintenance of high biodiversity also contributes to the regulation of pests by reducing invasion by weeds or pathogens, and of the climate by enhancing carbon storage5. However, there are many sources of uncertainty in several of the relationships between biodiversity and ecosystem services4, including mismatches between the ecosystem functions measured and the ecosystem services of interest, trade-offs between positive and negative effects of biodiversity on the supply of ecosystem services, and context-dependent patterns. The direct contributions of biodiversity to a large number of ecosystem services, including those related to cultural identity and aesthetic inspiration, remain under-explored. There is evidence, however, that people appreciate a high richness and evenness of plant species87.
Efforts to estimate the contributions of biodiversity to the monetary value of some ecosystem services21,43,88 have also begun, revealing that, if well-directed, the benefits of conserving biodiversity could be much greater than the costs. For example, the value of biodiversity in maintaining carbon storage is estimated as being on the order of US$0.3 trillion–3.1 trillion43 and the value of tree diversity in commercial forest productivity is about $166 billion–490 billion per year21. These values are much greater than the current conservation expenditure worldwide (estimated to be $21.5 billion per year89) or the cost of meeting global biodiversity conservation targets (estimated to be $76.1 billion per year90). Estimates of the monetary value of maintaining natural habitats are even larger91 than the value of maintaining biodiversity within habitats. Furthermore, we emphasize that biodiversity contributes substantially to many valuable benefits to society that cannot be monetized accurately, including aesthetic inspiration87. As the benefits of conservation are increasingly weighed against their costs, it will be important to account for both the indirect dependence of ecosystem services on biodiversity, which is mediated by ecosystem functioning, and the direct contributions of biodiversity to other ecosystem services, many of which are difficult or impossible to monetize. Both of these contributions of biodiversity to ecosystem services are missing from most valuation studies at present.
To further include the role of biodiversity in assessments of ecosystem services, an important next step will be to identify biodiversity components that are crucial for ecosystem functions that underlie ecosystem services. This is not an easy task because no species is able to maximize all ecosystem functions or services27,81,92,93. Trade-offs limit the extent to which species that have traits associated with particular functions (for example, high primary productivity) can also provide other functions (for example, drought resistance). Although a carefully chosen monoculture may perform as well as a mixture of species for a single function under a particular set of environmental conditions8, many species contribute to many ecosystem functions under a wider range of conditions58,81,92,93. Therefore, multifunctional ecosystems across space and time depend not only on a few dominant species94, but also on the contributions of many rare species55,95 at several trophic levels93. Depending on whether the aim is to maximize a particular ecosystem service under carefully controlled environmental conditions (for example, maize yield) or a larger bundle of services across a wider range of conditions (for example, forage production and carbon storage across extensive landscapes), the best option might be to retain either a subset of species with particular traits or a diverse community of species with a wide range of traits.
Strengthening biodiversity science for policy
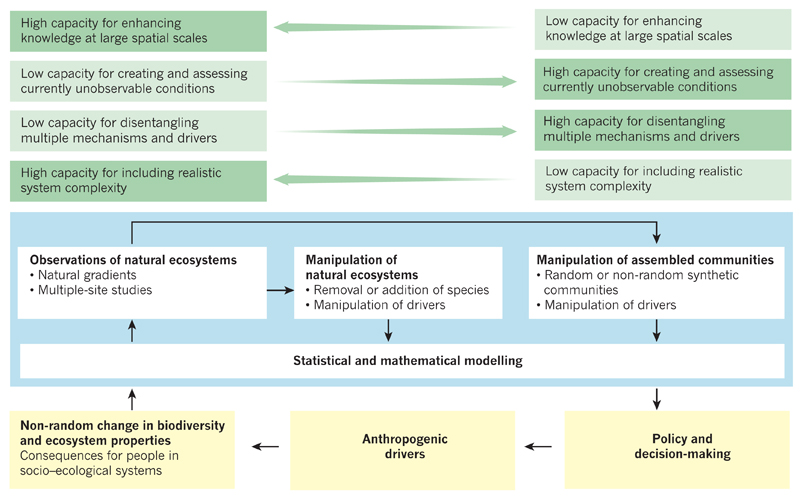
As well as developing multiscale knowledge (Fig. 1b), biodiversity science will need to expand in several new directions to support emerging policy priorities. The combination of increasing pressures from anthropogenic drivers of biodiversity loss together with growing demands for all types of ecosystem services in the coming decades will produce unprecedented challenges for policy and decision-makers. Well-designed research that addresses the impacts of biodiversity changes could help to explore potential solutions to these challenges now, using combinations of theory, observation and experiment (Fig. 4). Observations, but not experiments, are uniquely able to assess relationships at large spatial scales in natural ecosystems that are undergoing non-random changes in biodiversity. Observational studies are increasingly able to use statistical approaches to disentangle the effects of changes in biodiversity and abiotic factors on ecosystems19, bringing the conclusions of empirical studies closer to the spatial scales at which populations and species are lost, and at which the societal benefits of nature are delivered to people. Experiments, but not observations, are able to create and assess future conditions that are unobservable at present. Both types of empirical studies will therefore be needed to consider the large spatial and temporal scales at which human impacts on biodiversity are expected to undermine the dependence of people on biodiversity the most. Furthermore, functional trait and phylogenetic approaches71,73,75 (Box 1) are uniquely able to generalize across types of species, removing the need to study whether each species is vulnerable to anthropogenic drivers and crucial for ecosystem functioning. An important next step will be to predict the types of species that will be most vulnerable and crucial across spatially heterogeneous, temporally fluctuating and globally shifting environmental conditions.
Figure 4. Complementary approaches for understanding the ecosystem consequences of human-driven biodiversity change.
Four main approaches to understanding the effects of anthropogenic biodiversity change on ecosystems are shown (blue). Each approach has certain strengths and weaknesses (green) and enriches the others in several ways, and it is the combination of their results that best informs policy and decision-making at the scales at which populations and species are changed, and at which ecosystem services are delivered (yellow). The simultaneous use of all four approaches is therefore crucial for improving our knowledge of socio–ecological systems and to inform policy and decision-making.
Biodiversity science is also expanding to consider the dynamic interactions between people and nature in socio–ecological systems96 (Fig. 1a). For example, the conceptual framework of the biodiversity–policy interface outlined by IPBES10 includes many of the complex interactions that occur between the natural world and people. The expansion has emerged partly from shifts in the way that conservation has been framed, first moving from protecting nature from human threats towards conserving nature for its potential benefits to humans, and now towards emphasizing the interdependence of nature and people97. It has also coincided with increased recognition by the policy community that biodiversity supports human development and must be protected to fulfil fundamental human needs. For example, 2 of the 17 UN Sustainable Development Goals address marine and terrestrial biodiversity and natural resources directly, and several other goals address biodiversity through their specific targets, including Goal 2, which aims to end hunger and malnutrition. Biodiversity science will also need to consider the wider range of instrumental and relational values of biodiversity that are now being recognized98, as well as the contribution that biodiversity makes to the quality of life beyond its role in ecosystem functioning. Expanding in these ways adds both breadth and complexity to biodiversity science and policy. One way to make such endeavours tractable will be to focus on the biodiversity and ecosystem functions that underpin crucial ecosystem services, perhaps by working backwards from human well-being to services, functions and biodiversity in the interacting elements that are shown in Fig. 1a.
There is now abundant evidence that human-driven biodiversity changes can substantially affect several ecosystem services by altering ecosystem functioning and stability at multiple scales of space and time. Environmental policy needs to account for these important effects by considering biodiversity not only as an output but also as an input of environmental policy scenarios99, including future climate scenarios. In this way, well-directed biodiversity research and policy design could together secure the valuable, and often irreplaceable, benefits of biodiversity for future generations, even under conditions of rapid global change.
Acknowledgements
F.I. acknowledges support from the US National Science Foundation (award number 1234162). A.G. is supported by a Killam Research Fellowship and a Canada Research Chair. M.L. is grateful for funding from the Laboratoires d’Excellence programme (Project TULIP, grant ANR-10-LABX-41) and an Advanced Grant (BIOSTASES project, grant agreement number 666971), funded by the European Research Council under Horizon 2020, the European Union Framework Programme for Research and Innovation. S.D. acknowledges support from Fondo para la Investigación Científica y Tecnológica (FONCyT), Secretaría de Investigación, Ciencia y Técnica (SECyT) at Universidad Nacional de Córdoba and the National Scientific and Technical Research Council (CONICET) of Argentina. D.W. acknowledges support from the EU BiodivERsA Forecasting Future Invasions and their Impacts (FFII) programme and the Swedish Research Council.
References
- 1.Barnosky AD, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471:51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 2.Ceballos G, et al. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci Adv. 2015;1:e1400253. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimm SL, et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science. 2014;344:1246752. doi: 10.1126/science.1246752. [DOI] [PubMed] [Google Scholar]
- 4.Balvanera P, et al. Linking biodiversity and ecosystem services: current uncertainties and the necessary next steps. Bioscience. 2014;64:49–57. [Google Scholar]
- 5.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [This Review connects research on biodiversity and ecosystem functioning with research on ecosystem services.] [DOI] [PubMed] [Google Scholar]
- 6.Chapin FS, et al. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 7.Tilman D, Isbell F, Cowles JM. Biodiversity and ecosystem functioning. Annu Rev Ecol Evol Syst. 2014;45:471–493. [Google Scholar]
- 8.Cardinale BJ, et al. The functional role of producer diversity in ecosystems. Am J Bot. 2011;98:572–592. doi: 10.3732/ajb.1000364. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor MI, et al. A general biodiversity–function relationship is mediated by trophic level. Oikos. 2017;126:18–31. [Google Scholar]
- 10.Díaz S, et al. The IPBES Conceptual Framework — connecting nature and people. Curr Opin in Env Sust. 2015;14:1–16. [Google Scholar]
- 11.Newbold T, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [This article quantifies past changes and projects future changes in local species richness in response to land-use changes using an unparalleled global database of biodiversity observations.] [DOI] [PubMed] [Google Scholar]
- 12.Tilman D, May RM, Lehman CL, Nowak MA. Habitat destruction and the extinction debt. Nature. 1994;371:65–66. [Google Scholar]
- 13.Haddad NM, et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv. 2015;1:e1500052. doi: 10.1126/sciadv.1500052. [This paper synthesizes the short-term and long-term impacts of experimental habitat loss on biodiversity and ecosystem functioning.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butchart SHM, et al. Global biodiversity: indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 15.Capinha C, Essl F, Seebens H, Moser D, Pereira HM. The dispersal of alien species redefines biogeography in the Anthropocene. Science. 2015;348:1248–1251. doi: 10.1126/science.aaa8913. [DOI] [PubMed] [Google Scholar]
- 16.Reich PB, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336:589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 17.Tilman D, Lehman CL, Thomson KT. Plant diversity and ecosystem productivity: theoretical considerations. Proc Natl Acad Sci USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loreau M. From Populations to Ecosystems: Theoretical Foundations for a New Ecological Synthesis. Princeton Univ. Press; 2010. [Google Scholar]
- 19.Grace JB, et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature. 2016;529:390–393. doi: 10.1038/nature16524. [This article shows that productivity depends on plant diversity, especially across sites, in naturally assembled grasslands worldwide.] [DOI] [PubMed] [Google Scholar]
- 20.Hautier Y, et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- 21.Liang J, et al. Positive biodiversity–productivity relationship predominant in global forests. Science. 2016;354:aaf8957. doi: 10.1126/science.aaf8957. [This paper describes how the loss of tree diversity will lead to the loss of productivity in forests worldwide and quantifies the potential economic costs.] [DOI] [PubMed] [Google Scholar]
- 22.Paquette A, Messier C. The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob Ecol Biogeogr. 2011;20:170–180. [Google Scholar]
- 23.Gamfeldt L, et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nature Commun. 2013;4:1340. doi: 10.1038/ncomms2328. [This article shows that a greater number of tree species is more effective at delivering multiple ecosystem services in the forests of Sweden.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maestre FT, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy JE, Lefcheck JS, Stuart-Smith RD, Navarrete SA, Edgar GJ. Biodiversity enhances reef fish biomass and resistance to climate change. Proc Natl Acad Sci USA. 2016;113:6230–6235. doi: 10.1073/pnas.1524465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull LA, Isbell F, Purves DW, Loreau M, Hector A. Understanding the value of plant diversity for ecosystem functioning through niche theory. Proc R Soc B. 2016;283 doi: 10.1098/rspb.2016.0536. 20160536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross K, et al. Species richness and the temporal stability of biomass production: a new analysis of recent biodiversity experiments. Am Nat. 2014;183:1–12. doi: 10.1086/673915. [DOI] [PubMed] [Google Scholar]
- 29.Isbell F, et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature. 2015;526:574–577. doi: 10.1038/nature15374. [DOI] [PubMed] [Google Scholar]
- 30.Lehman CL, Tilman D. Biodiversity, stability, and productivity in competitive communities. Am Nat. 2000;156:534–552. doi: 10.1086/303402. [DOI] [PubMed] [Google Scholar]
- 31.Allan E, et al. More diverse plant communities have higher functioning over time due to turnover in complementary dominant species. Proc Natl Acad Sci USA. 2011;108:17034–17039. doi: 10.1073/pnas.1104015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [This paper and ref. 54 provide the theoretical basis for temporal and spatial insurance effects, which are now being tested empirically.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- 34.Nelson E, et al. Modeling multiple ecosystem services, biodiversity conservation, commodity production, and tradeoffs at landscape scales. Front Ecol Environ. 2009;7:4–11. [Google Scholar]
- 35.Bateman IJ, et al. Bringing ecosystem services into economic decision-making: land use in the United Kingdom. Science. 2013;341:45–50. doi: 10.1126/science.1234379. [DOI] [PubMed] [Google Scholar]
- 36.Hughes JB, Daily GC, Ehrlich PR. Population diversity: its extent and extinction. Science. 1997;278:689–692. doi: 10.1126/science.278.5338.689. [DOI] [PubMed] [Google Scholar]
- 37.Sax DF, Gaines SD. Species diversity: from global decreases to local increases. Trends Ecol Evol. 2003;18:561–566. [Google Scholar]
- 38.Hill SLL, et al. Reconciling biodiversity indicators to guide understanding and action. Conserv Lett. 2016;9:405–412. [Google Scholar]
- 39.Dornelas M, et al. Assemblage time series reveal biodiversity change but not systematic loss. Science. 2014;344:296–299. doi: 10.1126/science.1248484. [DOI] [PubMed] [Google Scholar]
- 40.Elahi R, et al. Recent trends in local-scale marine biodiversity reflect community structure and human impacts. Curr Biol. 2015;25:1938–1943. doi: 10.1016/j.cub.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 41.Vellend M, et al. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc Natl Acad Sci USA. 2013;110:19456–19459. doi: 10.1073/pnas.1312779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez A, et al. Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology. 2016;97:1949–1960. doi: 10.1890/15-1759.1. [DOI] [PubMed] [Google Scholar]
- 43.Isbell F, Tilman D, Polasky S, Loreau M. The biodiversity-dependent ecosystem service debt. Ecol Lett. 2015;18:119–134. doi: 10.1111/ele.12393. [DOI] [PubMed] [Google Scholar]
- 44.Murphy GEP, Romanuk TN. A meta-analysis of declines in local species richness from human disturbances. Ecol Evol. 2014;4:91–103. doi: 10.1002/ece3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wardle DA, Bardgett RD, Callaway RM, van der Putten WH. Terrestrial ecosystem responses to species gains and losses. Science. 2011;332:1273–1277. doi: 10.1126/science.1197479. [This review examines worldwide evidence of the ecosystem effects of losses and gains of particular species or groups of species with a certain function.] [DOI] [PubMed] [Google Scholar]
- 46.Bellingham PJ, et al. Browsing by an invasive herbivore promotes development of plant and soil communities during primary succession. J Ecol. 2016;104:1505–1517. [Google Scholar]
- 47.Buckley YM, Catford J. Does the biogeographic origin of species matter? Ecological effects of native and non-native species and the use of origin to guide management. J Ecol. 2016;104:4–17. [Google Scholar]
- 48.Wilsey BJ, Daneshgar PP, Polley HW. Biodiversity, phenology and temporal niche differences between native- and novel exotic-dominated grasslands. Perspect Plant Ecol Evol Syst. 2011;13:265–276. [Google Scholar]
- 49.Zuppinger-Dingley D, et al. Selection for niche differentiation in plant communities increases biodiversity effects. Nature. 2014;515:108–111. doi: 10.1038/nature13869. [DOI] [PubMed] [Google Scholar]
- 50.Ewers RM, Didham RK. Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc. 2006;81:117–142. doi: 10.1017/S1464793105006949. [DOI] [PubMed] [Google Scholar]
- 51.Bertrand R, et al. Ecological constraints increase the climatic debt in forests. Nature Commun. 2016;7:12643. doi: 10.1038/ncomms12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez A, Mouquet N, Loreau M. In: Biodiversity, Ecosystem Functioning, and Human Wellbeing. Naeem S, Bunker DE, Hector A, Loreau M, Perrings C, editors. Oxford Univ. Press; 2009. pp. 134–146. Ch. 10. [Google Scholar]
- 53.Gonzalez A, Chaneton EJ. Heterotroph species extinction, abundance and biomass dynamics in an experimentally fragmented microecosystem. J Anim Ecol. 2002;71:594–602. [Google Scholar]
- 54.Loreau M, Mouquet N, Gonzalez A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci USA. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson PL, Gonzalez A. Ecosystem multifunctionality in metacommunities. Ecology. 2016;97:2867–2879. doi: 10.1002/ecy.1502. [DOI] [PubMed] [Google Scholar]
- 56.Laurance WF, et al. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol. 2002;16:605–618. [Google Scholar]
- 57.Staddon P, Lindo Z, Crittenden PD, Gilbert F, Gonzalez A. Connectivity, non-random extinction and ecosystem function in experimental metacommunities. Ecol Lett. 2010;13:543–552. doi: 10.1111/j.1461-0248.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 58.Isbell F, et al. High plant diversity is needed to maintain ecosystem services. Nature. 2011;477:199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 59.Venail PA, et al. Diversity and productivity peak at intermediate dispersal rate in evolving metacommunities. Nature. 2008;452:210–214. doi: 10.1038/nature06554. [DOI] [PubMed] [Google Scholar]
- 60.Wang S, Loreau M. Biodiversity and ecosystem stability across scales in metacommunities. Ecol Lett. 2016;19:510–518. doi: 10.1111/ele.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schindler DE, et al. Population diversity and the portfolio effect in an exploited species. Nature. 2010;465:609–612. doi: 10.1038/nature09060. [DOI] [PubMed] [Google Scholar]
- 62.de Mazancourt C, et al. Predicting ecosystem stability from community composition and biodiversity. Ecol Lett. 2013;16:617–625. doi: 10.1111/ele.12088. [DOI] [PubMed] [Google Scholar]
- 63.Cowles JM, Wragg PD, Wright AJ, Powers JS, Tilman D. Shifting grassland plant community structure drives positive interactive effects of warming and diversity on aboveground net primary productivity. Glob Change Biol. 2016;22:741–749. doi: 10.1111/gcb.13111. [DOI] [PubMed] [Google Scholar]
- 64.Reich PB, et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature. 2001;410:809–812. doi: 10.1038/35071062. [DOI] [PubMed] [Google Scholar]
- 65.Craven D, et al. Plant diversity effects on grassland productivity are robust to both nutrient enrichment and drought. Phil Trans R Soc B. 2016;371 doi: 10.1098/rstb.2015.0277. 20150277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooper D, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 67.McKinney ML. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu Rev Ecol Syst. 1997;28:495–516. [Google Scholar]
- 68.Hobbie SE. Effects of plant species on nutrient cycling. Trends Ecol Evol. 1992;7:336–339. doi: 10.1016/0169-5347(92)90126-V. [DOI] [PubMed] [Google Scholar]
- 69.Hooper DU, Vitousek PM. The effects of plant composition and diversity on ecosystem processes. Science. 1997;277:1302–1305. [Google Scholar]
- 70.Díaz S, Cabido M. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol. 2001;16:646–655. [Google Scholar]
- 71.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol. 2002;16:545–556. [Google Scholar]
- 72.Suding KN, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Change Biol. 2008;14:1125–1140. [Google Scholar]
- 73.Díaz S, et al. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol Evol. 2013;3:2958–2975. doi: 10.1002/ece3.601. [This article provides a conceptual basis and case studies for linking functional traits and phylogenetic diversity to ecosystem services.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naeem S, Duffy JE, Zavaleta E. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 75.Cadotte MW, Cardinale BJ, Oakley TH. Evolutionary history and the effect of biodiversity on plant productivity. Proc Natl Acad Sci USA. 2008;105:17012–17017. doi: 10.1073/pnas.0805962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Payne JL, Bush AM, Heim NA, Knope ML, McCauley DJ. Ecological selectivity of the emerging mass extinction in the oceans. Science. 2016;353:1284–1286. doi: 10.1126/science.aaf2416. [DOI] [PubMed] [Google Scholar]
- 77.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 78.Larsen TH, Williams NM, Kremen C. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol Lett. 2005;8:538–547. doi: 10.1111/j.1461-0248.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 79.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA. 2008;105:17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duffy JE, Richardson JP, Canuel EA. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol Lett. 2003;6:637–645. [Google Scholar]
- 81.Lefcheck JS, et al. Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nature Commun. 2015;6:6936. doi: 10.1038/ncomms7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dirzo R, et al. Defaunation in the Anthropocene. Science. 2014;345:401–406. doi: 10.1126/science.1251817. [DOI] [PubMed] [Google Scholar]
- 83.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci USA. 2013;110:11911–11916. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suding KN, et al. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fornara DA, Tilman D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J Ecol. 2008;96:314–322. [Google Scholar]
- 86.Keeler BL, et al. The social costs of nitrogen. Sci Adv. 2016;2:e1600219. doi: 10.1126/sciadv.1600219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindemann-Matthies P, Junge X, Matthies D. The influence of plant diversity on people’s perception and aesthetic appreciation of grassland vegetation. Biol Conserv. 2010;143:195–202. [Google Scholar]
- 88.Mace GM, Norris K, Fitter AH. Biodiversity and ecosystem services: a multilayered relationship. Trends Ecol Evol. 2012;27:19–26. doi: 10.1016/j.tree.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 89.Waldron A, et al. Targeting global conservation funding to limit immediate biodiversity declines. Proc Natl Acad Sci USA. 2013;110:12144–12148. doi: 10.1073/pnas.1221370110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCarthy DP, et al. Financial costs of meeting global biodiversity conservation targets: current spending and unmet needs. Science. 2012;338:946–949. doi: 10.1126/science.1229803. [DOI] [PubMed] [Google Scholar]
- 91.Costanza R, et al. Changes in the global value of ecosystem services. Glob Environ Change. 2014;26:152–158. [Google Scholar]
- 92.Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448:188–190. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- 93.Soliveres S, et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature. 2016;536:456–459. doi: 10.1038/nature19092. [DOI] [PubMed] [Google Scholar]
- 94.Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol. 1998;86:902–910. [Google Scholar]
- 95.Soliveres S, et al. Locally rare species influence grassland ecosystem multifunctionality. Phil Trans R Soc B. 2016;371 doi: 10.1098/rstb.2015.0269. 20150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carpenter SR, et al. Science for managing ecosystem services: beyond the Millennium Ecosystem Assessment. Proc Natl Acad Sci USA. 2009;106:1305–1312. doi: 10.1073/pnas.0808772106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mace GM. Whose conservation? Science. 2014;345:1558–1560. doi: 10.1126/science.1254704. [DOI] [PubMed] [Google Scholar]
- 98.Chan KMA, et al. Opinion: why protect nature? Rethinking values and the environment. Proc Natl Acad Sci USA. 2016;113:1462–1465. doi: 10.1073/pnas.1525002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.IPBES. The Methodological Assessment Report on Scenarios and Models of Biodiversity and Ecosystem Services: Summary for Policymakers. IPBES; 2016. [This report evaluates scenarios and models to explore plausible future changes in biodiversity, and the societal consequences, that result from human activities, and provides a road map for the use of these models.] [Google Scholar]
- 100.Purvis A, Hector A. Getting the measure of biodiversity. Nature. 2000;405:212–219. doi: 10.1038/35012221. [DOI] [PubMed] [Google Scholar]